Abstract
Over-reliance on synthetic pesticides in insect pest control has caused widespread public and scientific concerns for human health and the environment, especially since many insect pests have already developed resistances to conventional pesticides and Bt products. For this reason, there is a considerable interest in development of alternative control methods for insect pest management. Based on laboratory studies, we report that methyl benzoate (MB), a naturally-occurring compound in many plants, may possess toxicity against various stages of a variety of insect pests, including the brown marmorated stinkbug, Halyomorpha halys, diamondback moth, Plutella xylostella, and tobacco hornworm, Manduca sexta, as well as the spotted wing drosophila, Drosophila suzukii. Based on our laboratory toxicity data, MB was at least 5 to 20 times more toxic than the conventional pyrethroid (β-cyfluthrin), sulfur & pyrethrin mixture, and some organic commercial products available on the market against H. halys, P. xylostella, and M. sexta, eggs. Because MB is considered an environment-friendly, it has great potential to be used as an alternative tool to synthetic pesticide for insect pest management in crop production, thereby, reducing threats to natural ecosystems and human health caused by over-application of conventional synthetic pesticides.
Several studies have suggested that the increasing global food demand for direct human consumption as result of world population growth, poses unique and huge challenges for the sustainability of crop production1,2,3. Although about 99% of agricultural crop pests, worldwide, are control by natural enemies4, crop production can still be severely reduced (in the range of 25–50%) by insects, weeds, and pathogens5. Application of synthetic pesticides in pest control has been shown to provide significant economic benefits, allowing farmers to reduce human labor costs in crop production, and made it possible to produce a great volume of food for global consumers5. However, this has not been all positive as many insect pests have developed resistances to major classes of synthetic insecticides, and serious damage to human health, agriculture, and natural ecosystems has been attributed to the continuing application of synthetic pesticides. In addition, because insecticides and herbicides are sprayed or spread across entire agricultural fields, it has been estimated that over 98% of sprayed insecticides and 95% of herbicides reach destinations other than their target species, often resulting in harm to non-target wildlife and the environment6. Recent estimates have even suggested that pesticides are responsible for more than 20,000 human fatalities, yearly, primarily in developing countries7. Thus, there is an urgent need to curtail pesticide use and reduce the human and environmental impacts of synthetic pesticides.
Botanical pesticides have emerged as attractive alternatives to synthetic pesticides presumable because botanicals are perceived as less threatening to human health and the environment8,9,10. A number of botanical pesticides, based on pyrethrum and neem, have been successfully commercialized; however their use in agricultural and veterinary pest control commands only about 1% of the global pesticide market11. Therefore, there is still significant opportunity for development of botanical pesticides as environmental-friendly tools in pest management.
Recent investigations have indicated that a volatile organic compound (VOC), methyl benzoate (MB), from the fermented apple juice, exhibits significant repellent activity against an invasive fruit fly, Drosophila suzukii Matsumura, also known as spotted wing drosophila (Zhang et al. unpublished data). As part of an effort aimed at development of green pesticides based on plant origin, the potential acute toxicity and sublethal effect of MB against D. suzukii have been investigated by comparing MB with some monoterpenes, including α-terpinene12, γ-terpinene13, α-terpineol11, α-pinene14, and 1,8-cineole15 (considered ‘minimum risk pesticides’). The toxicities of these latter compounds have been well demonstrated against several different insect pests. Among all the compounds tested, MB was found to be the most toxic for D. suzukii. Since pesticides are usually active against a broad spectrum of pests, MB was also tested against several other pest species, including brown marmorated stinkbug Halyomorpha halys, diamondback moth Plutella xylostella, and tobacco hornworm Manduca sexta, for both acute toxic efficacy and/or sublethal effect. Our results indicated that MB not only effectively prevented egg hatch and inhibited nymph and/or larvae developments of H. halys, P. xylostella, and M. sexta with contact ovicidal effect, but also had contact insecticidal activity against H. halys nymphs.
Results
Toxicity of MB to D. suzukii
MB, at a concentration of 1%, exhibited potent toxicity against D. suzukii when blueberries were exposed to D. suzukii four days prior to treatment (“pre-infested”). Exposure to MB resulted in 100% mortality as no larvae and pupae had developed nor adult flies emerged after 12 days incubation at room temperature (Fig. 1) (N = 6, F = 25.472; df = 5,30, p < 0.0001).
Figure 1. Impact of MB on emergence of D. suzukii from pre-infested blueberries (100 berries pre-infested with 100 mixed-adult for 4 days/treatment, 50 berries were then dipped with 1% MB solution and water respectively.
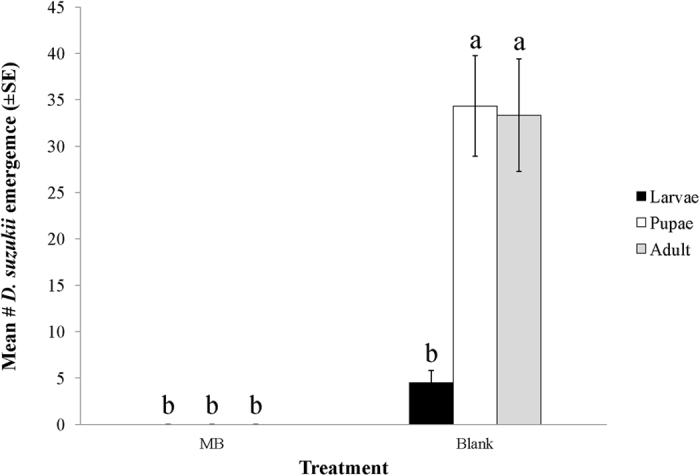
Assessment was conducted after 10 days incubation at room temperature. Means flowed by the different letters are significantly different at α = 0.05 (N = 6, F = 25.472; df = 5,30, p < 0.0001).
Comparison of MB to ‘minimum risk’ pesticides against D. suzukii
Of all the compounds tested, MB exhibited the most toxicity against D. suzukii (Fig. 2). MB exhibited complete mortality and no adult flies survived after two days exposure to pre-treated blueberries (N = 6, F = 10.691; df = 6,35, p < 0.0001). All other essential oils (‘minimum risk pesticides’) tested did not show significant toxicity, when compared to the control. Following further incubation at ambient (room) temperature for 10 days, no adults emerged and significantly fewer pupae developed from MB-treated berries when compared to the blank control or other essential oil treatments (Fig. 3). (N = 6, df = 6,35; for adult, F = 4.843, p < 0.01; for pupae, F = 3.586, p < 0.01), suggesting that MB also possessed an oviposition deterrent property.
Figure 2. Impact of different VOCs against adult D. suzukii (60 flies/treatment).
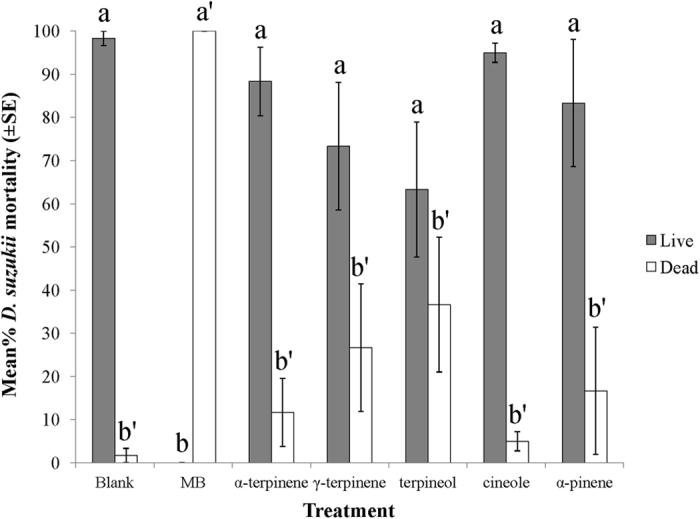
Mortality assessed after 48 hrs exposure to pre-treated blueberries (60 berries/treatment, 1% solution dipping). Means flowed by the different letters and superscripts are significantly different at α = 0.05 (N = 6, F = 10.691; df = 6,35, p < 0.0001).
Figure 3. Impact of different VOCs on subsequent D. suzukii infestation and development on pre-treated blueberries.
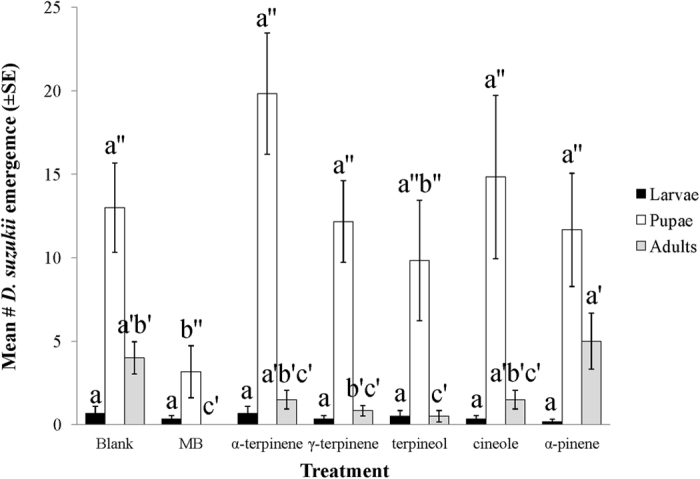
Numbers assessed after 10 days incubation at room temperature. Means flowed by the different letters and superscripts are significantly different at α = 0.05 (Log transformed; N = 6, df = 6,35; for larvae, F = 0.248, p > 0.05; for pupae, F = 3.586, p < 0.01; for adult, F = 4.843, p < 0.01).
The toxicity of MB is concentration dependent. After two days exposure to pre-treated blueberries, MB exhibited potent activity against adult D. suzukii at 1% and 5% concentrations. Little activity at 0.5% and no significant activity at 0.1% concentrations were observed (Fig. 4). (N = 6, F = 12.151; df = 4,25, p < 0.0001). Following further incubation at room temperature for 10 days, no adults emerged and significantly fewer pupae developed from 1% and 5% MB treated berries comparing to the blank control (Fig. 5). (N = 6, df = 6,35; for adult, F = 27.981, p < 0.001; for pupae, F = 5.982, p < 0.01; for larvae, F = 0.458, p > 0.05).
Figure 4. Dose response of MB against adult D. suzukii (60 flies/treatment).
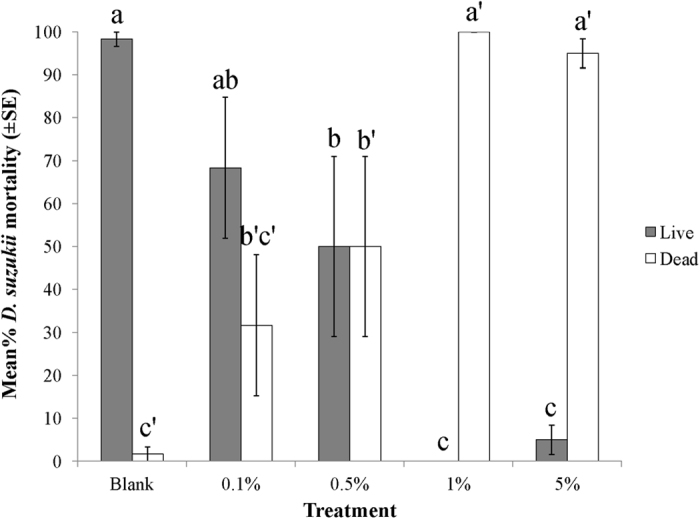
Mortality assessed after 48 hrs exposure to pre-treated blueberries (60 berries/treatment). Means flowed by the different letters and superscripts are significantly different at α = 0.05 (N = 6, F = 12.151; df = 4,25, p < 0.0001).
Figure 5. Impact of MB with different doses on subsequent D. suzukii infestation and development on pre-treated blueberries.
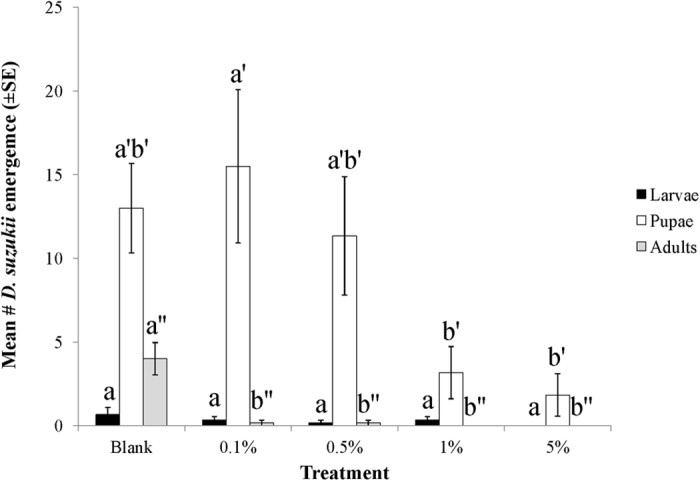
Numbers assessed after 10 days incubation at room temperature. Means flowed by the different letters and superscripts are significantly different at α = 0.05 (Log transformed; N = 6, df = 4,25; for larvae, F = 1.038, p > 0.05; for pupae, F = 5.982, p < 0.01; for adult, F = 27.981, p < 0.001).
Toxicity of MB to H. halys nymphs
MB also showed contact nymphicidal effect against H. halys nymphs. Of the five different stages tested, MB exhibited LC50 values from 1.01 to 2.39 μL/vial (Table 1).
Table 1. Nymphicidal toxicity of MB to different stages of H. halys.
| Stage | n* | LC50 (95% CL) μL/vial | Slope ± SE |
|---|---|---|---|
| 1st | 270 | 1.03 (0.93–1.10) | 7.69 ± 1.07 |
| 2nd | 270 | 1.01 (0.86–1.12) | 6.73 ± 1.11 |
| 3rd | 270 | 1.23 (1.12–1.33) | 5.28 ± 0.60 |
| 4th | 270 | 2.39 (2.19–2.60) | 6.10 ± 0.72 |
| 5th | 270 | 1.77 (1.60–1.93) | 6.00 ± 0.67 |
*Number of nymphs tested, 30 nymphs/concentration and nine different concentration solutions (including blank control) were tested. Acetone was used as blank control.
Ovicidal toxicity of MB and commercial pesticides
The ovicidal action of MB was compared to several commercially available organic pest control products (Table 2). The evaluations were conducted by measuring hatchability in direct spray bioassay on three species of eggs, including H. halys, M. sexta, and P. xylostella. Our results indicated that the MB had potent ovicidal effects with an LC50 value at 0.020 mg/cm2 and LC95 value at 0.048 mg/cm2 on H. halys (Table 3). A lower concentration of MB (0.0637 mg/cm2 active ingredient) was needed to reach 100% egg mortality for H. halys comparing to the other products used in the study (Table 4). At 0.0318 mg/cm2, MB was as potent as deltamethrin (0.0019 mg/cm2), ζ-cypermethrin (0.0223 mg/cm2), carbaryl (0.0080 mg/cm2), pyrethroid (β-cyfluthrin, 0.1592 mg/cm2), sulfur/pyrethroid (sulfur/pyrethrin, 0.6525 mg/cm2), and one of the organic essential oil products (2-phenethyl propionate, clover oil, rosemary oil, and thyme oil, at 0.3979 mg/cm2). Commercially available pesticides, λ-cyhalothrin (0.0016 mg/cm2), and acetamiprid, (0.0004 mg/cm2) and another organic essential oil product tested containing rosemary oil and peppermint oil (0.0637 mg/cm2) were almost ineffective. MB not only exhibited excellent ovicidal toxicity, but also had contact nymphicidal effect against H. halys nymphs (Table 1).
Table 2. Commercially available pesticides tested in laboratory bioassay.
| Trademark | Product | Active Ingredient (AI) | C%* |
|---|---|---|---|
| Spectracide | Bug Stop | Gamma-cyhalothrin | 0.025 |
| Bayer Advanced | Carpenter Ant & Termite Killer Plus | Beta-cyfluthrin | 2.5 |
| Hot Shot | Bedbug & Flea Home Insect Killer | Lambda-cyhalothrin | 0.03 |
| Raid Max | Bug Barrier | Deltamethrin | 0.03 |
| Amdro Quick Kill | Lawn & Landscape Insect Killer | Zeta-cypermethrin | 0.35 |
| Ortho | Bug B Gon | Bifenthrin | 0.3 |
| Zeta-cypermethrin | 0.075 | ||
| Natria | Insect, Disease & Mite Control | Sulfur | 10 |
| Pyrethrin | 0.25 | ||
| Bayer Advanced | Complete Insect Killer | Imidacloprid | 0.72 |
| Beta-cyfluthrin | 0.36 | ||
| Ortho | Flower, Fruit & Vegetable Insect Killer | Acetamiprid | 0.006 |
| Sevin | GardenTech | Carbaryl | 0.126 |
| EcoSmart | Organic Home Pest Control | 2-phenethyl propionate | 5 |
| Clover oil | 0.5 | ||
| Rosemary oil | 0.5 | ||
| Thyme oil | 0.25 | ||
| EcoSmart | Organic Garden Insect Killer | Rosemary oil | 0.5 |
| Peppermint oil | 0.5 |
*Aqueous solution.
Table 3. Ovicidal toxicity of MB to three species of insect eggs.
| Insect | n* | LC50 (95% CL) mg/cm2 | LC95 (95% CL) mg/cm2 | Slope ± SE |
|---|---|---|---|---|
| H. halys | 270 | 0.020 (0.012–0.026) | 0.048 (0.036–0.090) | 4.36 ± 1.11 |
| M. sexta | 270 | 0.015 (0.011–0.020) | 0.060 (0.042–0.112) | 2.77 ± 0.46 |
| P. xylostella | 2100 | 0.001 (0.001–0.002) | 0.005 (0.004–0.025) | 7.32 ± 1.14 |
*Number of eggs tested. 30 eggs/concentration and nine different concentration solutions (including blank control) were tested for H. halys and M. sexta. 300 eggs/concentration and seven different concentration solutions (including blank control) were tested for P. xylostella. DI water solution contained 0.5% Tween 20 (v/v) and 0.5% Tween 80 (v/v) was used as blank control.
Table 4. Ovicidal effect of MB and tested commercially available pesticides on different species of eggs after 10 days exposure.
| Treatment* | AI Dose (mg/cm2) | Hatchability (%) (mean ± SE) |
||
|---|---|---|---|---|
| H. halys** | M. sexta** | P. xylostella*** | ||
| Blank Control**** | 0.0000 | 70 ± 5.8d | 87 ± 8.8c | 78 ± 3.8d |
| MB 0.025%**** | 0.0016 | 67 ± 8.8d | 67 ± 8.8c | 38 ± 5.5c |
| MB 0.05%**** | 0.0032 | 63 ± 17.6 cd | 63 ± 8.8c | 13 ± 5.0ab |
| MB 0.1% **** | 0.0064 | 60 ± 10.0 cd | 50 ± 17.3c | 0 ± 0.3a |
| MB 0.25 **** | 0.0159 | 43 ± 6.7abcd | 40 ± 15.3c | 1 ± 0.0a |
| MB 0.5% **** | 0.0318 | 17 ± 12.0ab | 20 ± 10.0abc | 0 ± 0.0a |
| MB 1% **** | 0.0637 | 0 ± 0.0a | 3 ± 3.3ab | 0 ± 0.0a |
| MB 2% **** | 0.1273 | 0 ± 0.0a | 0 ± 0.0a | |
| MB 4% **** | 0.2546 | 0 ± 0.0a | 0 ± 0.0a | |
| Bug Stop (γ-cyhalothrin) | 0.0016 | 83 ± 8.8d | 0 ± 0.0a | 9 ± 5.4a |
| Carpenter Ant & Termite Killer Plus (β-cyfluthrin) | 0.1592 | 3 ± 3.3a | 0 ± 0.0a | 7 ± 1.2a |
| Bedbug & Flea Home Insect Killer (λ-Cyhalothrin) | 0.0019 | 53 ± 3.3bcd | 0 ± 0.0a | 2 ± 0.9a |
| Bug Barrier (Deltamethrin) | 0.0019 | 10 ± 5.8ab | 0 ± 0.0a | 3 ± 1.0a |
| Lawn & Landscape Insect Killer (ζ-cypermethrin) | 0.0223 | 0 ± 0.0a | 0 ± 0.0a | 1 ± 0.9a |
| Bug B Gon (Bifenthrin & ζ-cypermethrin) | 0.0239 | 33 ± 14.5abcd | 43 ± 14.5c | 1 ± 0.7a |
| Insect, Disease & Mite Control (Sulfur & Pyrethrin) | 0.6525 | 3 ± 3.3a | ||
| Complete Insect Killer (Imidacloprid & β-cyfluthrin) | 0.0688 | 0 ± 0.0a | ||
| Flower, Fruit & Vegetable Insect Killer (Acetamiprid) | 0.0004 | 67 ± 20.3d | 16 ± 7.0ab | |
| GardenTech (Carbaryl) | 0.0080 | 0 ± 0.0a | 28 ± 9.2bc | |
| Organic Home Pest Control | ||||
| (2-phenethyl propionate & clover oil & Rosemary oil & Thyme oil) | 0.3979 | 17 ± 3.3ab | 30 ± 17.3bc | 1 ± 0.3a |
| Organic Garden Insect Killer (Rosemary Oil & Peppermint Oil) | 0.0637 | 70 ± 25.2d | ||
*0.5 ml volume applied. ** 30 eggs per treatment. *** 300 eggs per treatment. **** DI water solution contained 0.5% Tween 20 (v/v) and 0.5% Tween 80 (v/v). Means within the same column flowed by the different letters are significantly different at α = 0.05 (Log transformed, N = 3; for H. halys, df = 20, 42, F = 9.41, p < 0.001; for M. sexta, df = 15, 32, F = 14.14, p < 0.001; for P. xylostella, df = 15, 32, F = 29.18, p < 0.001).
The MB was also ovicidal against M. sexta eggs at 0.0637 mg/cm2 dose with an LC50 value at 0.015 mg/cm2 and LC95 value at 0.060 mg/cm2 (Table 3). It was significantly better than the mixture of bifenthrin & ζ-cypermethrin (0.0239 mg/cm2) and an essential oil product containing 2-phenethyl propionate, clover oil, rosemary oil, and thyme oil (0.3979 mg/cm2) (Table 4).
For P. xylostella, MB demonstrated potent ovicidal activity at a dose as low as 0.0032 mg/cm2, with an LC50 value at 0.001 mg/cm2 and LC95 value at 0.005 mg/cm2 (Table 3). Interestingly, the carbaryl was one of the most effective compounds against H. halys egg at 0.0080 mg/cm2, but it was one of the most ineffective ovicidal compounds against P. xylostella (Table 4).
Discussions
This current study demonstrates that methyl benzoate (MB) is an efficient green pesticide against invasive insect pest D. suzukii, and several other agricultural pests. MB not only effectively prevented D. suzukii from oviposition and inhibited subsequent larvae/pupae development, but also caused complete mortality of adult flies on pre- and post-treated blueberries at a concentration as low as 1%. Moreover, MB possessed ovicidal activity against several different species of eggs, when compared to some commercially available pesticides. On the basis of toxicity data, MB was five times more toxic than the conventional pyrethroid (β-cyfluthrin), 20 times more toxic than sulfur & pyrethrin mixture, and 12 times more toxic than one of the organic commercial products (2-phenethyl propionate, clover oil, rosemary oil, and thyme oil) against H. halys eggs. Neither γ-cyhalothrin nor acetamiprid exhibited ovicidal toxicity against H. halys at tested doses. For M. sexta and P. xylostella, similar toxic results were obtained, but P. xylostella appeared to be more sensitive to MB treatment. To reach 100% egg mortality, only 0.0064 mg/cm2 was needed, which was 10 times less than H. halys and 20 times less than M. sexta eggs needed for the same results.
In nature, many plant species emit a great amount of VOCs into atmosphere, which are related to plant ecology, physiology, and atmospheric chemistry16,17. Some of these VOCs may act as defensive compounds against insect herbivores and plant pathogens; while others may act as chemical signals involved in plant-plant, plant-animal, and plant-microorganisms interactions18. MB naturally occurs as an aroma and scent of many plants19, including flowers20,21 and fruits22,23,24,25,26,27, and plays important roles in plant communication with the surrounding environment. Particularly, MB is one of the more abundant scents emitted from petunia, Petunia hybrid and snapdragon, Antirrhinum majus, functioning as a long-range attractant to lure bees for pollination28,29,30,31. MB has also been used by many insect species as a semiochemical that carries a message for purpose of communication between individuals of the same species (intraspecific) or between different species (interspecific)32. Moreover, MB is known for its sweet, balsamic, spicy, and heady floral odor; and it has been used as a fragrance ingredient and preservative in many personal care applications, such as shampoos, shower products and face/neck care, liquid soaps, mouthwash, perfume, hair colorants and cosmetics33. MB is of low to moderate human toxicity by ingestion and inhalation34,35, and it is approved by the US Food and Drug Administration (21 CFR 172.515)36 and the European Union (EU Regulation 1334/2008 & 178/2002)37 for food use as a food-grade flavor ingredients. While MB is also considered environment-friendly, slowly biodegrading in the atmosphere38, it would still need to be registered as a pesticide with the EPA. To the best of our knowledge, the pesticidal activity of MB has not been previously reported. Overall, our research findings demonstrated that the methyl benzoate was an effective green pesticide against some invasive species, especially, H. halys and D. suzukii, with low concentration and high mortality; therefore, providing an alternative tool to synthetic pesticides for insect pest management in crop production.
Methods
Chemicals
Methyl benzoate, α-terpinene, γ-terpinene, terpineol, cineole, α-pinene, Tween 20, and Tween 80 were purchased from Sigma-Aldrich (St. Louis, MO). Acetone was used as solvent and purchased from Sigma-Aldrich (St. Louis, MO). All chemicals were used without further purification. Commercial pesticides were purchased from the Home Depot (College Park, MD) and used directly: Spectracide® Bug Stop (St. Louis, MO), Bayer Advanced® Carpenter Ant & Termite Killer Plus (Research Triangle Park, NC), Hot Shot® Bedbug & Flea Home Insect Killer (St. Louis, MO), Raid Max® Bug Barrier (Racine, WI), Amdro Quick Kill® Lawn & Landscape Insect Killer (Atlanta, GA), Ortho® Bug B Gon (Marysville, OH), Natria® Insect Disease & Mite Control (Research Triangle Park, NC), Bayer Advanced® Complete Insect Killer (Research Triangle Park, NC), Ortho® Flower, Fruit & Vegetable Insect Killer (Marysville, OH), Sevin® Garden Tech(Atlanta, GA), EcoSmart® Organic Home Pest Control (Roswell, GA), and EcoSmart® Organic Garden Insect Killer (Roswell, GA). Some commercial pesticides contained either a synthetic pyrethroid, a neonicotinoid or a combination of these. One (Sevin) contained a carbamate, while several other contained compounds (essential oils; and 2-phenylethy proprionate) exempt from EPA registration (see: https://www.epa.gov/minimum-risk-pesticides/minimum-risk-pesticide-definition-and-product-confirmation). Active ingredients and corresponding concentrations are listed in Table 2.
Insects
The H. halys adults, nymphs, and eggs were obtained from colony maintained in the facility located in USDA, ARS, Beltsville, MD. The H. halys colony was established in 2007 from adults collected in Allentown, PA, USA. Insects were reared on a diet of organic green beans and shelled sunflower and buckwheat seeds (2:1, w/w) in ventilated plastic cylinders (21 cm × 21 cm OD, BioQuip Inc, Rancho Dominguez, CA) and maintained in Percival incubator (Percival Scientific Inc, Perry, IA) at 25 °C and 60% RH, under a 16 L:8D photoperiod39. Eggs were collected weekly and hatched in plastic Petri dishes with a water vial, and after molting to second-instars, the nymphs were transferred to the ventilated plastic cylinders for the remaining four instars39. Adult males and females were separated 1 or 2 days after emergence and subsequently maintained in different containers.
The colony of M. sexta was originally obtained from the University of Arizona, Tucson, AZ, reared, and maintained on an artificial wheat germ diet40 in an insectary located in the same USDA, Beltsville facility at 24 °C and 40% RH. Eggs and young larvae were covered by glass trays. Older larvae were kept in ventilated plastic boxes (27 × 17.5 × 10 cm, BioQuip Inc, Rancho Dominguez, CA). Adults were kept in screened cages (45.75 × 45.75 × 45.75 cm, BioQuip). After small tomato plants introduced into the screened cages for 3–4 days, deposited eggs on the plants were removed by hand.
The P. xylostella colony was obtained from Benzon Research, Carlisle, PA, reared, and maintained on an artificial wheat germ diet41 at the same USDA facility. Eggs and larvae were put in closed cardboard cups (236 mL, 8.9 cm diameter, 5.7 cm height, Solo Cup Company, Lake Forest, IL) and kept in a Percival incubator at 25 °C, 34% RH, under a 16 L:8 D photoperiod in the same insectary. Adults were maintained in screened cage (30.5 cm × 30.5 cm × 30.5 cm, BioQuip Inc). Eggs were deposited on aluminum foil strips (approx. 5.0 × 30.5 cm) dipped in cabbage juice and collected after 3–4 days.
The D. suzukii colony was provided by Rutgers University, originally obtained from D. suzukii-infested blueberry (Vaccininum corymbosum cv. Bluecrop) fruits in Burlington County, New Jersey. The colony was reared on cornmeal diet42 in polystyrene vials (height, 95 mm, diameter, 28.5 mm, Fisher Scientific, PA, USA) with plugs (height, 25 mm, diameter. 28.5 mm, Fisher Scientific, PA, USA) and kept in a Percival incubator at 25 °C, 34% RH, under a 16 L:8 D photoperiod in the same facility located in USDA, ARS, Beltsville, MD.
Organic green beans and blueberries (Cottle Farms, Cottle Strawberry Nursery, Inc, Faison, NC) were purchased from MOM’s organic market (College Park, MD, USA).
Laboratory bioassay
Bioassays were conducted in a USDA Beltsville laboratory at ~25 °C controlled by central air-condition system, ~60% RH by a dehumidifier (WHYnter the Home Depot, MD), under a 16 L:8D photoperiod (TORK 1101 Time Switch, Amazon.com) with ∼1700 lux light illuminance (a 100 watt bulb). A fume hood was maintained at same condition with face velocity at 129 FPM. The plastic cups (32 oz, diameter 4.5 inches, deep 5 inches, or 16 oz, diameter 4.5 inches, deep 2.5 inches) were purchased from papermart.com (CA). An 80 mm diameter hole was cut from each lid and replaced with an 85 mm diameter mesh (mesh size, 81 × 81, BioQuip, CA) screen that was glued into place. The polystyrene vials (height, 95 mm, diameter, 28.5 mm) and plugs were obtained from Fisher Scientific (Pittsburg, PA). The plastic cage (30 × 30 × 30 cm) was purchased from BugDorm (Rancho Dominguez, CA). Glass vial (20 mL), glass spray bottle (Amber glass with spray top, 30 mL), Petri dish (9 cm diameter), and Whatman filter paper (90 mm diameter) were obtained from VWR (Atlanta, GA). Deionized water (DI) containing 1% emulsifier (v/v), Tween 20 and Tween 80, at 1:1 ratio was used to make different VOCs water solutions and also used as blank control.
Toxicity of MB to D. suzukii
To investigate the acute toxicity of MB against D. suzukii, the bioassays were performed according to a published procedure43 with modifications. A total of 100 blueberries were placed in a plastic cage (30 × 30 × 30 cm) and infested by 100 mixed sex adults D. suzukii for 4 days. After removing all insects, half of pre-infested blueberries were dipped in100 mL MB aqueous emulsion at 1% concentration, while other half of pre-infested blueberries was dipped in deionized water (DI) water as a control. The blueberries were subsequently placed in two separate Petri dishes and allowed to air dry for 2 hr. After drying, the blueberries were stored separately in two plastic cups (32 oz) with ventilated lids and incubated at ambient room temperature on the bench top for 12 days. At this time, the presences of adult D. suzukii were recorded, and the berries were dissected to assess development of any larvae and pupae. The experiment was repeated six times.
Comparison of MB to ‘minimum risk’ pesticides against D. suzukii
To compare the pesticidal properties of MB to other essential oils (considered ‘minimum risk pesticides’) against D. suzukii, a series of bioassays were conducted according to the published procedure43 with modifications. 10 blueberries were separately dipped in either 100 mL MB, or other essential oil aqueous emulsions (1%) Blueberries dipped in 100 mL DI water served as controls. The blueberries were placed in different Petri dish bottoms and allowed to air dry for 2 hr. After drying, the treated blueberries were placed in plastic cups (32 oz) with ventilated lids, and 10 adult (males and females) D. suzukii were introduced into each cup. Mortality of the D. suzukii was examined after 48 hr. After removing all insects, the blueberries were maintained at room temperature on the bench top for another 10 days. Present of adults were subsequently assessed and development of larvae and pupae was further inspected by dissection of the berries.
Toxicity of MB to H. halys nymphs
The contact mortality bioassays were carried out in scintillation glass vials (20 mL, Wheaton Scientific Product, Millville, NJ), following published procedures with modifications44. Filter paper was cut into round pieces (2.4 cm diameter). MB was added to acetone to give different concentrations (0.025, 0.05, 0.10, 0.25, 0.5, 1.0, 2.0, 4.0%). 50 μL of each solution was applied to the filter papers, and the filter papers were dried for 1 min before being placed in the bottom of the vials. A small piece of green bean (1 cm) was put on the filter paper in each vial as food source. Different stages of H. halys nymphs were introduced into the vials and then the vials were capped with loosen cotton balls to prevent the nymphs from escaping. For each stage, 30 nymphs were used for each concentration of MB and nine different concentration solutions (including blank control) were tested. In total, 270 nymphs were tested for each stage. Due to the size and weight of H. halys increased significantly from first to fifth instar, the number of nymph tested per vial was decreased from 10 to 2 accordingly so that they had enough space and could move freely in the vial. For example, for the first instar, 10 nymphs were tested pre vial (3 vials per concentration); for the second and third instars, 5 nymphs were tested per vial (6 vials per concentration); for the fourth instar, 3 nymphs were tested pre vial (10 vials per concentration); and for the fifth instar, 2 nymphs were tested pre vial (15 vials per concentration). The vials were maintained in a fume hood and mortality was assessed after 24 hr. Mortality data was subjected to probit analysis using PoloPlus for LC50, LC95 with 95% confidence intervals calculation. In all tests acetone acted as the control.
Ovicidal toxicity of MB and commercial pesticides
Aqueous solutions of MB at varying concentrations (0.00, 0.025, 0.05, 0.10, 0.25, 0.5, 1.0, 2.0, 4.0%) and commercially available pesticides (see Table 2) were tested against several insect species. Eggs (10 for H. halys and M. sexta, 100 for P. xylostella) were collected and then placed on filter paper in Petri dishes. Different aqueous solutions were sprayed on the surfaces of the eggs three times (~ 0.5 mL total) using glass spray bottles (Amber glass with spray top, 30 mL) to completely cover the treatment areas (egg and filter paper). Then Petri dishes were covered with lids and maintained in a fume hood for 10 days. After this time, the Petri dishes were then inspected for presence of nymph or larvae, and the number of unhatched eggs, if any. An acetone spray was used as a control. The experiment was repeated three times.
Data analysis
Comparisons of different treatments were analyzed using one-way ANOVA followed by Ryan-Einot-Gabriel-Welsch F test (SPSS 10.0 for Windows)45 for significance at α = 0.05). Some data that ranges from being heavily positively skewed distribution (skewness = 1.37–2.38); therefore, log transformations were performed to remedy non-normality prior to the statistical analysis. PoloPlus software (LeOra Software, Berkeley, CA) was used to conduct probit analysis for mortality data, and LC50 and LC95 with 95% confidence intervals were estimated.
Additional Information
How to cite this article: Feng, Y. and Zhang, A. A Floral Fragrance, Methyl Benzoate, is An Efficient Green Pesticide. Sci. Rep. 7, 42168; doi: 10.1038/srep42168 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We would like to thank Dr. Cesar Rodriguez-Saona, Department of Entomology, Rutgers University, for providing SWD colony. We greatly appreciate Dr. Mark F. Feldlaufer, Invasive Insect Behavior and Biocontrol Laboratory, USDA, ARS, for his careful review of the manuscript. We are also deeply grateful for the technical assistance by Filadelfo Guzman on GC/GC-MS instrumentation and semiochemical collection.
Footnotes
The authors declare no competing financial interests.
Author Contributions All authors performed experiments. A.Z. and Y.F. conceived the idea. A.Z., Y.F. analyzed data and wrote the manuscript. All authors contributed to the discussion of the results and reviewed the manuscript.
References
- Alexandratos N. World food and agriculture: Outlook for the medium and longer term. Proc. Natl. Acad. Sci. USA 96(11), 5908–5914 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassman K. G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 96(11), 5952–5959 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D., Cassman K. G., Matson P. A., Naylor R. & Polasky S. Agricultural sustainability and intensive production practices. Nature 418, 671–677 (2002). [DOI] [PubMed] [Google Scholar]
- Debach P. & Rosen D. Biological Control by Natural Enemies, 2 ed. (London: Cambridge University Press, London, 1991). [Google Scholar]
- Pimentel D. et al. Environmental and economic effects of reducing pesticide use. Bioscience 41(6), 402–409 (1991). [Google Scholar]
- Miller G. T. Sustaining the Earth: An integrated Approach, 6 ed. (Pacific Grove: Brooks/Cole, Pacific Grove, CA, 2004). [Google Scholar]
- Forget G. Pesticides and the third world. J. Toxicol. Environ. Health 32(1), 11–31 (1991). [DOI] [PubMed] [Google Scholar]
- Isman M. B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66 (2006). [DOI] [PubMed] [Google Scholar]
- de Oliveira J. L., Campos E. V. R., Bakshi M., Abhilash P. C. & Fraceto L. F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 32, 1550–1561 (2014). [DOI] [PubMed] [Google Scholar]
- Adorjan B. & Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragrance J. 25, 407–426 (2010). [Google Scholar]
- Isman M. B. Plant essential oils for pest and disease management. Crop Protect. 19(8-10), 603–608 (2000). [Google Scholar]
- Palacios S. M., Bertoni A., Rossi Y., Santander R. & Urzua A. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca oomestica L. Molecules 14(5), 1938–1947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batish D. R., Singh H. P., Kohli S. K. & Kaur S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manage. 256, 2166–2174 (2008). [Google Scholar]
- Traboulsi A. F., Taoubi K., El-Haj S., Bessiere J. M. & Rammal S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera : Culicidae). Pest Manage. Sci. 58(5), 491–495 (2002). [DOI] [PubMed] [Google Scholar]
- Pavlidou V. et al. Insecticidal and genotoxic effects of essential oils of Greek sage, Salvia fruticosa, and mint, Mentha pulegium, on Drosophila melanogaster and Bactrocera oleae (Diptera : Tephritidae). J. Agric. Urban Entomol. 21(1), 39–49 (2004). [Google Scholar]
- Lerdau M., Guenther A. & Monson R. Plant production and emission of volatile organic compounds. Bioscience 47(6), 373–383 (1997). [Google Scholar]
- Guenther A. et al. A global-model of natural volatile organic-compound emission. J Geophys Res Atmos 100(D5), 8873–8892 (1995). [Google Scholar]
- Penuelas J. & Llusia J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 44(4), 481–487 (2001). [Google Scholar]
- Choudhary M. I. et al. Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 69(4), 1018–1023 (2008). [DOI] [PubMed] [Google Scholar]
- Knudsen J. T. & Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in mothpollinated taxa. Bot. J. Linn. Soc. 113(3), 263–284 (1993). [Google Scholar]
- Effmert U. et al. Floral benzenoid carboxyl methyltransferases: From in vitro to in planta function. Phytochemistry 66(11), 1211–1230 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich O. & Schreier P. Additional volatile constituents of carambola (Averrhoa carambola L.) fruit. Flavour Fragrance J. 4(4), 177–184 (1989). [Google Scholar]
- Shaw G. J., Ellingham P. J. & Birch E. J. Volatile constituents of Feijoa - headspace analysis of intact fruit. J. Sci. Food Agric. 34(7), 743–747 (1983). [Google Scholar]
- Young H., Paterson V. J. & Burns D. J. Volatile aroma constituents of kiwifruit. J. Sci. Food Agric. 34(1), 81–85 (1983). [Google Scholar]
- Bartley J. P. & Schwede A. M. Production of volatile compounds in ripening kiwi fruit (Actinidia chinensis). J. Agric. Food Chem. 37(4), 1023–1025 (1989). [Google Scholar]
- Binder R. G. & Flath R. A. Volatile components of pineapple guava. J. Agric. Food Chem. 37(3), 734–736 (1989). [Google Scholar]
- Muchalal M. & Crouzet J. Volatile components of clove essential oil (Eugenia caryophyllus Spreng): neutral fraction. Agric. Biol. Chem. 49(6), 1583–1589 (1985). [Google Scholar]
- Kolosova N., Gorenstein N., Kish C. M. & Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13(10), 2333–2347 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. Bumblebee Economics: With a New Preface. (Cambridge: Harvard Univ Press, Cambridge, MA, 2004). [Google Scholar]
- Dudareva N. et al. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12(6), 949–961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre F. et al. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 15(12), 2992–3006 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed A. M. The pherobase: database of insect pheromones and semiochemicals, Available at http://www.pherobase.com., (2016) Data access: 09/14/2016.
- European-Commission, List of preservatives allowed in cosmetic products, Available at http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006D0257&from=EN., (2006) Data access: 09/14/2016. [Google Scholar]
- Clayton G. D. & Clayton F. E., Patty’s Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology, 3 ed. (New York: John Wiley Sons, New York, 1981–1982). [Google Scholar]
- Opdyke D. L. J. Monographs on Fragrance Raw Materials: A Collection of Monographs Originally Appearing in Food and Cosmetics Toxicology. (Oxford: Pergamon Press Ltd., Oxford, 1979). [Google Scholar]
- FDA, Code of Federal Regulations Title 21 - Food and Drugs. Sec. 172.515 Synthetic flavoring substances and adjuvants, Available at http://www.ecfr.gov/cgi-bin/text-idx?SID=19b722c36a7ddf6190681f27b911a80b&mc=rue&node=se21.3.172_1515&rgn=div8., (2016) Data access: 09/14/2016.
- European-Union, Food flavourings. EU Regulation 1334/2008 & 178/2002, Available at http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:sa0006., (2015) Data access: 09/14/2016.
- Atkinson R. A structure-activity relationship for the estimation of rate constants for the gas-phase reactions of OH radicals with organic compounds. Int J Chem Kinet 19(9), 799–828 (1987). [Google Scholar]
- Harris C., Abubeker S., Yu M., Leskey T. & Zhang A. Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha Halys. PLoS ONE 10(11), e0140876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R. T. Mass rearing of the tobacco hornworm. II. Larval rearing and pupation. J. Econ. Entomol. 62(6), 1427–2431 (1969). [Google Scholar]
- Shelton A. M., Cooley R. J., Kroening M. K., Wilsey W. T. & Eigenbrode S. D. Comparative analysis of two rearing procedures for diamondback moth (Lepidoptera: Plutellidae). J. Entomol. Sci. 26(1), 17–26 (1991). [Google Scholar]
- Dalton D. T. et al. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manage. Sci. 67(11), 1368–1374 (2011). [DOI] [PubMed] [Google Scholar]
- Cuthbertson A. G. S., Collins D. A., Blackburn L. F., Audsley N. & Bell H. A. Preliminary Screening of Potential Control Products against Drosophila suzukii. Insects 5(2), 488–498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A. L., Shearer P. W. & Hamilton G. C., Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. J. Econ. Entomol. 101(4), 1439–1442 (2008). [DOI] [PubMed] [Google Scholar]
- George D. & Mallery P., SPSS for Windows step by step: A simple guide and reference, 4th ed. (Allyn & Bacon, Boston, 2002). [Google Scholar]


