SUMMARY
The suspected link between infection by Zika virus (ZIKV), a re-emerging flavivirus, and microcephaly is an urgent global health concern. The direct target cells of ZIKV in the developing human fetus are not clear. Here we show that a strain of the ZIKV MR766, serially passaged in monkey and mosquito cells, efficiently infects human cortical neural progenitor cells (hNPCs) derived from induced pluripotent stem cells. Infected hNPCs further release infectious ZIKV particles. Importantly, ZIKV infection increases cell death and dysregulates cell cycle progression, resulting in attenuated hNPC growth. Global gene expression analysis of infected hNPCs reveals transcriptional dysregulation, notably of cell cycle-related pathways. Our results identify human cortical neural precursor cells as a direct ZIKV target. In addition, we establish a tractable experimental model system to investigate the impact and mechanism of ZIKV on human brain development and provide a platform to screen therapeutic compounds.
Graphical Abstract
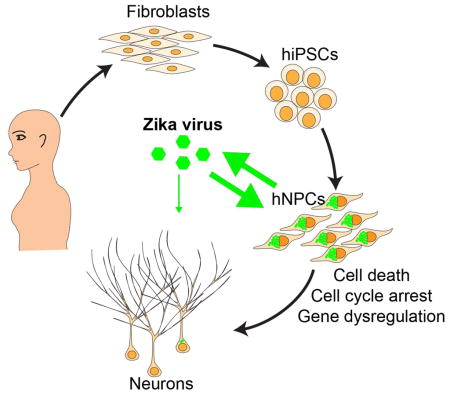
Zika virus (ZIKV), a mosquito-borne flaviviruss, is now reported to be circulating in 26 countries and territories in Latin America and the Caribbean (Petersen et al., 2016). While infected individuals can often be asymptomatic or have only mild symptoms, of mounting concern are reports linking ZIKV infection to fetal and newborn microcephaly and serious neurological complications, such as Guillain-Barré syndrome (Petersen et al., 2016). The World Health Organization declared a Public Health Emergency of International Concern on February 1 of 2016 (Heymann et al., 2016). ZIKV infects human skin cells, consistent with its major transmission route (Hamel et al., 2015). ZIKV was detected in the amniotic fluid of two pregnant women whose fetuses had been diagnosed with microcephaly (Calvet et al., 2016), suggesting that ZIKV can cross the placental barrier. ZIKV was also found in microcephalic fetal brain tissue (Mlakar et al., 2016). Because so little is known about direct cell targets and mechanisms of ZIKV, and access to fetal human brain tissue is limited, there is an urgent need to develop a new strategy to determine whether there is a causal relationship between ZIKV infection and microcephaly. Here we used human induced pluripotent stem cells (hiPSCs) as an in vitro model to investigate whether ZIKV directly infects human neural cells and the nature of its impact.
We obtained a ZIKV stock from an infected rhesus Macaca cell line LLC-MK2. We passaged the virus in the mosquito C6/C36 cell line and titered collected ZIKV on Vero cells, an interferon-deficient monkey cell line commonly used to titer viruses. Sequences of multiple RT-PCR fragments generated from this stock (Figure S1A) matched the sequence of MR766, the original ZIKV strain that likely passed from an infected rhesus monkey to mosquitos (Dick et al., 1952). We first tested several human cell lines and found varying levels of susceptibility to ZIKV infection (Table S1). Notably, the human embryonic kidney cell line HEK293T showed low permissiveness for ZIKV infection (Figure S1C).
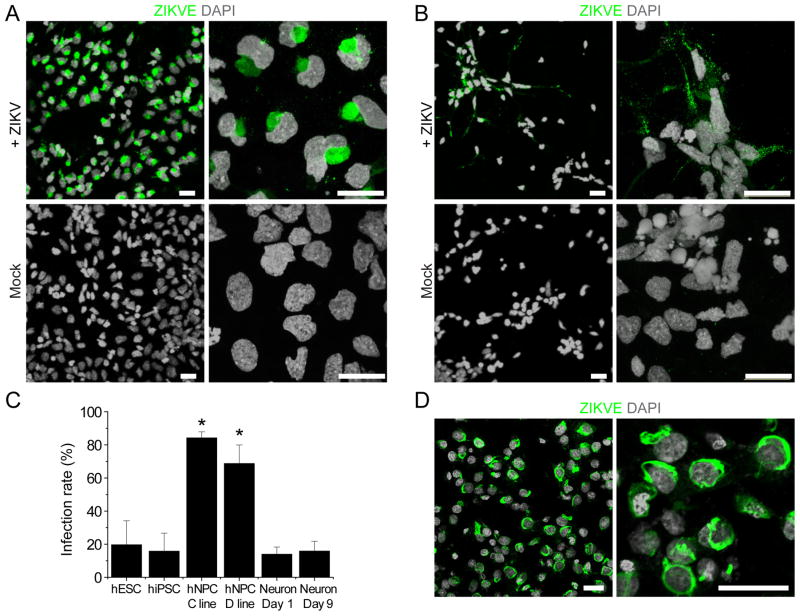
To identify direct target cells of ZIKV in the human neural lineage, we used an highly efficient protocol to differentiate hiPSCs into forebrain-specific human neural progenitor cells (hNPCs), which can be further differentiated into cortical neurons (Wen et al., 2014). The in vivo ZIKA concentration is currently unknown. We performed infections at a low multiplicity of infection (MOI < 0.1) and the medium containing virus inoculum was removed after a 2-hour incubation. Infection rates were then quantified 56 hours later with RT-PCR using MR766-specific primers (Figure S1A) and with immunocytochemistry using an anti-ZIKV envelope antibody (Figure 1A–B). The hNPCs were readily infected by ZIKV in vitro, with the infection spreading to 65–90% of the cells within three days of inoculation (Figure 1A, C). Quantitative analysis showed similar results for hNPCs derived from hiPSC lines of two different subjects (Figure 1C). As a control, we also exposed human embryonic stem cells (hESCs), hiPSCs, and immature cortical neurons to ZIKV under the same condition. hESCs and hiPSCs could also be infected by ZIKV, but the infection was limited to a few cells at the colony edge with reduced expression of the pluripotent marker NANOG (Figure 1C and S1D; Table S1). Immature neurons differentiated from hNPCs also exhibited lower levels of infection under our conditions (Figure 1B–C). Together, these results establish that hNPCs, a constitutive population of the developing embryonic brain, are a direct cell target of ZIKV.
Figure 1. ZIKV Infects hiPSC-derived Neural Progenitor Cells with High Efficiency.
(A–B) Sample confocal images of forebrain-specific hNPCs (A) and immature neurons (B) 56 hours after infection with ZIKV supernatant, and immunostained for ZIKV envelop protein (ZIKVE; green) and DAPI (gray). Cells were differentiated from the C1-2 hiPSC line. Scale bars: 20 μm.
(C) Quantification of infection efficiency for different cell types, including hESCs, hiPSCs, hNPCS derived from two different hiPSCs, and immature neurons 1 or 9 days after differentiation from hNPCs. Both hESCs and hiPSCs were analyzed 72 hours after infection, whereas all other cells were analyzed 56 hours after infection. Numbers associated with bar graphs indicate numbers of independent experiments. Values represent mean ± SD (*P < 0.01; Student’s t-test)
(D) Production of infectious ZIKV particles by infected hNPCs. Supernatant from hNPC cultures 72 hours after ZIKV infection was collected and added to Vero cells for 2 hours. The Vero cells were further cultured for 48 hours. Shown are sample images of ZIKVE immunostaining (green) and DAPI (gray). Scale bars: 20 μm.
ZIKV envelope immunostaining exhibited the characteristic intracellular “virus factory” pattern of flaviviruses (Romero-Brey and Bartenschlager, 2014) (Figure 1A). We therefore tested infectivity using supernatant from infected hNPCs and observed robust infection of Vero cells (Figure 1D), indicating that productive infection of hNPCs leads to efficient secretion of infectious ZIKV particles.
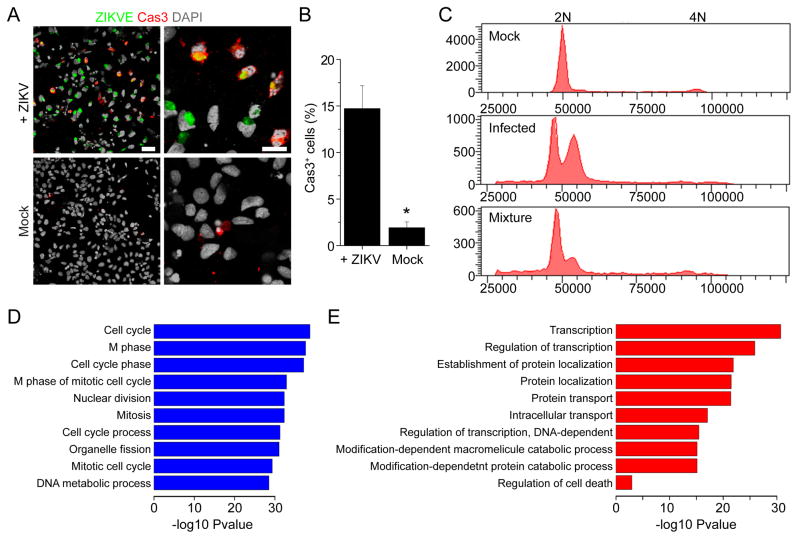
We next determined the potential impact of ZIKV infection on hNPCs. We found a 29.9 + 6.6% reduction in the total number of viable cells 66–72 hours after ZIKV infection, as compared to the mock infection (n = 3). Interestingly, ZIKV infection led to significantly higher caspase-3 activation in hNPCs 3 days after infection, as compared to the mock infection, suggesting increased cell death (Figure 2A–B). Furthermore, analysis of DNA content by flow cytometry suggested cell cycle perturbation of infected hNPCs (Figure 2C and S2A). Therefore, ZIKV infection of hNPCs leads to attenuated growth of this cell population that is due, at least partly, to both increased cell death and cell cycle dysregulation.
Figure 2. ZIKV-infected hNPCs Exhibit Increased Cell Death and Dysregulated Cell Cycle Progression and Gene Expression.
(A–B) Increased cell death of ZIKV-infected hNPCs. Shown in (A) are sample images of immunostaining of hNPCs for ZIKVE (green) and cleaved-caspase 3 (Cas3; red) and DAPI (gray) 72 hours after ZIKV infection. Scale bars: 20 μm. Shown in (B) is the quantification. Values represent mean ± SEM (n = 6; *P < 0.01; Student’s t-test).
(C) Cell cycle perturbation of hNPCs infected by ZIKV. Shown are sample flow cytometry analyses of distributions of hNPCs (from the C1-2 line) at different phases of cell cycle 72 hours after ZIKV or Mock infection. For the mixture sample, mock and infected hNPCs were mixed at a ratio of 1:1 following Propidium Iodide staining of each sample.
(D–E) RNA-seq analysis of hNPCs (C1-2 line) 56 hours after ZIKV or mock infection. Genes with significant differences in expression between infected and uninfected hNPCs were subjected to GO analyses. The top 10 most significant terms are shown for downregulated (D) and upregulated (E) genes, respectively. The −log10 p-values are indicated by bar plots. An additional term of regulation of programmed cell death is also shown for upregulated genes (E).
To investigate the impact of ZIKV infection on hNPCs at the molecular level, we employed global transcriptome analyses (RNA-seq). Our genome-wide analyses identified a large number of differentially expressed genes upon viral infection (Figure S2B and Table S2). Gene Ontology analyses revealed a particular enrichment of downregulated genes in cell cycle-related pathways (Figure 2D), which is consistent with our flow cytometry findings (Figure 2C). Upregulated genes were primarily enriched in transcription, protein transport and catabolic processes (Figure 2E). Consistent with increased caspase-3 activation observed by immunocytochemistry (Figure 2A–B), RNA-seq analysis revealed upregulation of genes involved in regulation of apoptotic pathway, including caspase-3 (Figure 2E). These global transcriptome datasets not only support our cell biology findings, but also provide a valuable resource for the field.
It is not known whether specific strains of ZIKV circulating in geographically diverse parts of the world differ in their ability to impact neural development and the stain we used had been discovered prior to the current reports of a potential epidemiologic link between ZIKV and microcephaly. Nevertheless, our results clearly demonstrate that ZIKV can directly infect human embryonic cortical neural precursor cells in vitro with high efficiency, and that infection of hNPCs leads to attenuated population growth through virally-induced caspase 3-mediated apoptosis and cell cycle dysregulation. Infected hNPCs also release infectious viral particles, which presents a significant clinical challenge for developing effective therapeutics to arrest or block the impact of infection. Future studies using the hiPSC/hNPC model can determine whether various ZIKV strains impact hNPCs differently and, conversely, whether a single ZIKV strain differentially affects hNPCs from hiPSCs of various human populations.
Flaviviruses tend to have broad cellular tropisms and multiple factors contribute to pathogenic outcomes, including specific cellular response and tissue accessibility. Dengue virus infects cells of several lineages and hematopoietic cells play an essential role in the associated pathogenesis (Pham et al., 2012). West Nile virus infects epithelial cells of multiple tissues and can be neuroinvasive (Suthar et al., 2013). We note that ZIKV also infects other human cell types, including skin cells and fibroblasts (Hamel et al., 2015), and it remains unknown how ZIKV may gain access to the fetal brain (Mlakar et al., 2016). The capacity of ZIKV to infect hNPCs and attenuate their growth underscores the urgent need for more research into the role of these cells in putative ZIKV-related neuropathology. The finding that ZIKV also infects immature neurons raises critical questions about pathological effects on neurons and other neural cell types in the brain, as well as potential long-term consequences. Intriguingly, an early animal study showed ZIKV infection of neurons and astrocytes in mice and observed enlarged astrocytes (Bell et al., 1971). Our study also raises the question of whether ZIKV infects neural stem cells in adult humans (Bond et al., 2015).
In summary, our results fill a major gap in our knowledge about ZIKV biology and serve as an entry point to establish a mechanistic link between ZIKV and microcephaly. Our study also provides a tractable experimental system for modeling the impact of ZIKV on neural development and for investigating underlying cellular and molecular mechanisms. Of equal importance, our hNPC model and robust cellular phenotype comprise a readily scalable platform for high-throughput screens to prevent ZIKV infection of hNPCs and to ameliorate its pathological effects during neural development.
Supplementary Material
Highlights.
Zika virus (ZIKV) infects human embryonic cortical neural progenitor cells (hNPCs)
ZIKV-infected hNPCs produce infectious ZIKV particles
ZIKV infection leads to increased cell death of hNPCs
ZIKV infection dysregulates cell cycle and transcription in hNPCs
Acknowledgments
We thank Yichen Cheng, Taylor Lee, and Jianshe Lang of Tang lab, Lihong Liu and Yuan Cai of Ming and Song labs, Luoxiu Huang of Jin lab for technical assistance, Zhiheng Xu and additional lab members for suggestions, and Timothy Megraw for assistance with confocal imaging. H.T. thank the College of Arts and Sciences and Department of Biological Science at Florida State University for seed funding. This work was partially supported by NIH (AI119530/AI111250 to H.T.; NS047344 to H.S., NS048271/NS095348 to G-l.M. and NS051630/NS079625/MH102690 to P.J.), MSCRF (to H.S.) and start-up fund (to H.S.).
Footnotes
ACCESSION NUMBER
The accession number for RNA-seq data reported in this paper is GEO: GSE78711.
Supplemental information includes two figures, two tables, experimental procedures, and references.
AUTHOR CONTRIBUTIONS
H.T, H.S. and G-l. Ming conceived of the research, designed the study, and wrote the manuscript. C.H., S.C.O., Z.W. and X.Q. performed experiments, analyzed data and contributed equally to this study. Y.L., B.Y., J.S., F.Z. P.J. performed RNA-seq analysis, E.M.L., K.M.C., R.A.D. contributed to additional data collection. All authors commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35:183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- Bond AM, Ming G-l, Song H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonca MC, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PF, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387:719–21. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. Zika virus associated with microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, Dar O, Mattes F, Kidd M, Ippolito G, et al. Rapid spread of Zika virus in the Americas - Implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis. 2016;44:11–15. doi: 10.1016/j.ijid.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Pham AM, Langlois RA, TenOever BR. Replication in cells of hematopoietic origin is necessary for Dengue virus dissemination. PLoS Pathog. 2012;8:e1002465. doi: 10.1371/journal.ppat.1002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Brey I, Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6:2826–2857. doi: 10.3390/v6072826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Diamond MS, Gale M., Jr West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.