Progressive multifocal leukoencephalopathy (PML) is a serious complication of natalizumab treatment in patients with relapsing-remitting MS (RRMS)1 with 638 confirmed cases as of March 2016. Therapeutic re-establishment of cerebral immune surveillance in PML management is complicated by immune reconstitution inflammatory syndrome (IRIS), an exuberant inflammatory response that aggravates damage caused by John Cunningham virus (JCV) infection and ultimately leads to a combined PML/IRIS syndrome.2 Currently, plasma exchange (PLEX) for the elimination of natalizumab and reconstitution of immune surveillance is used as standard of care, although this might lead to rebound MS activity or enhanced IRIS. Here, we report 2 cases of patients with PML/IRIS who did not receive PLEX, but were instead treated with the CCR5 antagonist maraviroc that has been associated with an amelioration of IRIS.3
Classification of evidence.
This provides class IV evidence. It is an observational study without controls.
Case presentation.
Case 1.
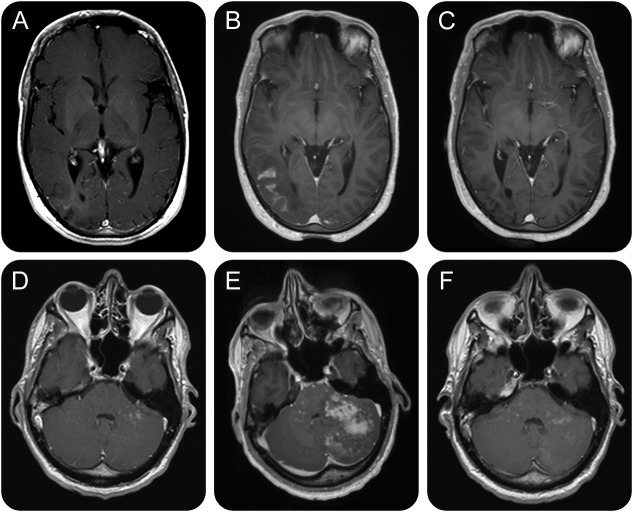
A 34-year-old man with RRMS (an Expanded Disability Status Scale (EDSS) score of 3.5) had been treated with natalizumab for 3 years without previous immunosuppression. Treatment was stopped because of a high JCV antibody index (3.21 in July 2014). Four weeks later, head MRI showed a right subcortical occipital contrast-enhancing T2-hyperintense lesion suggestive of PML-IRIS. However, JCV DNA was undetectable in the CSF on repeated lumbar punctures, and the patient was asymptomatic. Three months later, the patient was admitted with seizures, headaches, impaired memory, and subsequent hemianopia. MRI showed progression of the occipital lesion (figure). Brain biopsy confirmed clinically suspected PML. High-dose steroid therapy led to clinical improvement; however, 4 weeks later, MRI showed progression with multifocal PML-IRIS. Treatment with oral maraviroc at a dose of 600 mg daily was initiated. Cognitive abnormalities improved, and MRI showed regression of PML-IRIS 6 weeks later. At 12 months after diagnosis of PML-IRIS and 6 months after start of maraviroc, the EDSS score was 4.0. PML-IRIS lesions did resolve, and no new MS disease activity was detectable.
Figure. Serial MRIs of the brain.
Serial axial postcontrast T1-weighted magnetic resonance images of the brain of case 1 (A–C) and case 2 (D–F). (A) In February 2015, a T1-weighted image showed right subcortical occipital lesion (hyperintense in T2-weighted images) with subtle contrast enhancement suggestive of progressive multifocal leukoencephalopathy (PML) in a clinically asymptomatic patient. (B) Four months later, MRI showed progression of the contrast-enhancing occipital lesion, and maraviroc treatment was initiated. (C) Six months after start of maraviroc, MRI shows regression of PML–immune reconstitution inflammatory syndrome (IRIS) with no detectable contrast enhancement. (D) A routine MRI in February 2015 showed disseminated contrast-enhancing lesions in the cerebellum suggestive of PML in a clinically asymptomatic patient. (E) In May 2015, a T1-weighted image showed extensive IRIS after discontinuation of maraviroc. (F) Eleven months after the initial diagnosis of PML and after 8 months of maraviroc treatment, multiple disseminated contrast-enhancing lesions in the cerebellum are still detectable.
Case 2.
A 40-year-old man with RRMS, an EDSS score of 0, had received natalizumab treatment for 4 years as a second-line treatment following β-interferons. The patient had been JCV antibody–positive since November 2011. A routine MRI in February 2015 showed disseminated contrast-enhancing lesions in the cerebellum suggestive of PML while the patient was clinically asymptomatic. PCR detection of JCV in the CSF (60 copies/mL) confirmed the diagnosis of PML. Natalizumab treatment was stopped, but PLEX was not performed. Follow-up MRI 4 weeks after PML diagnosis showed stable MRI findings. Oral maraviroc was started at a dose of 300 mg twice daily. While the patient remained asymptomatic, JCV DNA in the CSF increased to 330 copies/mL, with increased Gd-enhancement on MRI. Maraviroc was stopped after 8 weeks, assuming that treatment might have interfered with viral clearance. The patient presented 3 weeks later (15 weeks after the initial diagnosis) to our clinic with headache, vertigo, ataxia, and diplopia. MRI showed progression of the cerebellar lesions with increasing mass effect suggestive of IRIS (figure). JCV DNA in the CSF was slightly decreased (174 copies/mL). High-dose IV methylprednisolone (1,000 mg once daily for 5 days) led to clinical improvement. Treatment with oral maraviroc at a dose of 300 mg twice daily was restarted and combined with oral prednisolone at an initial dose of 80 mg daily and then tapered off within 24 weeks. The patient's cerebellar syndrome further improved and signs of IRIS on MRI attenuated. However, 11 months after the initial diagnosis of PML, multiple disseminated contrast-enhancing lesions in the cerebellum were still detectable on MRI, indicating persisting PML-IRIS. No new MS disease activity was noted, and the clinical condition reached pre-PML status.
Discussion.
Improved risk stratification algorithms with increased clinical vigilance, repeated evaluation of JCV serology and JCV antibody index, and MRI monitoring can now successfully lead to early detection of PML-IRIS in natalizumab-treated patients with RRMS.4 However, recommendations for therapy management of early-identified and ideally still asymptomatic patients are currently not available. We hypothesize, based on our case reports, that omission of PLEX and treatment with maraviroc might contribute to the prevention of complete PML-IRIS. Withholding natalizumab, treatment with steroids and young age of both patients might have also beneficially influenced outcome in both cases. The CCR5 antagonist maraviroc successfully excludes CD8+ T cells from invading the CNS;3 however, increasing JCV DNA levels in the CSF and the still detectable contrast enhancement in MRI after several months of maraviroc therapy in the second case indicate that management of PML-IRIS can be a tightrope walk between the necessity of JCV clearance and the overshooting damaging immune reconstitution. Further studies are warranted for evidence-based recommendations on the management of patients with early-identified natalizumab-associated PML.
Footnotes
Author contributions: Sibylle C. Hodecker and Klarissa H. Stürner: drafting/revising the manuscript, acquisition of data, and analysis and interpretation of data. Veit Becker, Birte Elias-Hamp, Brigitte Holst, Manuel A. Friese, and Christoph Heesen: drafting/revising the manuscript.
Study funding: No targeted funding.
Disclosure: S.C. Hodecker received travel funding from Genzyme. K.H. Stuerner received research support from Biogen-Idec and BMBF. V. Becker served on the advisory board for Multiple Sclerosis, Biogen, Novartis, Genzyme, and Merck Serono; received speaker honoraria from Genzyme and Biogen; and received research support from Biogen, Novartis, Merck Serono, Teva, Amgen, and Genzyme. B. Elias-Hamp served on the scientific advisory board for Biogen, Bayer Healthcare, Teva, Sanofi Genzyme, Novartis, Merck Serono, and Roche; received travel funding and/or speaker honoraria from Biogen, Bayer Healthcare, Teva, Sanofi Genzyme, Novartis, and Merck Serono; and received research support from Biogen, Bayer Healthcare, Sanofi Genzyme, and Merck Serono. B. Holst reports no disclosures. M.A. Friese received research support from the German Research Foundation and the German Ministry of Education and Research. C. Heesen received speaker honoraria from Biogen, Genzyme, Novartis, Merck, and Roche; is on the editorial board of the International Journal of MS Care; received research grants from Biogen, Genzyme, Novartis, Merck, and Roche; and received research support from Genzyme, Sanofi-Aventis, Biogen, Novartis, German Ministry of Research, Hertie Foundation, and NMSS. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Bloomgren G, Richman S, Hotermans C, et al. . Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880. [DOI] [PubMed] [Google Scholar]
- 2.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomini PS, Rozenberg A, Metz I, Araujo D, Arbour N, Bar-Or A. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med 2014;370:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plavina T, Subramanyam M, Bloomgren G, et al. . Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]