Abstract
Plasmodium vivax malaria is characterized by periodic relapses of symptomatic blood stage parasite infections likely initiated by activation of dormant liver stage parasites -hypnozoites. The lack of tractable animal models for P. vivax constitutes a severe obstacle to investigate this unique aspect of its biology and to test drug efficacy against liver stages. We show that the FRG KO huHep liver-humanized mice support P. vivax sporozoite infection, development of liver stages, and the formation of small non-replicating hypnozoites. Cellular characterization of P. vivax liver stage development in vivo demonstrates complete maturation into infectious exo-erythrocytic merozoites and continuing persistence of hypnozoites. Primaquine prophylaxis or treatment prevents and eliminates liver stage infection. Thus, the P. vivax/FRG KO huHep mouse infection model constitutes an important new tool to investigate the biology of liver stage development and dormancy and might aid in the discovery of new drugs for the prevention of relapsing malaria.
Keywords: Malaria, Plasmodium vivax, relapse, liver stages, hypnozoite, humanized mouse, FRG KO mouse
Introduction
The majority of human malaria is caused by infections with two Plasmodium parasite species: Plasmodium falciparum and Plasmodium vivax. Research efforts are predominantly focused on P. falciparum malaria because of the high mortality the disease causes in sub-Saharan Africa. However, P. vivax malaria affects more people in a wider geographical range (95 countries), and puts 2.85 billion people at risk of disease every year (Guerra et al., 2010). Furthermore, recent studies indicate that P. vivax infections are more pathogenic than previously appreciated (Price et al., 2009). Two major attributes contribute to P. vivax’s unique epidemiology: first, its ability to develop in mosquitoes at lower temperatures and second, the existence of dormant liver stages termed hypnozoites that can be activated weeks, months or even years after the primary mosquito-transmitted infection. Activated hypnozoites are thought to complete liver stage development, leading to a relapse of symptomatic blood stage infection (White, 2011). Thus, it is of great importance to develop experimental animal models that allow for the study of the biological features associated with the unique epidemiology of this parasite.
Unfortunately, studies of the complex P. vivax liver stage biology are encumbered by the parasite’s strong preference for human and nonhuman primate tissue. The first studies describing P. vivax liver stages were performed on either human liver biopsies of a patient undergoing experimental malaria fever therapy for neurosyphilis (Shortt et al., 1948), or the liver biopsies of chimpanzees infected by intravenous inoculation of a large numbers P. vivax sporozoites (Krotoski et al., 1982b; Rodhain, 1956). It was the latter study (Krotoski et al., 1982b) that demonstrated the existence of small, non-replicating forms –hypnozoites (Markus, 2011) in the P. vivax infected liver for the first time. Since these studies were undertaken, P. vivax liver stage research has been sparse and mostly limited to in vitro studies in primary hepatocytes (Mazier et al., 1984) or hepatoma cell lines (Hollingdale et al., 1985; Sattabongkot et al., 2006). Overall, little additional knowledge has been gained to date that has yielded a better understanding of the biology of P. vivax hypnozoites and their role in malaria relapse. These shortcomings also negatively impact the development of new anti-malarial drugs and as a result primaquine, an 8-aminoquinoline, is still the only licensed drug that eliminates hypnozoites and offers causal prophylaxis and radical cure treatment for P. vivax infection (Fernando et al., 2011). Additionally, P. vivax blood stages only replicate in reticulocytes and continuous in vitro blood stage culture remains extremely challenging. This further impedes studies of the parasite life cycle (Carlton et al., 2011). In consequence, researchers have in the past turned to the relapsing, nonhuman primate malaria parasite Plasmodium cynomolgi to model the biology of hypnozoites (Galinski et al., 2013). P. cynomolgi is genetically closely related to P. vivax and research on its liver stages led to the first description of hypnozoites (Krotoski et al., 1982c; Shortt and Garnham, 1948). Recently, an improved in vitro culture system for P. cynomolgi liver stages and hypnozoites was described (Dembele et al., 2014). Further refinements of such systems will certainly contribute to drive a better understanding of the biology of hypnozoites.
In search of new P. vivax in vivo liver stage models, we took advantage of a mouse that supports engraftment and long term survival of human primary hepatocytes (Azuma et al., 2007). The severely immunocompromised FRG KO mouse (with deletions in fumarylacetoacetate hydrolase (FAH), recombination-activating gene 2 (Rag2), and interleukin-2 receptor subunit gamma (Il2rg) gene deletions) can be transplanted with human hepatocytes (FRG KO huHep). We have recently shown that this mouse model supports the complete development of P. falciparum liver stages, culminating in transition to blood stage infection (Vaughan et al., 2012). Here we use the FRG KO huHep mouse to show, for the first time since the chimpanzee studies in the 1980s, complete P. vivax liver stage development as well as the formation and persistence of hypnozoites in vivo.
Results
Infection of FRG KO huHep mice with Plasmodium vivax sporozoites
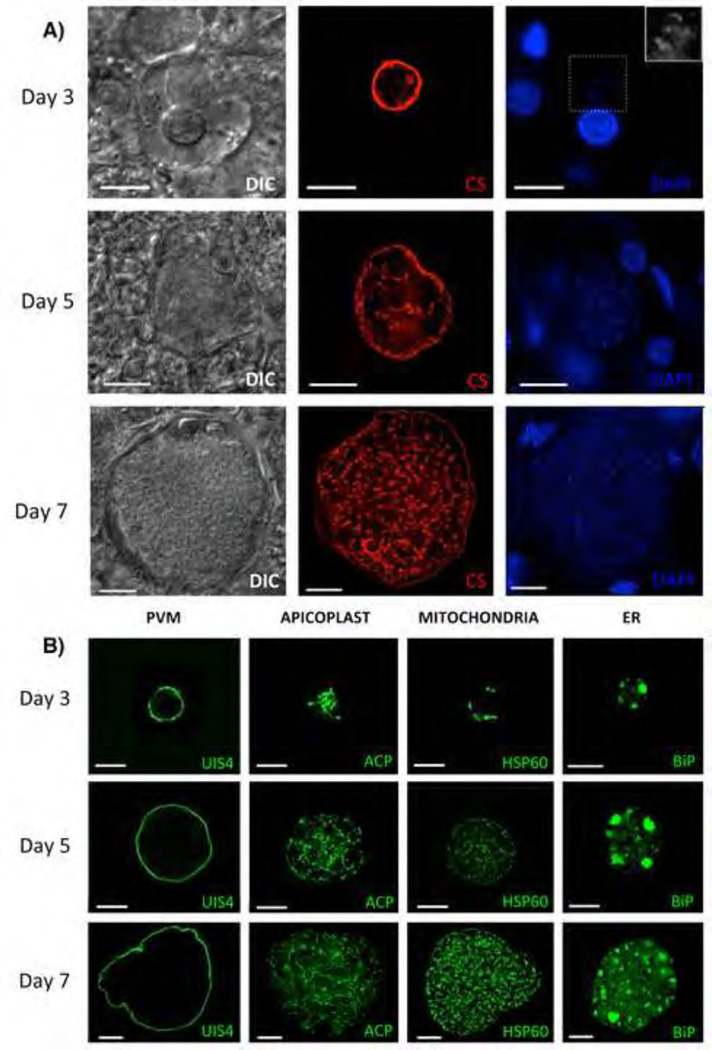
FRG KO huHep mice were injected intravenously with 3.5–5 x 105 P. vivax sporozoites derived from mosquitoes that had been infected with parasite isolates from Thailand. Mice were sacrificed, three-, five-, and seven-days post infection. Infected liver tissue was collected for histological evaluation and immunofluorescence assays (IFA). The liver stages were initially localized with a mouse monoclonal antibody (mAb) to the P. vivax circumsporozoite protein (CS, genotype VK247) (Rongnoparut et al., 1995). Infections appeared robust as indicated by the presence of numerous liver stage parasites in individual liver sections (Figure S1A). CS was expressed on the liver stage parasite plasma membrane (PPM) and strongly confined to the parasite periphery in a circumferential pattern at day three post infection (Figure 1A). It showed a more complex distribution at days five- and seven post infection (Figure 1A, Figure S1B) presumably due to the invagination of the PPM (Uni et al., 1985), which precedes the formation of exo-erythrocytic merozoites. In contrast, CS protein expression was not reported at late time points of P. falciparum liver stage development in FRG KO huHep mice (Vaughan et al., 2012), but it has previously been observed for P. vivax liver stage development in vitro (Hollingdale et al., 1985). To establish that P. vivax sporozoites infected and developed only in human hepatocytes within the mouse liver, we co-stained the infected liver sections with a human FAH specific polyclonal antibody, which only identifies human hepatocytes (FRG KO mouse hepatocytes lack FAH). Indeed, liver stages were exclusively detected in human hepatocytes at all time points of development (Figure S1C).
Figure 1. Development of P. vivax liver stages in the FRG KO huHep mouse.
(A) Liver stage parasites in infected liver sections three-, five- and seven days after sporozoite infection were visualized with differential interference contrast (DIC) imaging (left panel), with a monoclonal antibody specific for P. vivax circumsporozoite protein (CS; VK247) (central panel), and DAPI for DNA content (right panel). To visualize the DNA content in the small three-day old parasite the area inside the dotted box is magnified in the top right corner. (B) Liver stages at three-, five- and seven days after sporozoite infection were analyzed with monoclonal mouse antibodies to P. vivax Upregulated in Infectious Sporozoites protein 4 (UIS4, localizes to the PVM), acyl carrier protein (ACP, localizes the apicoplast), heat shock protein 60 (HSP60, localizes to the mitochondria) and binding immunoglobulin protein (BiP, localizes to the ER). Scale bar: 10 µm.
The complex cellular organelle and membrane development in P. vivax liver stages is revealed with antibodies
To reveal the cellular features of P. vivax liver stages, we developed polyclonal rabbit and monoclonal mouse antibodies specific to P. vivax proteins or conserved Plasmodium proteins that have a high amino acid sequence identity among orthologs (Figure 1B, Figure S2A). One prominent feature of Plasmodium liver stages is the establishment of the parasitophorous vacuole membrane (PVM), which separates the parasite from the cytoplasm of the host hepatocyte. To visualize the P. vivax PVM, we produced a mouse mAb against the P. vivax ortholog (PvUIS4) of P. yoelii UIS4, which was previously shown to localize to the PVM of rodent malaria parasite liver stages (Mueller et al., 2005). The PvUIS4 mAb revealed a circumferential staining pattern that ensconced the liver stages (Figure 1B) and was distinct from CS (a PPM marker) (Figure S2B), indicating that PvUIS4 localizes to the PVM. Furthermore, previously unstudied organelles of P. vivax liver stages were detected using mouse mAbs to: (i) the relict plastid (apicoplast)-targeted acyl carrier protein (ACP), (ii) the mitochondrial heat shock protein, HSP60, and (iii) the endoplasmic reticulum (ER)-targeted protein binding immunoglobulin protein (BiP) (Figure 1B). As liver stage schizogony progressed, the organelle structures became highly complex, consistent with their extensive replication. The PV lumen was localized with a rabbit polyclonal antibody to falstatin (P. vivax ortholog of P. yoelii falstatin (Pei et al., 2013)), the parasite cytoplasm was visualized with rabbit polyclonal antibodies to macrophage inhibitory factor (P. vivax ortholog of P. yoelii MIF (Miller et al., 2012)), and the Golgi apparatus localized with the ER lumen protein retaining receptor (ERD2) (Elmendorf and Haldar, 1993) (Figure S2A).
Complete maturation of P. vivax liver stages, infectious exo-erythrocytic merozoite release and reticulocyte invasion
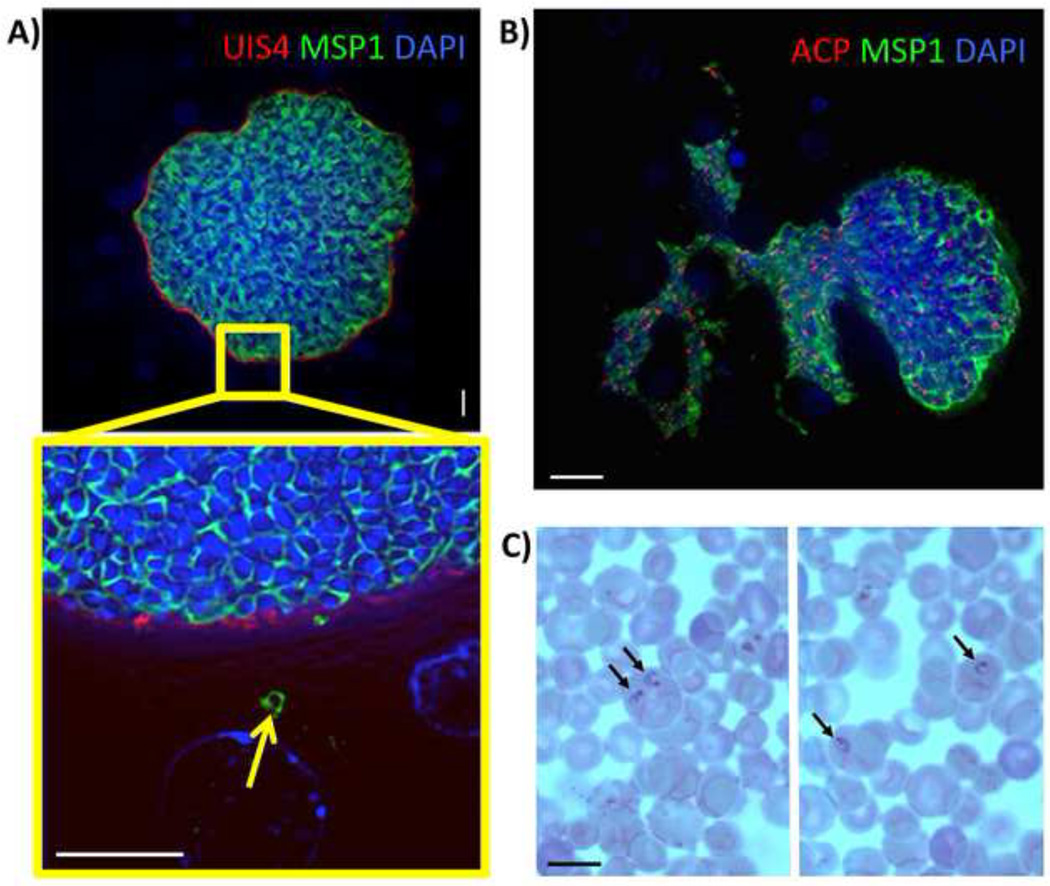
The duration of P. vivax liver stage development culminating in the formation and release of infectious exo-erythrocytic merozoites remains imprecisely defined. Analysis of P. vivax liver stage schizogony in the FRG KO huHep mice at day seven- and day eight post sporozoite infection showed that liver stage maturation was not yet complete. This contrasts with observations of P. falciparum liver stage maturation in this mouse model where release of exo-erythrocytic merozoites occured between six and seven days post sporozoite infection (Vaughan et al., 2012). In P. falciparum, the expression of MSP-1 precedes exo-erythrocytic merozoite formation and clearly identifies the surface of individual exo-erythrocytic merozoites (Vaughan et al., 2012). We thus analyzed P. vivax MSP-1 expression in liver stage schizonts and observed it as early as day five post sporozoite inoculation, with increased expression at day eight (Figure S2C). However, individual merozoites were not yet observed. Continued analysis of liver stage schizogony showed numerous mature liver stage schizonts at day nine that contained individual exo-erythrocytic merozoites (Figure 2A). Some mature schizonts had released merozoites in tightly packed masses that were observed in the surrounding tissue, beyond the confines of what appeared to be a ruptured PVM (Figure S3A). The majority of mature liver stage schizonts lost their characteristic spherical structure and released a substantial fraction of their exo-erythrocytic merozoites into the surrounding tissue, including the liver sinusoids (Figure 2B). Mature liver stage schizonts were also detected at day ten-post sporozoite infection, but they were fewer when compared to day nine (data not shown). The remaining liver stages had clearly differentiated apicoplasts, suggesting that they were close to maturation (Figure S3B). The decline in overall liver stage schizont numbers at day ten compared to day nine was likely the result of mature parasite egress from hepatocytes, leaving fewer schizonts in the tissue. Therefore, we conclude that the complete maturation of P. vivax liver stages and exo-erythrocytic merozoite release occurs between day nine- and day ten-post sporozoite infection in the FRG KO huHep mouse model. To test whether exo-erythrocytic merozoites released from the liver can invade red blood cells to continue the parasite life cycle, mice were intravenously injected with reticulocytes at day nine and ten after sporozoite inoculation (this experiment was performed twice with two mice per group). Four hours after the second reticulocyte injection, blood was removed from each mouse by cardiac puncture and analyzed for parasites by Giemsa-stained thin blood smears. Ring stage parasite-infected reticulocytes were readily observed for each mouse tested (at least 20 separate thin blood smears were made from each mouse and parasite-infected reticulocytes were seen in all smears (Figure 2C). Thus, P. vivax liver stage maturation and release of reticulocyte-infectious exo-erythrocytic merozoites can be modeled using FRG KO huHep mice.
Figure 2. Complete maturation of P. vivax liver stages and persistence of hypnozoites in the FRG KO huHep mouse.
(A) Indirect immunofluorescence assay of P. vivax liver stages at ten days after sporozoite infection using Pv merozoite surface protein-1 (MSP-1) polyclonal rabbit antibody (green), PvUIS4 mouse monoclonal antibody (red) and DAPI (blue, DNA). MSP-1 expression reveals the presence of differentiated exo-erythrocytic merozoites within the liver stage schizont. The magnification of the area in the yellow box shows a MSP-1- positive exo-erythrocytic merozoite (arrow) outside the confines of the mature liver schizont. Scale bar: 10 µm. (B) IFA of a nine-day old P. vivax liver stage stained with PvMSP-1 polyclonal rabbit antibody (green) to visualize the merozoite surfaces, ACP monoclonal mouse antibody (red) to visualize the apicoplast and DAPI (DNA stain, blue). The mature liver stage is in the process of releasing exo-erythrocytic merozoites into the surrounding liver tissue. Scale bar: 20 µm. (C) Human red blood cells enriched for reticulocytes were injected into FRG KO huHep mice nine days post sporozoite infection. Four hours later, the blood was removed and microscopically analyzed by Giemsa-stained thin blood smear. Black arrows point to P. vivax ring stage parasites within reticulocytes. Scale bar: 10 µm.
Formation of P. vivax hypnozoites in the FRG KO huHep mouse liver
During the analysis of liver stage schizont growth at days five to eight post sporozoite infection, we also detected a subset of liver stage parasites that were small at all time points and appeared not to increase significantly in size (Figure 3). We concluded that these were small, non-replicating hypnozoite forms and for the ease of terminology will refer to these small forms as hypnozoites. At the time of exo-erythrocytic merozoite release at days nine and ten post infection, hypnozoites continued to persist (Figure S3A). To confirm that hypnozoites resided within human hepatocytes and not to remaining mouse hepatocytes, which could retard liver stage development, we used an antibody to detect human FAH in the infected liver sections. Indeed, all observed hypnozoites were located within human hepatocytes (Figure S1C).
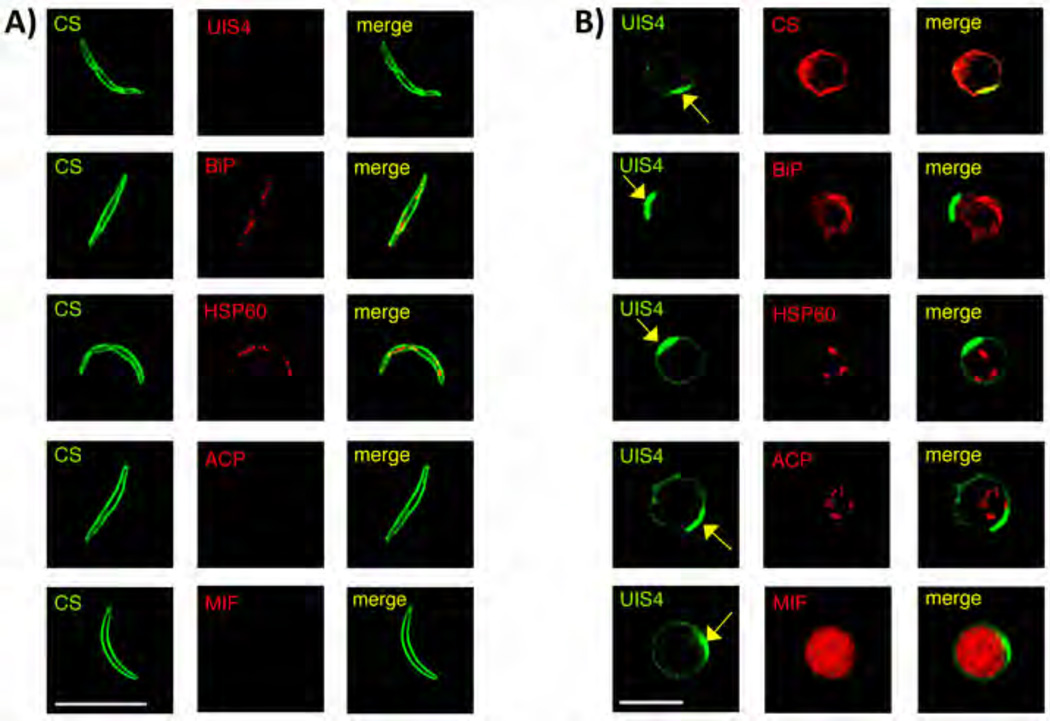
Figure 3. Characterization of P. vivax hypnozoites in FRG KO huHep mouse and comparison to sporozoites.
(A) IFA of P. vivax sporozoites stained with mouse monoclonal antibody to PvCS (green) and rabbit polyclonal antibodies to PvUIS4, BiP, PvHSP60, ACP and Pv macrophage inhibitory factor (MIF, red). (B) Hypnozoites at day seven post sporozoite infection were localized with antibodies to PvUIS4 (rabbit polyclonal and mouse monoclonal) (green), monoclonal mouse antibody to PvCS (red) and rabbit polyclonal antibodies to BiP, PvHSP60, ACP and PvMIF (red). The yellow arrow points to a PvUIS4-postive PVM prominence that is notable in the PVM of all hypnozoites. Scale bar: 10 µm.
To determine if P. vivax hypnozoites display any unique organellar features that would differentiate them from sporozoites and replicating liver stage schizonts, we performed IFAs on sporozoites (Figure 3A) and hypnozoites at day five-, seven-, and eight- post sporozoite infection (Figure 3B). Mouse mAbs and rabbit polyclonal antibodies to PvUIS4, BiP, PvHSP60, ACP, and PvMIF were used to characterize protein expression and localization. As expected, the ER marker BiP and the mitochondrial marker HSP60 were localized to the sporozoite interior (Figure 3A), as has been described for sporozoites of other Plasmodium species. Interestingly, PvUIS4 was not detected in P. vivax sporozoites, in contrast to observations made for P. yoelii UIS4 (Kaiser et al., 2004) and P. falciparum UIS4 (Mackellar et al., 2010) but in agreement with P. berghei UIS4 (Silvie et al., 2014). Expression of ACP and PvMIF was also not observed in P. vivax sporozoites (Figure 3A). As opposed to sporozoites, UIS4 was readily detected in hypnozoites in a circumferential pattern, indicative of PVM localization (Figure 3B). Intriguingly, PvUIS4 staining always revealed a polarized densely fluorescent prominence of the PVM (Figure 3B, yellow arrows), which was never observed on the PVM of liver stage schizonts at all time points examined (Figure 1B, Figure S2A and data not shown). Moreover, the PVM prominence was also not observed on liver stage trophozoites at day two-post sporozoite infection (two mice infected with two independent P. vivax isolates) (Figure S3C). Thus, the UIS4-positive PVM prominence might be a unique feature of P. vivax hypnozoites. The localization patterns for BiP, HSP60 and ACP, suggested that biogenesis of the ER and initial replication of mitochondria and apicoplast had commenced in hypnozoites (Figure 3B). However, the hypnozoite organelles were more limited in their replication when compared to early liver stage schizonts at day three post sporozoite infection (Figure 1B). Interestingly, ACP and PvMIF were expressed in hypnozoites but not in sporozoites (Figure 3). Furthermore, exported protein 1 (EXP-1), another PVM–resident protein (Doolan et al., 1996; Vaughan et al., 2012) was not expressed in sporozoites, hypnozoites or day three liver stage schizonts but clearly detected in liver stage schizonts starting at day five post sporozoite infection (Figure S4A). This expression pattern contrasted with the PVM protein, PvUIS4, which was expressed by all P. vivax liver stages observed, including hypnozoites (Figure 3B).
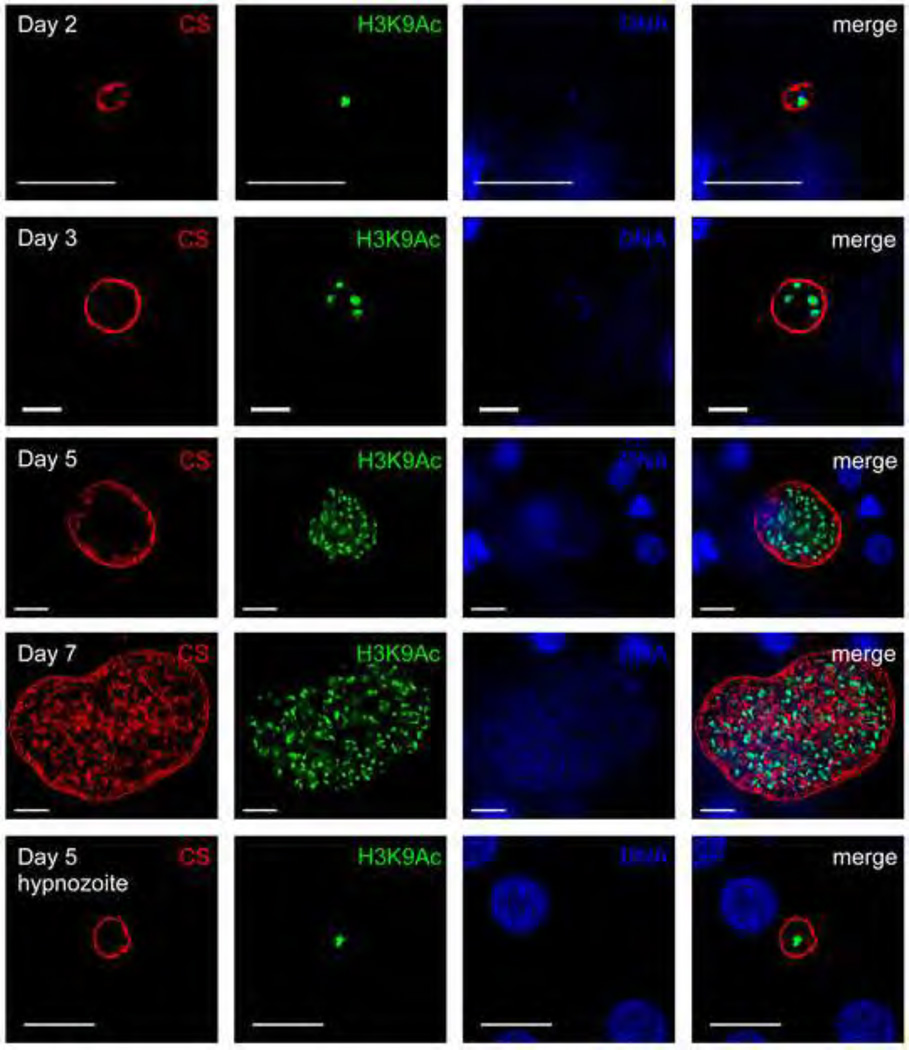
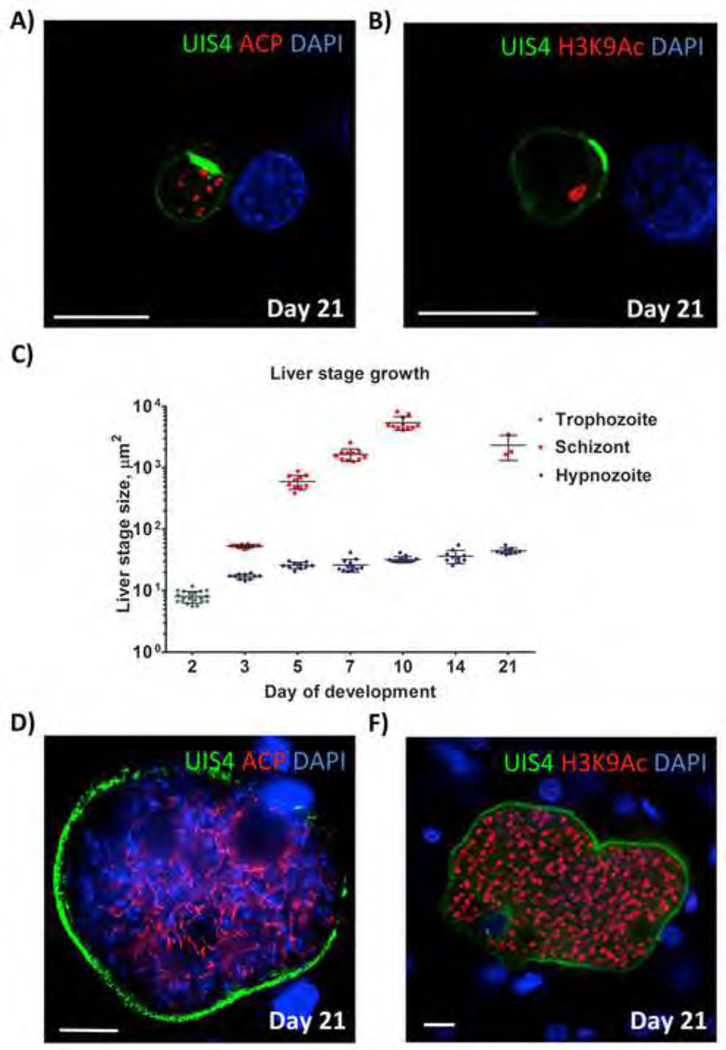
Next, the extent of genome replication was analyzed in hypnozoites. We encountered difficulties in visualizing hypnozoite DNA by DAPI due to the high fluorescent intensity of hepatocyte nuclei in the infected liver sections and comparatively minute DNA content of hypnozoites. Therefore, to indirectly analyze the extent of DNA replication in hypnozoites, a histone acetylation-specific antibody (recognizing acetylated lysine 9 of histone H3, H3K9Ac) was used for IFA (Figure 4). A single histone-positive structure was observed in hypnozoites, suggesting that they had not undergone significant DNA replication. This was in stark contrast to replicating liver stage schizonts, which exhibited an increasing number of histone positive structures with progressive growth over the course of infection, starting as early as day three after sporozoite infection (Figure 4).
Figure 4. P. vivax liver stage genome replication.
Antibodies to acetylated lysine 9 of histone H3 (H3K9Ac) (green) was used to analyze genome replication of liver stage parasites in infected FRG KO huHep mice. Schizonts and hypnozoites were also stained with antibody to CS (VK247) (red). DNA was visualized with DAPI (blue). A single H3K9Ac-positive structure was detected within liver stage trophozoites two days after sporozoite infection. Hypnozoites (shown here five days after sporozoite infection) also contained a single H3K9Ac-positive structure. Multiple H3K9Ac-positive structures were observed for replicating liver stage schizonts at day three-, five- and seven after sporozoite infection. Scale bar: 10 µm.
The persistence of P. vivax hypnozoites and hypnozoite activation
To investigate if hypnozoites persisted in the mouse model, we analyzed livers at day’s 14- and 21 post sporozoites infection. At day 14, persistent hypnozoites continued to be present (Figure S4B, Figure 5B)) and importantly, no liver stage schizonts were observed at this time point (Figure 5C). Furthermore, no parasite-infected human reticulocytes where recovered when the day 14-sporozoite-inoculated mice were transfused with human reticulocyte-enriched blood and analyzed as described above (data not shown). We also observed multiple hypnozoites at day 21 post infection (five mice evaluated; > 20 hypnozoites observed in 30 sections from each liver) (Figure 5A,B, Figure 5C). Day 21 hypnozoites were somewhat larger when compared to hypnozoites detected during the first ten days of infection (Figure 5C) but still showed no evidence of DNA replication based on detection of a single H3K9Ac positive structure in each hypnozoite (Figure 5B). Interestingly, day 21 hypnozoites exhibited multiple, apparently individualized apicoplasts. All persistent hypnozoites showed the unique UIS4-positive PVM prominence (Figure 5A, 5B, S4B). Overall, hypnozoites were not distinguishable at day two post infection, when all parasites were at trophozoite stage but could be clearly distinguished from replicating schizonts at day three post infection onward (Figure 5C). Hypnozoites from all analyzed time points of infection remained smaller in size than liver stage schizonts detected at day three post infection (Figure 5C).
Figure 5. P. vivax hypnozoite persistence and hypnozoite activation in FRG KO huHep mice.
(A, B) Persistent hypnozoites at day 21 after sporozoite infection. Parasites were visualized with antibodies to PvUIS4 (green), antibodies to acetylated lysine 9 of histone H3 (H3K9Ac) (red) and host hepatocyte nuclei were visualized with DAPI. The UIS4-postive PVM prominence is maintained and notable in the PVM of all persistent hypnozoites. Hypnozoites contained a single H3K9Ac-positive structure. (C) Size comparison of liver stage trophozoites, liver stage schizonts and hypnozoites at different time points of infection. The size (liver stage area at the greatest circumference of the parasite) was calculated. Measurements were taken for at least 10 liver stages at each time point. The average size +/− SEM is shown on the dot plots. Note that hypnozoites show some growth over time but remain smaller than the three-day old schizonts. No schizonts were detected in infections at 14 days after sporozoite infection biut were again detected at day 21. (D, E) show examples replicating liver stages at day 21 post sporozoite infection, suggesting that they originated from hypnozoites that activated and entered schizogony. Antibodies used for IFA were mouse monoclonal antibody to PvUIS4 (green) rabbit polyclonal antibodies to ACP in the left panel and H3K9Ac (red) in the right panel. DNA was stained with DAPI (blue). Scale bar: 10 µm.
Strikingly, we have also observed a total of three liver stage schizonts in multiple liver sections analyzed from day 21 infections (>30 sections analyzed in five independently infected mice) (examples are shown in Figures 5D, 5E). These schizonts were not yet fully mature and similar in size to schizonts observed at days five to seven post sporozoite infection (Figure 1B, 5C).
The frequency of hypnozoite formation in Thai P. vivax isolates
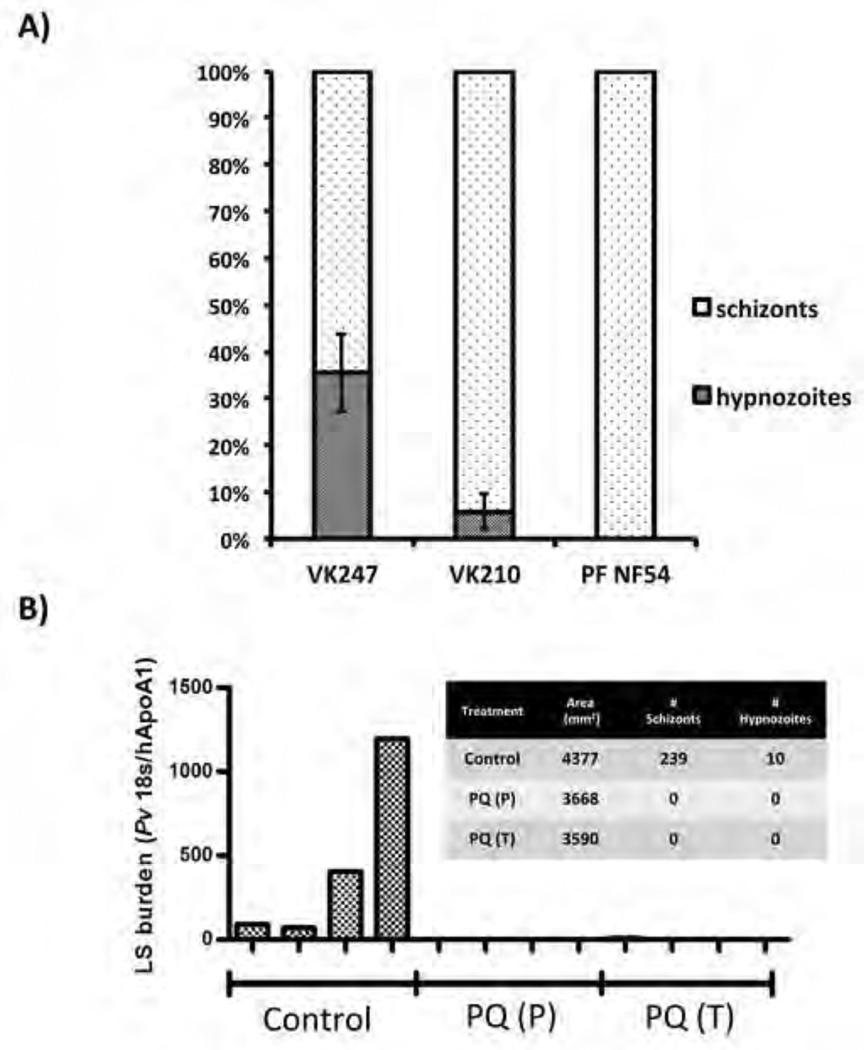
Hypnozoites remain non-replicating, much smaller than replicating liver stage schizonts and persist after commencement of the primary liver stage infection (Figure 5C). Hypnozoites also possess unique cellular characteristics that can be readily visualized by antibody staining (Figure 4). To determine hypnozoite frequencies for the Thai P. vivax isolates used for infections, we determined their fraction in the total number of liver stage parasites (schizonts + hypnozoites) at days five through seven of infection. We observed that infections performed with P. vivax isolates of the CS VK247 genotype (experiment performed twice with two mice per independent experiment and individual VK247 isolates), yielded a hypnozoite frequency of approximately 40% (Figure 6A), closely matching the hypnozoite frequency of 50% predicted (but never formally shown) for tropical strains of P. vivax (White, 2011). This hypnozoite frequency did not change significantly between five- and seven day infections or mice that were repopulated using different hepatocyte donors, indicating that hypnozoite frequencies are maintained with progression of infection in the FRG KO huHep model (Figure S5A). Strikingly however, in another set of infections with Thai P. vivax isolates of the CS VK210 genotype, the hypnozoite frequency was drastically different averaging only 8% (three independent infections with at least two mice per infection; Figure 6A, Figure S5A).
Figure 6. Thai P. vivax hypnozoite frequencies and outcomes of experimental drug treatments in FRG KO huHep mice.
(A) A graphical representation of hypnozoite and liver stage schizont abundance in infections. Liver stages are denoted as schizonts or hypnozoites. For quantitative assessment, liver stages observed on ten, non-serial liver sections were totaled for each infected mouse. Results for two Thai P. vivax isolates are shown (CS VK247 genotype (two independent experiments with different patient isolates) and CS VK210 genotype (four independent experiments with different patient isolates). Error bars: standard deviation. (B) Four mice were treated with primaquine (30 mg/kg) at days one- through day three post sporozoite infection (PQ prophylaxis (P)) or days three through seven post sporozoite infection (PQ treatment (T)). Parasites of the CS VK210 genotype were used for the experiments. Four untreated mice served as controls (Control). Eight days after sporozoite infection, all mice were sacrificed and cDNA was produced from three separate liver tissue samples for each mouse. Liver stage burden using qRT-PCR and was used to normalize P. vivax 18S rRNA transcription to human ApoAI transcription. The graph insert shows microscopic quantitation of liver stage schizonts (LS) and hypnozoites observed by IFA. Area of tissue refers to cumulative area of tissue analyzed for each treatment where similar number of sections from each technical replicate was processed.
Testing P. vivax liver stage drugs in the FRG KO huHep model
We next explored the FRG KO huHep model as a platform for testing drugs against P. vivax liver stages. In addition to evaluating liver stage burden by histological examination, we also developed a quantitative real-time PCR (qPCR) assay based on the detection of P. vivax 18S ribosomal RNA (normalized against human apolipoprotein AI to account for hepatocyte humanization of the liver). Primaquine is currently the only licensed drug that has demonstrated efficacy to prevent relapses and affords causal prophylaxis as well as treatment for P. vivax infections of the liver. We treated a group of four mice with primaquine (30 mg/kg; intravenously) for five days, starting one day before P. vivax sporozoite infection (VK210 genotype) as a prophylaxis regimen. A second group of mice was treated with an equivalent primaquine regimen starting on day three post sporozoite infection, to assess elimination of established hypnozoites. The mice were sacrificed at day eight and the efficacy of the drug to eliminate P. vivax liver stage infection relative to control – four untreated P. vivax-infected mice, was evaluated by qPCR. In untreated control mice, P. vivax liver stage burden was robustly detected by qPCR (Figure 6B). In seven out of eight primaquine-treated, P. vivax-infected mice, liver stage parasites were undetectable by qPCR (Figure 6B). One mouse in the prophylaxis group showed a very low signal for parasite 18S rRNA. Histological analysis of multiple liver tissue sections from all mice readily detected P. vivax liver stage schizonts and hypnozoites in untreated control livers. No schizonts or hypnozoites were detected in the livers of primaquine treated mice, including the liver that showed residual parasite 18S rRNA signal by qPCR analysis (Figure 6B). We also investigated the effect of atovaquone, a drug that eliminates replicating liver stages but does not prevent relapses, on P. vivax liver stages in the FRG KO huHep mouse model. We treated a group of three mice with atovaquone (10 mg/kg; orally) for 3 days, starting one day before P. vivax sporozoite infection (Figure S5B). The mice were sacrificed at day eight and the effect of the drug on P. vivax liver stage infection relative to control – two untreated P. vivax-infected mice - and primaquine prophylaxis regimen (three mice in a prophylaxis regimen described above) was analyzed. Histological analysis of multiple liver tissue sections from mice treated with atovaquone showed that P. vivax liver stage schizonts were almost completely absent (one schizont detected) in the mice. However, establishment of hypnozoites were not affected by atovaquone (Figure S5B). In contrast, primaquine prevented establishment of hypnozoites and replicating liver stages (Figure S5B).
Discussion
Activation of dormant hypnozoites, subsequent liver stage schizogony, exo-erythrocytic merozoite release and concomitant blood stage infection (relapse) constitutes a major determinant of the unique P. vivax epidemiology (White and Imwong, 2012). Yet, P. vivax hypnozoites, their occurrence, frequency, dormancy and activation, remains mostly unexplored. Since pioneering studies described P. vivax liver stages in humans and Aotus monkeys (Collins et al., 1973; Shortt et al., 1948) and hypnozoites in infected chimpanzees (Krotoski et al., 1982b), little progress has been made in this area of research, despite the global importance of vivax malaria.
Here, we have shown that the human liver-chimeric FRG KO huHep mouse model provides a unique opportunity to advance our understanding of P. vivax liver stage biology. This model supports robust infection with P. vivax sporozoites and the development of liver stages, both non-replicating hypnozoites that persist and replicating schizonts that develop to completion and release infectious exo-erythrocytic merozoites. One of the advantages of the model is its small size. In consequence, with only a few hundred thousand P. vivax sporozoites, a robust liver stage infection can be achieved and infection is amenable to quantitative analysis by microscopy and PCR. Thus, the model also enables testing of drugs that prevent the establishment of hypnozoites or eliminate established hypnozoites. Although in vitro models for P. vivax liver stage research have improved with the advent of micro-scale primary hepatocytes cultures (March et al., 2013) the FRG KO huHep mouse model will be extremely valuable to study P. vivax liver stage biology and test drugs in vivo.
We have presented numerous lines of evidence that P. vivax infections in the mouse model result in the formation and persistence of hypnozoites. Intriguingly, our data indicates that independent P. vivax isolates from the same geographical area in Thailand differ in their hypnozoite frequencies and this appears to be associated with their genotype. It is known that P. vivax isolates from temperate regions relapse with longer time intervals to first event and also show longer periodicity when compared to tropical strains, which show short relapse frequencies (reviewed in (White, 2011)). It is possible that longer relapse periodicity is a parasite adaptation to the seasonal availability of vectors for transmission. The significance of the different hypnozoite frequencies we have seen for Thai P. vivax isolates is currently not understood and it will be of interest to further explore the question of why parasites in the same geographic area would show heterogeneity in this important biological aspect of liver infection. The robust P. vivax liver stage infections we have observed will allow future quantitative investigation of hypnozoite frequencies from P. vivax strains of different geographical origin. This analysis should contribute to unraveling the relationship between clinically observed relapse patterns and hypnozoite frequencies in different parts of the world. Newly developed antibodies to a diversity of parasite proteins allowed for the first time a detailed cellular characterization of P. vivax liver stages. Previous studies relied on immune sera from patients living in P. vivax-endemic areas (Krotoski et al., 1982b) and more recently CS protein specific antibodies (Rosenberg et al., 1989). The new antibodies revealed the PVM, apicoplast, mitochondria, ER and Golgi throughout liver stage development and also allowed for an unprecedented characterization of hypnozoites. They exhibited a PVM with a unique UIS4-positive prominence, a feature not observed in developing liver stage schizonts or early trophozoite stages. It is tempting to speculate that this structure constitutes a unique point of interaction between the hypnozoite and its host hepatocyte but this remains to be investigated. Of equal interest, hypnozoites showed evidence for active cellular processes including development of the ER and initial replication of mitochondria and the apicoplast, Hypnozoites also increased, albeit modestly, in size during the time course of the study (three days to 21 days after sporozoite infection). Together, these findings challenge the prevailing notion that hypnozoites are truly dormant. Nevertheless, hypnozoites did not show evidence of DNA replication as determined by microscopic analysis of their histone content. Critically, hypnozoites persisted for at least 21 days post sporozoite infection and future experiments will determine if even longer persistence can be observed in this model. Our initial observation also suggests that hypnozoite activation occurs as we detected a new generation of replicating schizonts at day twenty-one post sporozoite infection but this will require more detailed study. It might be feasible to combine the persistence of hypnozoites in the model with the transitioning of exo-erythrocytic merozoites to blood stage infection, which would truly model relapsing infection. Furthermore, it should be feasible to address what in vivo stimuli can trigger hypnozoite activation. Recently, a new methodology for longer-term in vitro cultivation of P. cynomolgi liver stages in Macaca fascicularis primary hepatocytes has been described (Dembele et al., 2014). This enabled the observation that P. cynomolgi hypnozoites could persist, activate and enter liver stage schizogony. Additional pharmacological manipulation of the system with inhibitors of histone modification enzymes accelerated the rate of hypnozoite activation. Thus, it will be of interest to determine if manipulation of histone modifications leads to activation of P. vivax hypnozoites in the FRG KO huHep mouse model.
While the duration of complete liver stage development for other Plasmodium species has been experimentally determined (for example, six to seven days for P. falciparum (Vaughan et al., 2012), seven-11 days for P. cynomolgi (Krotoski et al., 1982a; Voorberg-van der Wel et al., 2013)), our work for the first time directly determined the time to complete P. vivax liver stage maturation and release of exo-erythrocytic merozoites using the FRG KO huHep mouse model. Previously, this had been extrapolated from the appearance of blood stage parasites after experimental P. vivax sporozoite infection of humans and nonhuman primates (Anstey et al., 2012). In the latter, peripheral blood stage parasitemia was detected as early as day nine (for the Chesson strain) post inoculation with very large numbers of sporozoites, suggesting that the release of exo-erythrocytic merozoites occurred at this time (Krotoski et al., 1982b). In agreement with this, we observed mature liver stage schizonts containing exo-erythrocytic merozoites as well as the release of merozoites at day nine-post sporozoite infection. We also observed mature schizonts on day ten post sporozoite infection but their numbers had declined when compared to day nine infections, indicating that the peak of liver stage schizont maturation occurs around day nine. To add credence to our observations in the liver, we showed that P. vivax exo-erythrocytic merozoites released from the liver on day nine and day ten after sporozoite inoculation infected reticulocytes. Thus, the model can be used for studying the liver stage-to-blood stage transition of P. vivax.
Successful prevention (causal prophylaxis) and treatment (radical cure) of P. vivax hypnozoite infection requires two components: 1) prevention/elimination of the blood stage infection that causes pathology and 2) prevention of future occurrences of relapses by prevention/elimination of hypnozoites in the liver. Currently, the only approved drug for causal prophylaxis and radical cure of relapsing P. vivax infections is primaquine. Primaquine however has a short half-life, requires a two-week dosage regimen and has incompatibility with glucose-6-phosphate-dehydrogenase deficiency, which requires pre-screening of drug recipients (Kevin Baird, 2013). Therefore, there is an ongoing search for new drugs that could replace primaquine. We showed that primaquine causal prophylaxis and treatment of established hypnozoites prevents/eliminates P. vivax liver stage infection in the FRG KO huHep model. We also showed that atovaquone has little effect on hypnozoites. Thus, this model will help in accelerating the discovery of next generation drugs that target hypnozoites.
Taken together, our data demonstrate that the FRG KO huHep mouse model constitutes an unprecedented, robust small animal model to support P. vivax sporozoite infection, liver stage development, complete maturation of schizonts, infectious exo-erythrocytic merozoite release, as well as hypnozoite formation and persistence. The advance is of critical importance, because questions regarding the biological basis of relapses and their relationship to hypnozoite frequencies and activation can now easily be addressed in vivo. This will reveal new insights into the unique biology of P. vivax and might accelerate the development of novel interventions for causal prophylaxis and radical cure of P. vivax infection.
Methods
Plasmodium vivax sporozoite production
Anopheles dirus mosquitoes (from the Mahidol University colony maintained at the Faculty of Tropical Medicine laboratories) were infected with blood collected from patients who were confirmed positive for only P. vivax malaria via microscopy at local health centers in close proximity to the Kanchanaburi Campus, Mahidol University. In brief, 150 µL of red blood cell pellet from blood samples was suspended in pooled normal AB serum to a packed cell volume of 50%. Then, the suspension was fed for 30 minutes to 100 female mosquitoes (5–7 days old) via an artificial membrane attached to a water-jacketed glass feeder maintained at 37 °C. Next, unfed mosquitoes were removed, and fed mosquitoes were maintained on a 10% sucrose solution and incubated at 26 °C and 80% humidity for at least 14 days. Salivary gland dissections were performed at days 14 through 19. Production of P. falciparum sporozoites and the infection of FRG KO huHep mice with P. falciparum were performed as previously described (Vaughan et al., 2012).
FRG KO huHep mice
Female FRG KO mice engrafted with human hepatocytes (FRG KO huHep) were purchased from Yecuris Corporation (Oregon, USA). The FRG KO mouse is a triple gene knockout (Azuma et al., 2007; Bissig et al., 2007). R stands for recombination-activating gene 2 (Rag2) and G stands for interleukin-2 receptor subunit gamma (Il2r⍰). The Rag2−/− and Il2r⍰−/− phenotype is a severely immunocompromised mouse lacking B-, T- and NK-cells that does not reject xenotransplanted huHep. The F stands for fumarylacetoacetate hydrolase (FAH). Due to the lack of FAH, the hepatocytes of FAH−/− mice suffer buildup of intracellular fumarylacetoacetate, resulting in their death. The phenotype is ablated by the addition of 2-(2-nitro-4-trifluoromethylbenzoyl)-1, 3-cyclohexanedione (NTBC) to the mouse diet, via the drinking water or food (Grompe et al., 1995). FRG KO huHep mice are cycled with NTBC, which allows for the repopulation of the mouse liver with huHeps. Repopulation levels can reach in excess of 90%. Mice were maintained periodically on NTBC throughout the experimental period according to the supplier’s methodology.
Enriched human reticulocyte preparations
Adult human whole blood was obtained from the Thai Red Cross and depleted from white blood cells by passage through Pall RN1 filters. The remaining reticulocytes and red blood cells were washed with RPMI1640 and concentrated by centrifugation (1000g for 10 min). The blood was then overlaid on a Nycondenz preparation (19%) in KCl buffer and centrifuged (3000g for 30 min) to separate reticulocytes from the mature red blood cells. The enriched reticulocyte preparation was collected from the Nycondenz interface, washed and concentrated again in RPMI1640. The enrichment of reticulocytes was estimated by methylene blue staining. For intravenous (IV) mouse injections, 300–400 µl of a 20–40% enriched reticulocyte preparation was used.
In vivo sporozoite infection and liver isolation
Mice were injected IV into the tail with 3.5x105 – 1x106 sporozoites isolated from the salivary glands of infected mosquitoes in 100 µL of RPMI media. Mice were euthanized at two (four mice), three (two mice), five (two mice), seven (three mice), eight (>10 mice), nine (three mice), 10 (four mice), 14 (two mice), and 21 (three mice) days after sporozoite infection. Livers were perfused with PBS through the hepatic portal vein, removed and separated into lobes. The lobes were fixed in 4% electron microscopy grade formaldehyde in PBS, which was replaced after 24 hours with TBS + 0.05% sodium azide. The liver lobes were then stored at 4°C in TBS containing 0.05% sodium azide. The fixed lobes were subsequently sliced into 25–50 µm sections for immunofluorescence assay (IFA). The sporozoites used for IFA were fixed in 4% formaldehyde in PBS for 30 min. After processing for IFA, the sporozoites were immobilized on glass slides for microscopy.
Immunofluorescence assay
IFAs were carried out as previously described (Vaughan et al., 2009). Human hepatocytes were detected with a rabbit anti-FAH antibody (a kind gift from Yecuris Corporation). P. vivax liver stages were detected with a CS protein mouse monoclonal antibody (obtained from MR4, ATCC, VA), as well as rabbit polyclonal antibodies to BiP (Noe et al., 2000), ERD2 (MR4, ATCC, VA), and MSP-1. The polyclonal and monoclonal antibodies created for this study were developed by GenScript. In brief, codon optimized plasmids encoding the whole or a fragment of the protein of interest (if not full length, the amino acids used are denoted with superscript numbers next to the name of the protein) were produced and used for immunization of animals according to the company protocols. Using this technology, antibodies to UIS479–166 (PVX_001715), HSP60 (PVX_095000), falstatin (PVX_099035), MIF (PVX_124095), AMA-144–487 (PVX_092275), and EXP-1100–148 (PVX_091700) were created. ProMab developed the mouse monoclonal antibodies to BiP (Roobsoong et al., 2014) and ACP (PY04779, whole protein) according to the company’s protocols. For P. vivax histone detection anti-acetylated histone H3 rabbit antibody (Millipore) was used. Fluorescence and differential interference contrast (DIC) images were acquired using an Olympus 1 × 70 Delta Vision microscope equipped with deconvolution software. All of the plasmids used to produce the proteins for immunizations as well as all the antibodies described above will be deposited in MR4 as a resource for the malaria research community.
Drug treatment studies
For primaquine treatment, 30 mg/kg of primaquine phosphate (Sigma) in PBS was injected intraperitoneally (IP) into mice. For primaquine treatments, mice were injected IP with primaquine at days −1 thru 3 post sporozoite inoculation or at days 3 thru 7 post sporozoite injection. For Atovaquone (Sigma) treatment, 10 mg/kg of drug was administered orally (in 100 µL of PEG450 as a carrier) at days −1 thru 1 post sporozoite inoculation. For all drug experiments, the P. vivax VK210 genotype was used. All mice were sacrificed at day eight for analysis. NTBC was not used.
qRT-PCR
Total RNA from liver lobe samples was extracted using Trizol (Invitrogen) and DNase treated using Turbo-DNase (Ambion). First-strand cDNA was synthesized from RNA using the Superscript III Platinum RT Kit (Invitrogen). The resulting cDNA was used for the amplification of human ApoAI and P. vivax 18S rRNA cDNA (human ApoAI primers: (5’AGCGTGACCTCCACCTTCAG’3 and 5’CCTTCACCTCCTCCAGATCCTT’3; Pv18S primers: 5’ GAAGAAAATATTGGGATACGTAACAG’3 and 5’ATCGGTAGGAGCGACGGGCG’3). The qPCR was carried out with SYBR green (Invitrogen) using the Applied Biosystems 7300 Real-Time PCR System and associated software. Relative copy numbers for the transcripts under study were calculated using the Δ Ct method. Each qPCR used identical quantities of first-strand cDNA generated from three small sections of liver. Mice used in the experiments were littermates, received the same donor hepatocytes and were injected with P. vivax sporozoites (0.8 x 106) on the same date.
Study approval
The human blood collection protocol was approved by the Ethical Committee of the Faculty of Tropical Medicine, Mahidol University. The study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. To this end, the Seattle Biomedical Research Institute has an Assurance from the Public Health Service (PHS) through the Office of Laboratory Animal Welfare (OLAW) for work approved by its Institutional Animal Care and Use Committee (IACUC). The PHS Assurance number is A3640-01. All of the work carried out in this study was specifically reviewed and approved by the Seattle Biomedical Research Institute IACUC.
Supplementary Material
Acknowledgments
We would like to thank John Bial and Elizabeth Wilson (Yecuris Corporation) for assistance with the humanized mouse model and members of the Prachumsri laboratory with help in mosquito rearing and sporozoite isolation. We would also like to thank Omar Vandal, Richard Elliot and Brice Campo for helpful discussions concerning research presented in this manuscript. The research presented here was funded from Seattle Biomedical Research Institute internal financial support to SHIK in addition to a Global Health Grant from the Bill and Melinda Gates Foundation to SHIK (#OPP10215171), SAM (#OPP1041422), JHA and JP (#OPP1023643) and the Medicines for Malaria Venture (MMV).
Footnotes
The authors declare no conflicts of interest.
Author contributions
SAM and AMV designed and performed experiments, analyzed data and wrote the manuscript. NK, WR, MF, NY, NR, VL, NS, AK, NC, MB, SEL performed experiments and/or were critical to the execution of experiments. JHA contributed reagents. SAM, JP and SHIK designed experiments, analyzed data and wrote the manuscript.
REFERENCES
- Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: Clinical Spectrum, Risk Factors and Pathogenesis. Advances in parasitology. 2012;80:151–201. doi: 10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig KD, Le TT, Woods NB, Verma IM. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci U S A. 2007;104:20507–20511. doi: 10.1073/pnas.0710528105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Sina BJ, Adams JH. Why is Plasmodium vivax a neglected tropical disease? PLoS neglected tropical diseases. 2011;5:e1160. doi: 10.1371/journal.pntd.0001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE, Contacos PG, Jumper JR, Smith CS, Skinner JC. Studies on human malaria in aotus monkeys. 3. Exoerythrocytic stages of the Salvador II strain of Plasmodium vivax. The Journal of parasitology. 1973;59:859–866. [PubMed] [Google Scholar]
- Dembele L, Franetich JF, Lorthiois A, Gego A, Zeeman AM, Kocken CH, Le Grand R, Dereuddre-Bosquet N, van Gemert GJ, Sauerwein R, et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med. 2014;20:307–312. doi: 10.1038/nm.3461. [DOI] [PubMed] [Google Scholar]
- Doolan DL, Hedstrom RC, Rogers WO, Charoenvit Y, Rogers M, de la Vega P, Hoffman SL. Identification and characterization of the protective hepatocyte erythrocyte protein 17 kDa gene of Plasmodium yoelii, homolog of Plasmodium falciparum exported protein 1. J Biol Chem. 1996;271:17861–17868. doi: 10.1074/jbc.271.30.17861. [DOI] [PubMed] [Google Scholar]
- Elmendorf HG, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993;12:4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski MR, Meyer EV, Barnwell JW. Plasmodium vivax: modern strategies to study a persistent parasite’s life cycle. Advances in parasitology. 2013;81:1–26. doi: 10.1016/B978-0-12-407826-0.00001-1. [DOI] [PubMed] [Google Scholar]
- Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS neglected tropical diseases. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale MR, Collins WE, Campbell CC, Schwartz AL. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am J Trop Med Hyg. 1985;34:216–222. doi: 10.4269/ajtmh.1985.34.216. [DOI] [PubMed] [Google Scholar]
- Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- Kevin Baird J. Malaria caused by Plasmodium vivax: recurrent, difficult to treat, disabling, and threatening to life - averting the infectious bite preempts these hazards. Pathogens and global health. 2013;107:475–479. doi: 10.1179/2047772413Z.000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski WA, Bray RS, Garnham PC, Gwadz RW, Killick-Kendrick R, Draper CC, Targett GA, Krotoski DM, Guy MW, Koontz LC, et al. Observations on early and late post-sporozoite tissue stages in primate malaria. II. The hypnozoite of Plasmodium cynomolgi bastianellii from 3 to 105 days after infection, and detection of 36- to 40-hour pre-erythrocytic forms. Am J Trop Med Hyg. 1982a;31:211–225. [PubMed] [Google Scholar]
- Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, Killick-Kendrick R, Wolf R, Sinden R, Koontz LC, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982b;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- Krotoski WA, Garnham PC, Bray RS, Krotoski DM, Killick-Kendrick R, Draper CC, Targett GA, Guy MW. Observations on early and late post-sporozoite tissue stages in primate malaria. I. Discovery of a new latent form of Plasmodium cynomolgi (the hypnozoite), and failure to detect hepatic forms within the first 24 hours after infection. Am J Trop Med Hyg. 1982c;31:24–35. [PubMed] [Google Scholar]
- Mackellar DC, O’Neill MT, Aly AS, Sacci JB, Jr, Cowman AF, Kappe SH. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot Cell. 2010;9:784–794. doi: 10.1128/EC.00336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, Carpenter AE, Thomas D, Sim BK, Mota MM, et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus MB. Malaria: origin of the term “hypnozoite”. Journal of the history of biology. 2011;44:781–786. doi: 10.1007/s10739-010-9239-3. [DOI] [PubMed] [Google Scholar]
- Mazier D, Landau I, Druilhe P, Miltgen F, Guguen-Guillouzo C, Baccam D, Baxter J, Chigot JP, Gentilini M. Cultivation of the liver forms of Plasmodium vivax in human hepatocytes. Nature. 1984;307:367–369. doi: 10.1038/307367a0. [DOI] [PubMed] [Google Scholar]
- Miller JL, Harupa A, Kappe SH, Mikolajczak SA. Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect Immun. 2012;80:1399–1407. doi: 10.1128/IAI.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe AR, Fishkind DJ, Adams JH. Spatial and temporal dynamics of the secretory pathway during differentiation of the Plasmodium yoelii schizont. Mol Biochem Parasitol. 2000;108:169–185. doi: 10.1016/s0166-6851(00)00198-5. [DOI] [PubMed] [Google Scholar]
- Pei Y, Miller JL, Lindner SE, Vaughan AM, Torii M, Kappe SH. Plasmodium yoelii inhibitor of cysteine proteases is exported to exomembrane structures and interacts with yoelipain-2 during asexual blood-stage development. Cell Microbiol. 2013;15:1508–1526. doi: 10.1111/cmi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- Rodhain J. Paradoxical behaviour of Plasmodium vivax in the chimpanzee. Trans R Soc Trop Med Hyg. 1956;50:287–293. doi: 10.1016/0035-9203(56)90036-0. [DOI] [PubMed] [Google Scholar]
- Rongnoparut P, Supsamran N, Sattabongkot J, Suwanabun N, Rosenberg R. Phenotype and genotype diversity in the circumsporozoite proteins of Plasmodium vivax in Thailand. Mol Biochem Parasitol. 1995;74:201–210. doi: 10.1016/0166-6851(95)02504-9. [DOI] [PubMed] [Google Scholar]
- Roobsoong W, Maher SP, Rachaphaew N, Barnes SJ, Williamson KC, Sattabongkot J, Adams JH. A rapid sensitive, flow cytometry-based method for the detection of Plasmodium vivax-infected blood cells. Malar J. 2014;13:55. doi: 10.1186/1475-2875-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, Waters AP, Prasittisuk C. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science. 1989;245:973–976. doi: 10.1126/science.2672336. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, Udomsangpetch R, Cui L, Brewer TG. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- Shortt HE, Garnham PC. Demonstration of a persisting exo-erythrocytic cycle in Plasmodium cynomolgi and its bearing on the production of relapses. British medical journal. 1948;1:1225–1228. doi: 10.1136/bmj.1.4564.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortt HE, Garnham PC, et al. The pre-erythrocytic stage of human malaria, Plasmodium vivax. British medical journal. 1948;1:547. doi: 10.1136/bmj.1.4550.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O, Briquet S, Muller K, Manzoni G, Matuschewski K. Post-transcriptional silencing of UIS4 in Plasmodium berghei sporozoites is important for host switch. Mol Microbiol. 2014;91:1200–1213. doi: 10.1111/mmi.12528. [DOI] [PubMed] [Google Scholar]
- Uni S, Aikawa M, Collins WE, Campbell CC, Hollingdale MR. Electron microscopy of Plasmodium vivax exoerythrocytic schizonts grown in vitro in a hepatoma cell line. Am J Trop Med Hyg. 1985;34:1017–1021. doi: 10.4269/ajtmh.1985.34.1017. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg-van der Wel A, Zeeman AM, van Amsterdam SM, van den Berg A, Klooster EJ, Iwanaga S, Janse CJ, van Gemert GJ, Sauerwein R, Beenhakker N, et al. Transgenic Fluorescent Plasmodium cynomolgi Liver Stages Enable Live Imaging and Purification of Malaria Hypnozoite-Forms. PLoS One. 2013;8:e54888. doi: 10.1371/journal.pone.0054888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Imwong M. Relapse. Advances in parasitology. 2012;80:113–150. doi: 10.1016/B978-0-12-397900-1.00002-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.