Abstract
Background
Bone differs from other organs in that it can regenerate and remodel without scar formation. There are instances of trauma, congenital bone disorder, bone disease and bone cancer where this is not possible. Without bone grafts and implants, deformity and disability would result.
Human bone grafts are limited in their management of large or non-union fractures. In response, synthetic bone grafts and implants are available to the Orthopaedic Surgeon. Unfortunately these also have their limitations and associated complications.
Nanotechnology involves the research, design and manufacture of materials with a grain size less than 100nm. Nano-phase materials follow the laws of quantum physics, not classical mechanics, resulting in novel behavioural differences compared to conventional counterparts.
Methods:
Past, present and future nanotechnology in bone healing literature is reviewed and discussed. The article highlights concepts which are likely to be instrumental to the future of nanotechnology in bone healing.
Results:
Nanotechnology in bone healing is an emerging field within Orthopaedic Surgery. There is a requirement for bone healing technologies which are biochemically and structurally similar to bone. Nanotechnology is a potential solution as the arrangement of bone includes nanoscopic collagen fibres and hydroxyapatite.
This review centers on the novel field of nanotechnology in bone healing with discussion focusing on advances in bone grafts, implants, diagnostics and drug delivery.
Conclusion:
The concept of nanotechnology was first introduced in 1959. Current nanoproducts for bone healing include nano-HA-paste-ostim and nano-beta-tricalcium phosphate-Vitoss.
Nanophase technologies are considered to be superior bone healing solutions. Limited safety data and issues regarding cost and mass scale production require further research into this exciting field.
Keywords: Bone graft, Bone Healing, Bone Implant, Diagnostics, Drug delivery, Fracture, Large fracture, Nanotechnology, Non-union
INTRODUCTION
Richard Feyman first introduced the concept of Nanotechnology in 1959 [2]. Nanotechnology can be defined as the research, design and manufacture of materials with a grain size less than 100nm. These materials follow the laws of quantum physics, not classical mechanics. As a result, they act very differently to their conventional counterparts with similar anatomical structure but grain size greater than 100nm [2]. Manipulation of nanotechnology in bone healing has the potential to be successful as the hierarchical structure of bone is derived from nanoscopic collagen fibres and hydroxyapatite.
Nanotechnology, an emerging field in Orthopaedic Surgery, is a response to the lack of an ideal management option for bone healing in large or non-union fractures. In the USA, trauma is the second most common cause of admission to the Accident and Emergency Department. It is thought that 7.7 million of these admissions will require emergency orthopaedic surgical management [3]. Around 6.5 million of these presentations involve a fracture, with 500,000 requiring a bone graft [4]. A further 700,00 patients will require total joint replacement. Modern joint implants have a 10-15 year life expectancy, with 12.8% of total hip replacements requiring revision [3].
Bone disease such as Osteoporosis and Osteogenesis imperfecta are associated with fracture and disability. Management with bone graft and implants can reduce bone disease associated morbidity [5]. Bone cancers, most notably Osteosarcoma, are rare. It accounts for 1% of all cancer diagnoses in the US and has a bimodal distribution; the first at adolescence and the second in those over sixty years old [6]. The surgical management usually involves large dissection of bone. Bone grafting to optimise pain control, stability and mobilisation and to minimise disability, especially in the adolescent population, is of particular importance in the management of this pathology [5]. Literature suggests that around 10% of fractures result in delayed healing or non-union which severely impedes bone function and may be debilitating for the patient [5].
Bone is biochemically and structurally complex. To a certain extent, implants are able to mimic the structural requirements of bone, and bone grafts the biochemical, but there is not an option currently, which can provide both. This review presents the basic science behind bone structure, formation, function and healing. Furthermore, how these factors are affected by fracture and the current management options when normal bone healing fails. Nanotechnology is discussed, with focus on bone grafts, implants, diagnostics and drug delivery; highlighting promising advances in this novel field.
BONE STRUCTURE
The structure of bone consists of cells and extracellular matrix (ECM) [7]. Cells include osteoblasts, osteocytes, osteoprogenitor cells and osteoclasts. The ECM is made up of three main constituents; inorganic Hydroxyapatite (60% dry bone weight), an organic matrix (30% dry bone weight) and water [5]. The organic matrix includes collagen molecules (90% Type I with some Type V) [8], peptides, glycoproteins, soluble growth factors and hormones [5].
Manipulation of nanotechnology in bone healing has the potential to be successful as the hierarchical structure of bone is derived from nanoscopic collagen fibres between 100nm -2,000nm in length [7] which provide the architecture for nanoscopic (20-40nm in length) [9] hydroxyapatite Ca2(PO4)3OH to build upon [8].
Type I collagen is derived from a triple helix of two alpha 1 chains and one alpha 2 chain, which first bundles into fibrils, then into collagen fibres [5]. The synthesis of this 300nm molecule is carried out by osteoblast cells [7]. Collagen fibres are bound by Hydroxyapatite (HAp) nanocrystals [9]. Nano composite fibers of these two molecules are formed when the C-axes of hydroxyapatite is parallel to longitudinal fibrils of Type I collagen [10]. These fibres provide stability, allowing bone to maintain form and function under both high tensile and compression stress [9].
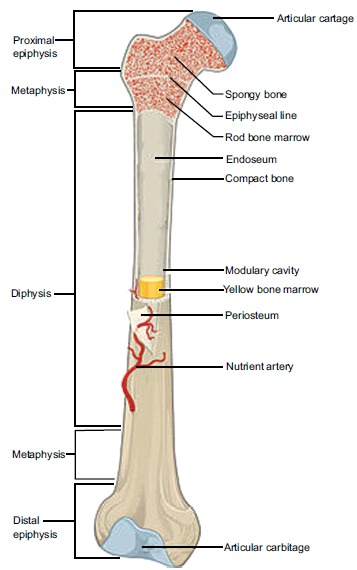
The adult skeleton is made up of 80% cortical bone and 20% trabecular (cancellous) bone [5]. Cortical bone is found in the shaft of long bones [9]. It is anisotropic in nature, as the main functional unit, the osteon, is made up of lamellae [9]. These osteons follow a longitudinal direction, parallel to collagen throughout the bone [5], providing strong skeletal support. Central to each osteon are Haversian canals, within which blood vessels, lymphatics and nerves traverse to provide nutrition to the bone [5].
The very porous trabecular bone is located in the epiphysis and metaphysis of long and cuboidal bones [9]. It is also found in the ribs and spine [5]. Compared to cortical bone, the density and weight of Trabecular bone is less due to its larger pores [7]. This increased pore size allows for the greater vascularity of trabecular bone [7].
Bone metabolism occurs more readily in trabecular bone than cortical bone [9]. Trabecular bone contains both woven and lamellae bone in addition to vascular bone marrow. Woven bone is apparent in metaphysial bone growth, fracture associated callus formation and Paget’s Disease. It is isotropic in nature, as it is formed from coarse, disordered collagen [9]. As a result, it provides poor structural support meaning that in times of physiological duress, trabecular bone will undergo compression force [9] (Fig. 1).
Fig. (1).
Anatomy of a long bone. (Taken and reproduced from [1]).
BONE FUNCTION
Consisting of 206 bones [7], the human skeleton provides structural support, protection of organs, movement of limbs via muscle attachment [9] and a site of calcium and phosphorus storage [8]. Bone, compared to other tissues, is almost unique in its ability to self regenerate when damaged [8]. It is an important buffering mechanism, especially in acidic conditions [9]. Bone marrow, which is found in flat bones and in epiphyseal region of long bones, contains pluripotent mesenchymal stem cells [5]. These cells have the potential to differentiate into bone, cartilage, muscle, skin, soft and adipose tissue [5]. Haemopoiesis is also conducted in the bone marrow [5].
BONE FORMATION
During embryogenesis, pluripotent mesenchymal cells differentiate into osteoprogenitor cells which are either osteoblasts, to produce bone, or chondrocytes, which form the template for endochondral ossification [4]. Bone formation and homeostasis is dependent on a number of different cells. Osteoblasts, Osteocytes and Osteoclasts are found within the bone itself [4]. Whilst Osteoprogenitor and bone lining cells influence bone function [11] (Fig. 2).
Fig. (2).
Bone cells. (Taken and reproduced from [1]).
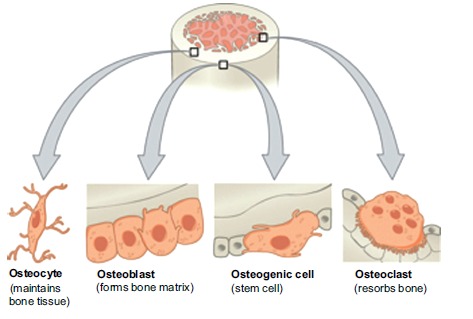
Found on the periosteal and endosteal surfaces, osteoblasts produce Type I collagen rich osteoid matrix [7]. This osteoid matrix is bound by non-collagenous proteins which promote calcium and phosphate adhesion [4], resulting in mineralisation and organisation of new bone. Osteoblasts transform into osteocytes if they are within the osteoid matrix when mineralisation takes place [4].
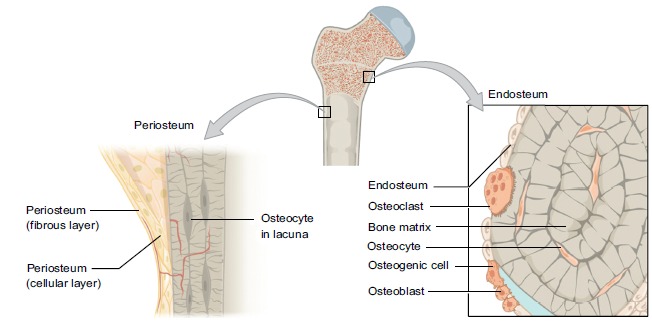
Osteocytes are located centrally within the osteon [7] and can communicate mechanical stresses with neighbouring osteocytes and bone lining cells through canaliculi [7]. This intercellular communication is thought to facilitate bone formation and resorption in addition to mineral transportation between bone and blood [4] (Fig. 3).
Fig. (3).
Periosteum and Endosteum. (Taken and reproduced from [1]).
Osteoclasts are differentiated, cytokine induced, macrophages [8]. Located at the bone surface [4] they resorb mature bone [7] by secreting acids and proteolytic enzymes [5]. Bone lining cells regulate the rate at which minerals from this dissolution are transported from the bone into the blood stream [4].
Non-collagenous proteins, as mentioned briefly, are important in bone formation and repair. Found in the extra cellular matrix, examples include growth factors (TGF beta, PDGF, IGF-1, FGF-a, FGF-b and IL-1), bone sialoprotein, osteopontin, osteonectin, osteocalcin and proteins (fibronectin, vitronectin and laminin) [12]. Osteopontin mediates adhesion of osteoblasts and osteoclasts to the osteoid matrix [13]. Bone sialoprotein, osteonectin and osteocalcin are required for bone mineralisation [14-16]. To note, these extracellular adhesion proteins have enhanced interaction with nanophase implant surfaces, compared to standard implant surfaces, which promotes osseointegration, thus reducing incidence joint loosening [2].
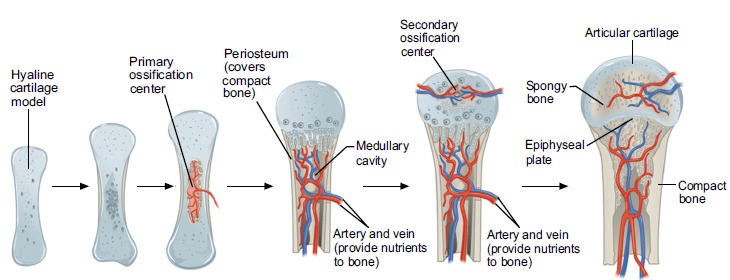
Bone formation follows two main pathways. The first, endochondral bone formation is seen in embryogenesis of long and short bones. Chondrocytes form a collagen rich matrix as they mature. On maturity these cells cease to proliferate and instead produce proteins which calcify the matrix. Resorption of this cartilaginous matrix is performed by phagocytes. At the primary ossification centre located mid-shaft, osteoblasts lay down osteoid to produce a ‘cartilage model’ of the bone. Vascularisation into the matrix facilitates MSC migration from the periosteum. This enables further osteoblastic osteoid deposition and calcification of the collagenous matrix framework forming spongy, trabeculae bone [5]. The medullary cavity of long bones, where the bone marrow is located, is formed by osteoclastic breakdown of this trabecular bone [17].
There is a secondary ossification centre, which is formed at birth. The epiphyseal plate, located between the first and second ossification centres, forms cartilage, which when ossified results in an increase in bone length. This plate is key to growth and development. By the age of twenty, the plate is usually ossified and therefore, ceases to function [17] (Fig. 4).
Fig. (4).
Endochondral ossification. (Taken and reproduced from [1]).
The second, intramembranous ossification is associated with formation of flat bones such as the skull. Although similar to endochondral ossification, it differs in that bone is directly formed from mesenchymal tissue without a cartilage framework [18]. During skull formation, neural crest cells group together, some will differentiate to form capillaries, other will become osteoblasts. These osteoblasts secrete the a collagen-proteoglycan (osteoid) matrix which encourages calcium binding and therefore calcification. Calcification results in the formation of bony ‘spicules’, needle like structures of bone, which radiate from the ossification centre and are surrounded by the periosteum, formed from condensed mesenchymal stem cells MSCs. Cells located in the layer between the spicules and the periosteum become osteoblasts. These osteoblasts deposit osteoid matrix in layers, resulting in the structure of flat bones [17].
The structure of bone can be directly related to its function. Weak, woven bone results from disorganised collagen deposition. When collagen fibres become more linear, strong lamellar bone results. Osteoblastic osteoid deposition, and therefore bone density, is greater at cortical bone than primitive cancellous bone.
NORMAL BONE HEALING AND REMODELLING
Bone healing differs from other human systems in that it does not result in scar formation [19]. Primary bone healing is uncommon. Occurrence requires closely opposed fractured bone fragments which are un-displaced with complete stability. Repair occurs via haversian remodelling [20]. Secondary or indirect bone healing occurs by callus formation via both endochondral and intramembranous ossification [21]. Intramembranous ossification recruits osteoprogenitor and MSCs from the periosteum distant from the fracture to form hard callus without a cartilage template [22]. Endochondral ossification follows a stepwise process; haematoma formation, inflammatory phase, repair phase and finally, remodelling [22]. In contrast to intramembranous ossification, healing is aided by periosteum close to the fracture site [22].
Formation of a haematoma is a result of blood vessel rupture in the medullary cavity at the fracture site [5]. The haematoma is replaced by granulation tissue, where cartilage deposition occurs to form a bridging, soft callus crucial for fracture site stabilisation [8]. The inflammatory phase follows when necrotic tissue at the fracture site releases inflammatory mediators promoting chemotaxis of cytokines, neutrophils, platelets, BMPs and MSCs [21]. This promotes osteoprogenitor cell activation, angiogenesis and osteoclast migration to remove necrotic tissue from the fracture site. Within the repair phase, osteoblasts deposit osteoid at the fracture site to form the disorganised weak woven bone [5]. The remodelling phase can, in large fractures, take up to a year to achieve pre-fracture bone strength [18]. Remodelling involves the transformation of woven bone into organised, strong lamellar bone [5].
BIOMATERIALS AND NANOTECHNOLOGY FOR BONE HEALING
Nanotechnology in Orthopaedic Surgery, and therefore bone healing, has the following three main avenues of exploration, bone graft and implants, diagnostic and drug delivery [3].
Bone grafts are the second most commonly transplanted tissue in humans [7]. Current bone graft and implant options are limited in their efficacy as bone is complex both in anatomical structure and biological activity. Therefore synthetic replication is not easy. Implants can, to a certain extent, imitate the structural support bone provides, but they cannot mimic the biological functions of bone. Bone grafts have the opposite problem. A material which can provide the structural, mechanical and biological complexities of bone in addition to being asceptic is required [3].
Aseptic joint replacement loosening, usually due to poor osseointegration, is one of the most common causes for implant failure [3]. The surface of nanophase materials imitates that of trabecular bone more than their conventional counterparts. Further more, the smaller material grain size seen in nanophase materials greatly increases their surface area to volume ratio. Both of these assets results in improved osseo-integration, which reduces the risk of joint replacement loosening [2] and fibrous encapsulation [23] compared to conventional implants.
TECHNIQUES CURRENTLY USED TO RESTORE BONE HEALING
Bone is almost unique in that it can regenerate and remodel without a scar [9]. It also has the ability to interpret mechanical stress such as exercise, which allows it to increase bone mineral content via osteocyte, osteoclast and osteoblast induction, reducing the risk of fracture [7].
There are clinical scenarios where bone can not regenerate and remodel. Large or non-union healing fractures, bone disease and congenital deformity are commonly managed with bone grafts to promote bone healing and therefore improve mechanical function and pain relief [4].
A successful synthetic bone graft, must be sterile, mechanically strong, promote bone growth including osteoid deposition, calcification, remodelling and vascularisation, and biocompatible, with any degradation products being non-toxic to reduce systemic inflammation and morbidity. In addition, replacement joint implants must promote osteointegration to reduce the risk of joint loosening and stress shielding [4, 5].
There are three main forms of bone grafting; autograft, allograft and synthetic [24]. Grafts may be Osteoconductive, osteoinductive or osteogenic. Osteoconductive bone grafts provide a physical scaffold for recruitment and growth of osteoprogenitor cells and neovascularization from host tissue. Examples are cancellous autografts and allografts, demineralized bone matrix, HAp, collagen and calcium phosphate [25]. Osteoinductive grafts are where osteoprogenitor cells are recruited resulting in osteoblastic bone formation [3]. Bone morphogenic proteins and demineralized bone matrix are two examples [25]. Osteogenic grafts contain osteoprogenitor cells or undifferentiated osteoblasts and have the ability to produce bone directly [5]. Only autografts and bone marrow cells are osteogenic [25].
Autografts
Autografts are considered the ‘gold standard’ approach as only live tissue contains osteogenic cells [7]. Other advantages include minimal risk of tissue rejection and transmitted disease [7]. Autograft efficacy is further improved if the periosteum is transplanted along with the bone, as its high content of mesenchymal stem cells promotes bone and cartilage formation [24]. The iliac crest is the most common autograft donor site. Disadvantages of autograft transplantation include prolonged anaesthetic time, pain, donor site defect, impaired healing, limited quantity of tissue available and infection [3]. A novel autograft, known as bone marrow aspirate, delivers osteoblastic stem cells from bone marrow to the recipient site. The risks associated with standard autograft is reduced as the cells are taken from the bone marrow via syringe thus reducing theatre and anaesthetic time and donor site morbidity [7].
Allografts
Allografts are human, genetically unmatched bone grafts. They are usually harvested from cadavers. Although not living tissue, these grafts have useful osteoconductive potential [3]. Allografts are widely used in spinal fusion, as they resorb effectively at the recipient site. Limitations asociated with allografts include availability, size and strength of donor tissue, rejection and transmission of viruses [3]. Allograft tissue lacks angiogenic and osteogenic factors, thus fibrous encapsulation is a problem [24] which contributes to the associated 30% graft failure [3].
The success of both allo and auto - bone grafts depend on the vascularity of the graft site, the quality of the bone graft and histo-immuno compatibility between the graft and the recipient [24].
Synthetic Grafts
Biomaterials for bone grafting and implants is a current area of focus for nanotechnology research and development. Traditionally, synthetic grafts are manufactured from metal, ceramics, polymers and composites in response to allograft and autograft associated disadvantages [26]. Synthetic bone grafts and implants are useful due to their availability, lack of associated infection, cost-effectiveness and ability to be accurately replicated [7].
Areas of biomaterial development include scaffold production and electrospinning [8, 27]. Scaffolds have the potential to exactly replicate the mechanical and biochemical functions of bone [28] to induce bone healing [26]. Manipulation using nanotechnology has resulted in composite scaffolds with more desirable mechanical qualities compared to their conventional counterparts and osteogenic cell and factor loaded porous scaffolds [28]. Electro-spinning involves “accelerating a jet of charged polymeric liquid under the presence of an electric field” [8]. A wide range of materials and scaffolds can be synthesised to allow for alteration in their structure and therefore function. Post-synthesis surface alteration is possible enabling deposition of nanophase particles, proteins and growth factors [8]. Due to their brittle nature, current electrospun scaffolds are not suitable for load baring implants, but may be useful in the management smaller bone defects or delayed non-union factures [8].
BONE HEALING REPLACEMENT STRATEGIES
Due to the inadequacy of current bone healing management options, novel strategies which are biochemically and structurally similar to bone are required. As the structure of bone is nanoscopic, Nanotechnology may provide the solution. Critically, nanophase materials show improved bone healing, due to their superior osteoblastic function, compared to their conventional counterparts. This has been noted in various studies of nanophase polymers, ceramics, metals, composites and carbon nanofibres and nanotubes as discussed below [3].
Synthetic And Natural Polymers
Polymers are currently widely used as synthetic bone graft. There are two forms; biodegradable and nonbiodegradable polymers. Biodegradable polymers include Polylactic acid (PLA), polyglycolic acid (PGA), gelatin, poly(lactic-co-glycolic acid) (PLGA), collagen, poly(E-caprolactone) (PCL) and copoly-L-lactide (CPLA).
Poly(ethylene) (PE) and, poly(ethylene terephthalate) (PET) and Poly(methyl methacrylate) (PMMA) fall under the non-biodegradable umbrella [26].
Polymers are particularly useful as they are biologically similar to bone, flexible, light-weight, can be modified easily and most will degrade into non-toxic constituents [26]. Unfortunately the more traditional polymers are not without their shortcomings; they do not adhere well to bone, may induce osteolysis or activate an immune response if wear debris is present [26] and are too brittle for load baring joint replacement [26]. Polymers are utilised within Orthopaedic surgery as scaffolds, pins, screws, plates, bone fillers and drug delivery systems [26].
The success of a scaffold is dependent on its ability to substitute or mediate the replacement of damaged or lost tissue. Scaffolds may be natural, synthetic or a composite of the two. Scaffold synthesis can be achieved by electrospinning, self-assembly or phase separation [9].
Important features of a bone healing scaffold are porosity and pore size. The scaffold structure must have interconnecting large pores to promote regeneration and delivery of nutrients, plus micropores to encourage osseointegration [26]. A number of authors have presented nanophase polymer scaffold which meet this specification. Stylios et al. present a Poly(L-lactic acid) nanofibrous scaffold [26]. Stankus et al. developed a scaffold of nanofibre biodegradable polymer and elastomeric poly(ester urethane)urea electrospun with vascular smooth muscle cells which showed promising strength and bone progenitor cell activity [29]
Electrospinning is the utilisation of an electric field to synthesise three dimensional nanophase polymer fibre matrices from a polymer solution or melt [30]. These matrices are a potential bone healing solution as they mimic the extracellular matrix of bone. Further more, the porous structure of these fibres have the potential to deliver growth factors, stem cells or drugs to the site of bone healing [8].
Electrospun synthetic polymer is flexible, cheap, predictable and provide good cell attachment [8]. Electrospun natural fibers such as collagen, gelatin, silk and chitosan exhibit excellent cell attachment to promote osteogenesis, but they are unpredictable in behaviour and carry a risk of infection and inflammation [8]. In particular, electrospun collagen fibres are strong and biochemically active but too brittle to support a joint replacement implant [8].
Electrospun composites of both natural and synthetic polymers have indicated positive bone healing properties. Lee at al. present a collagen and PCL composite with improved hydrophilic properties and degradation rate. Superior cell adhesion, growth and infiltration, and osteoprogenitor cell activity was also demonstrated [31]. Small intestine submucosa and PCL is associated with a fourfold increase in bone marrow derived stromal cell proliferation [8]. Heparan sulphate is a MSC carrier; when combined with PCL it displayed improved in vitro growth of osteoprogenitor cells [8].
Self-assembly is scaffold production without external manipulation. In comparison to electrospinning, fibres produced by self assembly are smaller and more closely replicate the ECM [9]. Peptide amphiphiles (PA) are an example of a self assembly nanofiber. They have been combined with inert titanium foam to form a bioactive titanium foam. This bioactive foam was introduced into a rat femur to promote implant fixation. Within four weeks, bone formation resulted [9]. Helical rosette nanotubes, produced by self assembly of DNA pairs with manipulable amino acid and peptide side chains, mediate osteoblastic adhesion and impede fibroblast adhesion. Resultant osseointegration and reduced fibrous tissue formation indicates a promising bone healing nanotechnology [9].
Incorporation of carbon nanotubes and carbon nanofibers within scaffolds is another emerging novel nanotechnology. Carbon nanofibers are linear, non-continuous carbon filaments [32] which are only 3-100 nanometers diameter and 0.1–1000 µm in length [33]. They provide flexibility and strength when incorporated within a scaffold [32]. Carbon nanotubes are hollow structures with a circumference of a few atoms which are part of the fullerene family. Their length can be far greater than their diameter [34]. As a result, carbon nanotubes are light weight, which enables their incorporation within the scaffold, and strong, providing enhanced mechanical strength to the end biomaterial [35]. Further more, their molecular organisation provides superior tensile strength [34].
In regards to bone healing carbon nanotubes and nanofibers have promising features. They imitate the ECM [36] and facilitate bone cell adhesion via increased adsorption of proteins such as fibronectin [35]. Their surface can be modified to allow for drug, enzyme and cytokine delivery [36]. Kim et al. found that there was less macrophage activation associated with carbon nanotube aligned polymers, compared to conventional polymer. Therefore, carbon nanotube aligned polymer implants may have reduced macrophage activated associated wound healing and implant failure [35]. Multi-walled carbon nanotubes with silk fibrin nanotubes, multi-walled carbonnanotubes with PLGA, multi-walled nanotubes blended with HAp within PLGA and a PCL grafted multi-walled nanotube have each shown bone healing properties. The technology requires further development as the percentage of nanotube within the blend is critical to mechanical property, degradation rate and fibre size optimisation [8].
Graphene, a nanophase carbon exhibits mechanical strength superior to carbon nanotubes [37]. Studies where Graphene was combined with PVA [38] or with chitosan [39] reported improved mechanical strength of the material. Graphene is a promising nanophase material for bone healing, specifically orthopaedic implants due to its mechanical strength, excellent biocompatibility and lack of metallic impurities [9].
There are examples of non-carbon nanofiber scaffolds which show promise in bone healing. The self-assembling peptides (SAP) is one such family. One SAP in particular, RAD16-I when transplanted, can assist with new callus formation and inhibit demineralization [40].
Manipulation, using nanotechnology, of existing bone graft materials may provide future bone healing solutions. One such example is Type X Collagen which naturally facilitates and regulates endochondral ossification of articular cartilage [2]. Further more, the architecture of nanophase collagen, compared to its conventional counterpart, more closely resembles the ECM due to its increased number of fibres, porosity and bioactivity [27].
Ceramics
Ceramic bone grafts are able to form robust bonds with soft and hard tissues, have anti-corrosive properties and depending on sub-type, are bioinert/bioactive/bioresorbable [26]. Of particular note, they have excellent osseointegration without fibrous tissue formation [9]. Unfortunately, their fragility makes them generally unsuitable for weight-loading joint replacement [26] or sizeable bone loss [9]. Current ceramic bone healing solutions include hydroxyapatite, titania, alumnia, zirconia and silicate and phosphate bioglass [9, 26].
Hydroxyapatite (HAp) is the most widely used ceramic bone graft [7]. It is available in two forms, natural hydroxyapatite harvested from coral or animal bones and synthetic HAp [7]. Although natural HAp is more closely matched to human bone composition, synthetic HAp is often opted for as its molecular structure is fully understood which in theory reduces HAp graft associated risks [7]. HAp is osteoconductive [41]. Current clinical uses include dental implants and as a bone cement [42]. Limitations of HAp include lack of durability and strength and risk of migration from the recipient site [7]. Nanophase hydroxyapatite is an exciting application. Its mineral structure is very similar to that of bone. Compared to conventional HAp, it is associated with greater osteoblastic adhesion and activity [3, 26]. It also has greater architectural, mechanical and bioactive stability resulting in a bone graft which is flexible, strong and predictable [3]. Kon et al. report that a tri-layered implant of a [Type I collagen layer, nano HAp 40%] and ]Type I Collagen 60% layer and nano-HAp 70%] and [Type I collagen 30% ‘bone’ layer] showed reduced morbidity when compared to current management options for osteochondral pathology [43].
Biphasic calcium phosphate (BCP) implants and scaffolds are associated with rapid bone regeneration [44, 45]. Stylios et al. present a nanocomposite BCP scaffold of β-tricalcium phosphate matrix and hydroxyapatite nanofibres which showed “high compressive modulus and strength”. Nano-hydroxyapatite tends to aggregate, thus impairing extra cellular matrix interaction. Bone deposition improved with the introduction of tricalcium phosphate as it disperses more readily, increasing take up by osteoclasts [8]. Further more, this scaffold was produced at low temperatures allowing protein and drug inclusion within the structure [26]. Similarly, bioglass has antimicrobial and anti inflammatory properties, can up regulate growth factor release and stimulate mineralisation of the matrix. Compared to conventional bioactive glass, nanofibres of bioactive glass are associated with greater mesenchymal stem cell activity [8]. Nanophase alumnia has greater vitronectin and fibronectin adsorption compared to conventional alumnia. Similar results have been noted for titania, HAp, titanium and PLGA. These two glycoproteins have calcium binding sites and are associated with osteoblast function [3].
Metals
Titanium, titanium alloys, stainless steel and cobalt-chromium alloys are current bone healing solutions within Orthopaedic Surgery [7]. Applications include bone plates and load bearing implants [26]. The main advantages of metallic implants are their strength and ability to sustain weight at load baring sites [9]. Unfortunately these implants do have significant shortcomings; bone does not adhere to metal and so implant infection, inflammation, fibrous encapsulation and systemic absorption of debris can lead to significant morbidity [3].
The average joint implant lifespan is ten to fifteen years [9]. Osteopenia associated joint loosening and stress shielding is usually responsible [3]. As an ageing population, incidence of revision joint replacement is likely to increase [3]. Unfortunately, joint replacement revision is associated with significant morbidity and expenditure.
Nanotechnology may provide the solution to this challenge. Increased osteoblastic adhesion was noted in titanum, titanium alloy and cobalt chromium molybdenum scaffolds with nanoscale altered topography [9]. These nanophase scaffolds present an exciting solution to the requirement for enhanced orthopaedic implants
There have been a number of developments within dental implant technology which have the potential to be utilised in orthopaedic surgery. Titanium dental implants coated with nanophase calcium phosphate show improved osteoblastic adhesion and differentiation, collagen synthesis, mineralisation and resorption. Similar findings were discovered for HAp coated titanium dental implants. Compared to conventional dental implants, the enhanced resorption leads to increased osteoblast activation, bone formation and osseointegration which ultimately improves bone healing [23]. Within the same study, an implant of titanium dioxide nanotubes within titanium exhibited an increased production of alkaline phophate by osteoblastic cells. The authors hypothesised that this could result in improved osteointegration of the implant with the surrounding tissue, thus reducing joint loosening [23]. There may be potential for these findings to be replicated within Orthopaedic Surgery.
Composite Materials
The architecture of bone is biochemically and structurally complex. One biomaterial alone is often unable to mimic this, thus two or more are combined. The result is known as a composite. These bone grafts aim to retain the desirable and negate the undesirable features of each biomaterial. The undesirable features of a biomaterial can often be detrimental to their effectiveness as a suitable bone graft or implant. Composite materials also allow for novel applications such as drug delivery and bone regeneration [26].
HAp has been synthesised as a composite scaffold with the following materials; collagen, gelatin, chitosan, alginate, PLGA, PLLA and PE. The result is a bone graft which replicates the molecular structure of bone due to its hydroxyapatite content whilst the polymer provides strength, flexibility and the ability to withstand mechanical strain [26].
Chitosan is a natural polymer derived from the exoskeleton of shrimp and crab. It is useful within bone healing as it is biocompatible, biodegradable, osteoconductive, has little inflammatory effects and is an effective anti-bacticide [46]. When blended with a polymer such as poly methylmethacrylate, the poor mechanical function of chitosan is reduced. Furthermore, HAp – chitosan nanocomposites are an exciting biomaterial in bone reconstruction due to the biocompatibility and biodegradability properties of chitosan and the enhanced calcification by HAp [7].
Nanophase polymer and ceramic composites are promising bone healing solutions due to their improved osteoconductive and osteoinductive properties. The literature presents nanophase HAp, carbon nanotubes, bioactive glass and tricalcium phosphate combined with electrospun biodegradable polymer to form a composite scaffold [26].
Carbon nanotubes, act as a building block whilst interacting with their implanted environment, have been added to nano-composite scaffolds to provide mechanical strength with success [28] (Tables 1 and 2).
Table 1.
Current and nano-phase biomaterials for bone grafting in Orthopaedic Surgery [2, 3, 7-9, 23, 26, 28, 40, 44, 45].
| Polymer | Ceramic | Metal and Alloy | Composite | |
|---|---|---|---|---|
| Advantages | Biologically similar to bone, flexible, light-weight, easily modified, non-toxic [25] |
Anti-corrosive, bioinert/bioactive/bioresorbable, HAp is Osteoconductive [25, 40] |
Strength, sustain weight at load bearing sites [8] |
Modified to be biochemically and structurally similar to bone. [25] |
| Disadvantages | Poor osteointegration, debris can induce osteolysis/immune response, too brittle for load baring joints [25] |
Fragile, HAp has risk of migration [6] |
Poor osteointegration, risk of infection, inflammation, fibrous encapsulation, systemic absorption of debris, joint loosening, stress shielding, 10-15 year longevity – revision surgery, bio-inert, bio-active, bio [2, 8] |
Characteristics dependant on manufacture. [25] |
| Application | Scaffolds, pins, screws, plates, bone filler, drug delivery [25] |
Hip joints and load baring implants, implant coating, bone filler. [25] |
Bone plates, load baring implants [25] |
Bone grafts, scaffolds, bone regenerative membranes, drug delivery [25] |
| Examples | Biodegradable polymers ; PLA, PGA, gelatin, PLGA, collagen, PCL, CPLA. Non-biodegradable; PE, PET, PMMA [25] |
HAp, alumnia, zirconia and bioglass [25] |
Titanium, stainless steel, titanium alloy, cobalt-chromium [25] |
HAp with collagen, gelatin, chitosan, alginate, PLGA, PLLA, PE. Chitosan- methylmethacrylate. [25] |
| Nanotechnology | Carbon nanotubes, carbon nanofibre and graphene polymer scaffolds. Electrospun polymer scaffolds. SAP scaffolds. Nanophase collagen. Peptide Amphiphiles. Helical rosette nanotubes. [7, 8, 25, 28-30, 34, 37, 38] |
Nano HAp. BCP implants and scaffolds. Bioactive glass nanofibers. Nanophase alumnia. [2, 7, 25, 42, 44] |
Titanium denatal implants coated with nanophase hydroxyapatite. [22] |
Chitosan & nanophase HAp. Electrospun biodegradable polymer plus nanophase HAp, carbon nanotubes, bioactive glass, tricalcium phosphate. Carbon nanotubes within nano-composite scaffold. [6, 25, 27] |
Table 2.
In vitro and in vivo nanotechnology in bone healing studies.
| In Vitro Studies | In Vivo Studies |
|---|---|
| Heparan sulphate, a MSC carrier, combined with electrospun PCL displayed improved in vitro growth of osteoprogenitor cells. [8] |
Peptide amphiphiles nanofibre combined with inert titanium foam to form a bioactive titanium foam. Bone formation within four weeks resulted after introduction into a rat femur. [47] |
| Collagen with an electrospun PCL is associated with significantly increased cell adhesion and growth in vitro [31] |
Collagen with an electrospun PCL is associated with significantly increased cell infiltration in vivo [35] |
| Electrospun β-TCP and HAp composite deposited greater MSC bone, both in vivo and in vitro, compared to each constituent alone. [48] |
Enhanced new bone formation in nanocrystalline HAp coated tantalum scaffolds, compared to conventional HAp coated tantalum scaffold [49] |
| Nanoscale surface modification with biphasic calcium phosphate on titanium dental implants induced early osteoblastic differentiation and bone apposition both in vitro and in vivo [23] |
- |
| Osteoblasts cultured in vitro on nano-topographical surfaces are associated with increased adhesion, induction of metabolic activity, and release of osteoinductive factors [28] |
Cementless implants with microtextured surfaces, rather than smooth surfaces, have greater osteoid tissue and less fibrous tissue adhesion [28] |
| Polymer/calcium phosphate nanocomposites demonstrated superior osteoblast alkaline phosphatase activity and osteoblast marker gene expression, promoting bone maturation in both in vitro and in vivo studies. [50] |
- |
DIAGNOSTIC
The second area of development for nanotechnology in bone healing is diagnostic. It is has been hypothesized that by altering existing metal implants with microsized pores, the ability for nano-sensors to sit within them would be possible. A multiwall carbon nanotube developed from anodized nanotubular titanium demonstrated promotion of protein redox reactions via direct electron transfer. The potential for development into an implant with inbuilt biological sensor and controlled drug delivery is promising. If successful, implant lifespan could be extended significantly [12].
DRUG AND GENE DELIVERY
Research into the third exploratory field of Nanotechnology, drug, protein and gene delivery, has also made advances for bone healing. Nanoparticles are ideal as drug delivery devices as they exhibit high surface area to volume ratio. Their novel size allows their incorporation within, and methodical release from, bone graft scaffolds and other delivery systems [28].
Zhang et al. hypothesise that both genes and proteins could be potentially delivered by a nanoparticle material to promote osteogenesis and therefore bone healing [27]. Examples include, nanosized polymers transporting growth factors, which are released as the polymer degrades. Other reported successful delivery systems include HAp, beta-tricalcium phosphate, carbonate apatite, collagen, hyaluronic acid, chitosan and alignate, micelles [28], PEG-ylation modified particles, liposomes and dendrimers [28, 51].
Implant infection leading to poor osseointegration is a leading cause of implant, and therefore bone healing, failure [3]. Li et al. present a polypeptide nanofilm with the ability to deliver antibiotics. This nanofilm is associated with reduced bacterial load, enhanced osteoblastic activity and promotion of bone healing. The pharmacokinetics of cefazolin release from this nanofilm can be tightly controlled, especially during the critical postimplant period, which is within the first two hours [52]. It is interesting to note that even without drug delivery of antibiotics, the polypeptide nanofilm alone showed markedly reduced Staph Aureus adhesion [52].
Other exciting areas of drug delivery include a silver titanium nanotube coated titanium orthopaedic implant which shows reduced post operative implant associated infection [53]. Scaffolds imbedded with nanophase silver is a future area of development. There introduction into a known or potential site of infection is associated with improved clinical outcomes without the need for long courses of intravenous antibiotics [54].
Yan et al. developed a nanostructured mesoporous bioactive glass (MPG). Compared to its non-mesoporous counterpart, MPG induced greater apatite mineralisation. MPG synthesised as porous 3D scaffolds exhibit enhanced bone regeneration and drug delivery compared to standard MPG forms. In one study, the sustained release of Dexamethasone from an MPG scaffold augmented osteoblastic activity with the potential to induce osteogenesis. The same study looked at a MPG scaffold, loaded with vascular endothelial growth factor (VEGF), which found the mesoporous structure potentiated the bioactivity of VEGF, therefore stimulating angiogenesis, an important aspect of bone healing. The study concluded that both Dexamethasone and VEGF loaded MPG scaffolds are potential nanotechnology bone healing solutions [55].
BONE HEALING PROMOTION
Harvey et al. studied the behaviour of osteoblasts cultured on 2D surfaces whose topography had been manipulated at the nanoscale level. Examples include alumina, titania, HA, PLGA. These “functionalized allografts” showed improved bone healing compared to surfaces with conventional topography. Surface appearance is thought to be more influential than surface chemistry in promoting bone healing. The authors suggest that rough nano topography induces greater release and adhesion of osteoinductive factors. The paper hypothesises that 3D scaffolds embedded with osteoblasts and that have rough nano-topography, should have similar results dependent that key characteristics, such as pore size, are maintained [28].
Another avenue of exploration within stimulation of bone healing is bone morphogenic proteins (BMPs). They are Food and Drug Association approved [28]. These growth factors are one of the transforming growth factor beta proteins involved in osteogenesis [56]. The disadvantage of using BMPs is their associated cost which is considerable [28].
Nanophase ceramics, metals and polymers are associated with reduced activity of fibroblasts and endothelial cells, which are implicated in the pathophysiology of joint loosening and fibrous encapsulation [3].
FUTURE OF NANOTECHNOLOGY
During the inflammatory phase of implant introduction, growth and platelet factors are released which attract mesenchymal stem cells. In vitro studies report that MSC migration, proliferation and differentiation to the site of the new implant results in cell adhesion to the extracellular matrix and to the implant. Fibrous tissue may result, which is associated with poor bone healing and implant failure. Nanoscale bone implants have enhanced bone healing properties as they exhibit reduced fibroblast adhesion and proliferation and greater osseointegration. Lavenus et al. hypothesise that the surface topography of nano scale implants may regulate the behaviour of MSCs during the inflammatory phase [23].
Nanoelectromechanical systems (NEMS) are a future direction of study for nanotechnology. Manipulation of this technology may allow for the production of nanosized systems with communication and monitoring functions. Implementation of NEMs at a fracture site could provide important information regarding bone healing for both clinical and research purposes [28].
Core-shell electrospinning is a future direction for nanotechnology. Current electrospun nano-composite fibres are often random in spatial organisation, prohibiting them from mimicking the complex organisation of natural bone. Core-shell electrospinning differs from electrospinning in that the spinneret has two jets, resulting in a dual layered nanofibre. Synthesis of polymers, metals and ceramics using this technology, could potentially further develop bone healing strategies [8].
Documented disadvantages of scaffolds include inflammation, limited cell turnover and growth factor expression [28]. Multiwall carbon nanotubes are associated with mild reversible damage to the liver and tubular damage to the kidneys. Control of these undesirable factors is required for this novel technology is to be clinically viable.
CONCLUSION
Although nanotechnology is considered to be in its infancy, its manipulation and utilization has developed since its introduction in 1959. Current nanoproducts for bone healing include nano-HA-paste-ostim and nano-beta-tricalcium phosphate-Vitoss [57].
Nanophase synthetic bone grafts, implants and scaffolds are considered superior bone healing solutions to their conventional counterparts. Unfortunately, design and production of these solutions is often expensive and difficult to replicate on mass scale. Further more, the evidence surrounding their biocompatibility, and therefore safety, is conflicting. This is compounded by the fact that many studies are in fact in vitro and not in vivo [9]. It would be prudent for the community to provide greater safety data, as it is estimated that only 3% of the capita spent on nanotechnology research has been devoted to healthy and safety [2].
Nanophase bone healing solutions produced on mass scale with clear safety data is required if Nanotechnology is to fulfill its very promising impact on Orthopaedic Surgery.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- BCP
= Biphasic calcium phosphate
- BMPs
= Bone morphogenic proteins
- CPLA
= Copoly-L-lactide
- ECM
= Extracellular matrix
- HAp
= Hydroxyapatite
- MSCs
= Mesenchymal stem cells
- NEMs
= Nanoelectromechanical systems
- PCL
= Poly(E-caprolactone)
- PE
= Polyethylene
- PET
= Poly(ethylene terephthalate)
- PGA
= Polyglycolic acid
- PLA
= Polylactic acid
- PLGA
= Poly(Lactide-co-Glycolide)
- PLLA
= Poly-L-lactide
- PMMA
= Poly(methyl methacrylate)
- PLGA
= Poly(lactic-co-glycolic acid)
- SAP
= Self assembly peptide
- PA
= Peptide amphiphiles
- MPG
= Mesoporous bioactive glass
- VEGF
= vascular endothelial growth factor
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.OpenStax College. CC BY 3.0. Available from: http://creativecommons.org/licenses/by/3.0. ) vWC.
- 2.Sullivan M.P., McHale K.J., Parvizi J., Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569–573. doi: 10.1302/0301-620X.96B5.33606. [DOI] [PubMed] [Google Scholar]
- 3.Webster T.J., Ahn E.S. Nanostructured biomaterials for tissue engineering bone. Tissue Engineering II. Springer; 2007. pp. 275–308. [DOI] [PubMed] [Google Scholar]
- 4.James R., Deng M., Laurencin C.T., Kumbar S.G. Nanocomposites and bone regeneration. Front. Mater. Sci. 2011;5(4):342–357. doi: 10.1007/s11706-011-0151-3. [DOI] [Google Scholar]
- 5.Mistry A.S., Mikos A.G. Tissue engineering strategies for bone regeneration. Regenerative Medicine II. Springer; 2005. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 6.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta P.K., Dutta J. Multifaceted development and application of biopolymers for biology, biomedicine and nanotechnology. In: Bhowmick A., Banerjee S., Kumar R., Kundu P.P., editors. Hydroxyapatite-packed chitosan-PMMA nanocomposite: A promising material for construction of synthetic bone. Springer; 2013. pp. 135–167. [DOI] [Google Scholar]
- 8.Bernstein H.S. Tissue engineering in regenerative medicine. In: Boyd N.R., Boyd R.L., Simon G.P., Nisbet D.R., editors. Synthetic multi-level matrices for bone regeneration. Springer; 2011. pp. 99–122. [Google Scholar]
- 9.Vajtai R. Springer Handbook of Nanomaterials. In: Kumar V., Tripathi B., Srivastava A., Saxena P.S., editors. Nanocomposites as Bone Implant Material. Springer; 2013. pp. 941–976. [DOI] [Google Scholar]
- 10.Kuhn-Spearing L., Rey C., Kim H-M., Glimcher M.J. Carbonated apatite nanocrystals of bone. Synthesis and processing of nanocrystalline powder Warrendale. PA, USA: The Minerals, Metals and Materials Society; 1996. [Google Scholar]
- 11.Sau P., Lupton G.P., Graham J.H. Pilomatrix carcinoma. Cancer. 1993;71(8):2491–2498. doi: 10.1002/1097-0142(19930415)71:8<2491::AID-CNCR2820710811>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Sirivisoot S., Webster T.J. In situ bone growth detection using carbon nanotubes–titanium sensors. Bionanoscience. 2013;3(2):184–191. doi: 10.1007/s12668-013-0079-4. [DOI] [Google Scholar]
- 13.Termine JD. Cell and molecular biology of vertebrate hard tissues. Chichester, UK: John Wiley & Sons Ltd; 1988. Non-collagen proteins in bone. pp. 178–90. Available from: http://samples.sainsburysebooks.co.uk/9780470513644_sample_388194.pdf . [Google Scholar]
- 14.Roach H.I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994;18(6):617–628. doi: 10.1006/cbir.1994.1088. [DOI] [PubMed] [Google Scholar]
- 15.Golub E.E. Role of matrix vesicles in biomineralization. Biochimica et Biophysica Acta (BBA)-. General Subjects. 2009;1790(12):1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann M., Eastell R., Hoyle N., Wieczorek L. Bone markers: biochemical and clinical perspectives. Taylor & Francis; 2001. [Google Scholar]
- 17.Gilbert SF. Osteogenesis: the development of bones. 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10056/
- 18.Yaszemski M.J., Payne R.G., Hayes W.C., Langer R., Mikos A.G. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17(2):175–185. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 19.Carano R.A., Filvaroff E.H. Angiogenesis and bone repair. Drug Discov. Today. 2003;8(21):980–989. doi: 10.1016/S1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 20.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl. 4):S3–S6. doi: 10.1016/S0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 21.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Einhorn T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998;(355) Suppl.:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 23.Lavenus S, Louarn G, Layrolle P. Nanotechnology and dental implants. Int. J. Biomater. 2010;2010 doi: 10.1155/2010/915327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Awad H.A., OKeefe R.J., Guldberg R.E., Schwarz E.M. A perspective: engineering periosteum for structural bone graft healing. Clin. Orthop. Relat. Res. 2008;466(8):1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalfas I.H. Principles of bone healing. Neurosurg. Focus. 2001;10(4):E1. doi: 10.3171/foc.2001.10.4.2. [DOI] [PubMed] [Google Scholar]
- 26.Stylios G., Wan T., Giannoudis P. Present status and future potential of enhancing bone healing using nanotechnology. Injury. 2007;38(1) Suppl. 1:S63–S74. doi: 10.1016/j.injury.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z-G., Li Z-H., Mao X-Z., Wang W-C. Advances in bone repair with nanobiomaterials: mini-review. Cytotechnology. 2011;63(5):437–443. doi: 10.1007/s10616-011-9367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey E.J., Henderson J.E., Vengallatore S.T. Nanotechnology and bone healing. J. Orthop. Trauma. 2010;24(Suppl. 1):S25–S30. doi: 10.1097/BOT.0b013e3181ca3b58. [DOI] [PubMed] [Google Scholar]
- 29.Stankus J.J., Guan J., Fujimoto K., Wagner W.R. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews J.A., Wnek G.E., Simpson D.G., Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 31.Lee J-J., Yu H-S., Hong S-J., Jeong I., Jang J-H., Kim H-W. Nanofibrous membrane of collagen-polycaprolactone for cell growth and tissue regeneration. J. Mater. Sci. Mater. Med. 2009;20(9):1927–1935. doi: 10.1007/s10856-009-3743-z. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y.A., Hayashi T., Endo M., Dresselhaus M.S. Carbon nanofibers. Springer Handbook of Nanomaterials. Springer; 2013. pp. 233–262. [DOI] [Google Scholar]
- 33.De Jong K.P., Geus J.W. Carbon nanofibers: catalytic synthesis and applications. Catal. Rev. 2000;42(4):481–510. doi: 10.1081/CR-100101954. [DOI] [Google Scholar]
- 34.Yakobson B.I., Smalley R.E. Fullerene nanotubes: C 1,000,000 and beyond: Some unusual new molecules—long, hollow fibers with tantalizing electronic and mechanical properties—have joined diamonds and graphite in the carbon family. Am. Sci. 1997:324–337. [Google Scholar]
- 35.Kim J.Y., Khang D., Lee J.E., Webster T.J. Decreased macrophage density on carbon nanotube patterns on polycarbonate urethane. J. Biomed. Mater. Res. A. 2009;88(2):419–426. doi: 10.1002/jbm.a.31799. [DOI] [PubMed] [Google Scholar]
- 36.Jayarama R V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013;21(1):01–16. doi: 10.1111/j.1524-475X.2012.00861.x. [DOI] [PubMed] [Google Scholar]
- 37.Rafiee M.A., Rafiee J., Wang Z., Song H., Yu Z-Z., Koratkar N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano. 2009;3(12):3884–3890. doi: 10.1021/nn9010472. [DOI] [PubMed] [Google Scholar]
- 38.Rao CeNeR, Sood AeK, Subrahmanyam KeS, Govindaraj A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009;48(42):7752–7777. doi: 10.1002/anie.200901678. [DOI] [PubMed] [Google Scholar]
- 39.Fan H., Wang L., Zhao K., Li N., Shi Z., Ge Z., Jin Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules. 2010;11(9):2345–2351. doi: 10.1021/bm100470q. [DOI] [PubMed] [Google Scholar]
- 40.Kirkham J., Firth A., Vernals D., Boden N., Robinson C., Shore R.C., Brookes S.J., Aggeli A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007;86(5):426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa H., Myoui A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs. 2005;8(3):131–136. doi: 10.1007/s10047-005-0292-1. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz J.P., Hollinger J.O., Milam S.B. Reconstruction of bone using calcium phosphate bone cements: a critical review. J. Oral Maxillofac. Surg. 1999;57(9):1122–1126. doi: 10.1016/S0278-2391(99)90338-5. [DOI] [PubMed] [Google Scholar]
- 43.Kon E., Delcogliano M., Filardo G., Altadonna G., Marcacci M. Novel nano-composite multi-layered biomaterial for the treatment of multifocal degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 2009;17(11):1312–1315. doi: 10.1007/s00167-009-0819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoki H. Science and medical applications of hydroxyapatite: Ishiyaku Euroamerica. Available from: http://www.scirp.org/ (S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=506290 . 1991.
- 45.Frayssinet P., Trouillet J.L., Rouquet N., Azimus E., Autefage A. Osseointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials. 1993;14(6):423–429. doi: 10.1016/0142-9612(93)90144-Q. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesan J., Kim S-K. Chitosan composites for bone tissue engineeringan overview. Mar. Drugs. 2010;8(8):2252–2266. doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sargeant T.D., Guler M.O., Oppenheimer S.M., Mata A., Satcher R.L., Dunand D.C., Stupp S.I. Hybrid bone implants: self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials. 2008;29(2):161–171. doi: 10.1016/j.biomaterials.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Arinzeh T.L., Tran T., Mcalary J., Daculsi G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials. 2005;26(17):3631–3638. doi: 10.1016/j.biomaterials.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L., Webster T.J. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4(1):66–80. doi: 10.1016/j.nantod.2008.10.014. [DOI] [Google Scholar]
- 50.Osathanon T., Linnes M.L., Rajachar R.M., Ratner B.D., Somerman M.J., Giachelli C.M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29(30):4091–4099. doi: 10.1016/j.biomaterials.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim K., Fisher J.P. Nanoparticle technology in bone tissue engineering. J. Drug Target. 2007;15(4):241–252. doi: 10.1080/10611860701289818. [DOI] [PubMed] [Google Scholar]
- 52.Li H., Ogle H., Jiang B., Hagar M., Li B. Cefazolin embedded biodegradable polypeptide nanofilms promising for infection prevention: a preliminary study on cell responses. J. Orthop. Res. 2010;28(8):992–999. doi: 10.1002/jor.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao L., Wang H., Huo K., Cui L., Zhang W., Ni H., Zhang Y., Wu Z., Chu P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32(24):5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Nair L.S., Laurencin C.T. Nanofibers and nanoparticles for orthopaedic surgery applications. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):128–131. doi: 10.2106/JBJS.G.01520. [DOI] [PubMed] [Google Scholar]
- 55.Gu W., Wu C., Chen J., Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomedicine. 2013;8:2305–2317. doi: 10.2147/IJN.S44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen D., Zhao M., Mundy G.R. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 57.Wagner V., Dullaart A., Bock A-K., Zweck A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24(10):1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]


