Abstract
Background
The use of left ventricular assist devices (LVADs) has gained significant importance for treatment of end-stage heart failure. Fast-track procedures are well established in cardiac surgery, whereas knowledge of their benefits after LVAD implantation is sparse. We hypothesized that ultra-fast-track anesthesia (UFTA) with in-theater extubation or at a maximum of 4 h. after surgery is feasible in Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level 3 and 4 patients and might prevent postoperative complications.
Methods
From March, 2010 to March, 2012, 53 LVADs (50 Heart Mate II and 3 Heart Ware) were implanted in patients in our department. UFTA was successfully performed (LVADultra) in 13 patients. After propensity score matching, we compared the LVADultra group with a matched group (LVADmatch) receiving conventional anesthesia management.
Results
Patients in the LVADultra group had significantly lower incidences of pneumonia (p = 0.031), delirium (p = 0.031) and right ventricular failure (RVF) (p = 0.031). They showed a significantly higher cardiac index in the first 12 h. (p = 0.017); a significantly lower central venous pressure during the first 24 h. postoperatively (p = 0.005) and a significantly shorter intensive care unit (ICU) stay (p = 0.016). Kaplan-Meier analysis after four years of follow-up showed no significant difference in survival.
Conclusion
In this pilot study, we demonstrated the feasibility of ultra-fast-track anesthesia in LVAD implantation in selected patients with INTERMACS level 3–4. Patients had a lower incidence of postoperative complications, better hemodynamic performance, shorter length of ICU stay and lower incidence of RVF after UFTA. Prospective randomized investigations should examine the preservation of right ventricular function in larger numbers and identify appropriate selection criteria.
Electronic supplementary material
The online version of this article (doi:10.1186/s13019-017-0573-9) contains supplementary material, which is available to authorized users.
Keywords: Fast-track-anesthesia, Left ventricular assist device, Right ventricular failure, Postoperative complication
Background
Fast-track anesthesia (FTA) in cardiac surgery had been around long before the nineties but did first gain popularity and acceptance after the 1990s. Many studies showed that, in selected patients, FTA is feasible and safe and reduces the occurrence of ventilator-induced complications, thereby decreasing intensive care unit (ICU) stay, resource use and cost [1–4]. The feasibility of ultra-fast-track anesthesia with in-theater extubation (UFTA) has even been described following heart transplantation and in high-risk patients [5–8]. Prolonged mechanical ventilation is associated with poor outcomes and mortality [9, 10], and it has a deleterious hemodynamic effect first and foremost on right heart function [11–13]. Patients with advanced heart failure requiring left ventricular assist device (LVAD) implantation are particularly prone to many postoperative complications such as respiratory failure, prolonged mechanical ventilation, psychiatric events and right ventricular failure (RVF) leading to high morbidity and mortality [14–16]. Despite ample knowledge of the risk factors promoting right heart dysfunction, RVF remains a serious and dreaded postoperative complication with high mortality rates [14, 15, 17, 18]. In this retrospective study, we aimed to investigate the impact of UFTA following LVAD implantation on ICU and overall hospital stay, and to assess the effect of UFTA in reducing postoperative complication.
Methods
Design and data collection
A retrospective data search and analysis of prospectively collected data from all patients who underwent implantation of LVAD between March, 2010 and March, 2012 was performed. Informed consent was waived by our ethical board (Ethik-Komission RWTH) due to the retrospective nature of the analysis. The following data were collected from the electronic database: demographics, comorbidities, preoperative diagnostic results from left and right heart catheterization, echocardiographic findings, spirometry, radiographic finding, laboratory results, perioperative surgical and anesthesia protocols, hemodynamic and ventilation parameters from monitoring during the operation and in the ICU, and packed red blood cells (PRBCs) given in the operating room (OR) and during the remaining hospital stay. European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) and Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) were calculated for all patients. Ambulatory patients were routinely followed up every two months from March, 2010 until March, 2016 according to our standardized follow-up protocol for LVAD patients.
Surgical procedures
All patients underwent cardiac surgery through full median sternotomy. LVAD implantation and, if necessary, concomitant tricuspid valve repair (TVR) and/or coronary artery bypass grafting (CABG) were performed with on-pump beating heart in 38 cases. In 5 cases with concomitant aortic valve replacement (AVR), myocardial protection was ensured through antegrade crystalloid cardioplegia with mild hypothermia (32–34 °C). Prior to cardiopulmonary bypass (CPB), heparin was given to achieve an activated clotting time (ACT) of ≥ 400 s. Patients, who underwent UFTA, were rewarmed to a minimal body temperature of >36.5 °C before weaning from CPB. At the end of surgery, all patients were transferred to the ICU.
Patients groups
Within the mentioned period, the patients were individually selected for the UFTA protocol at the discretion of the attending anesthetists and cardiac surgeons. The exclusion criteria for UFTA included age ≥ 70 years, INTERMACS levels 1 and 2, chronic obstructive pulmonary disease (COPD) > grade II, body mass index ≥ 30 kg/m2 (BMI), impaired preoperative pulmonary function with a reduced forced expiratory volume in 1 s (FEV1)/Forced vital capacity (FVC) ratio = FEV1% < 65%, cerebrovascular accident (CVA) in medical history and preoperative hemodialysis. Due to the fact that this is the first systematic approach to this new technique, we deliberately chose a pilot design: Only patients deemed suitable for this new strategy on an expert consensus between surgeon and anesthetist were recruited. This of course poses a source of bias, yet from an ethical point of view, it remains the only plausible strategy to determine non-inferiority before entering a randomized controlled trial design.
To avoid inappropriate comparison, patients classified as INTERMACS Level 1 or 2, were excluded, due to the fact that patients with INTERMACS level 1 and 2 are high risk patients, hemodynamically unstable, some of them already are intubated and on positive inotropic support preoperatively, all other LVAD patients who were extubated according to our regular institutional protocol during the first 12 h. postoperatively or later in the ICU formed our historical control group (LVADconv). Patients, who had successful UFTA, formed (LVADultra) group and were retrospectively compared to matched patients from the LVADconv group (LVADmatch) (Fig. 1), for detailed information of patient's data please refer to the Additional file 1. This excludes the two patients who failed UFTA despite an intention to treat. Those cases are further described in the following paragraph UFTA failure.
Fig. 1.
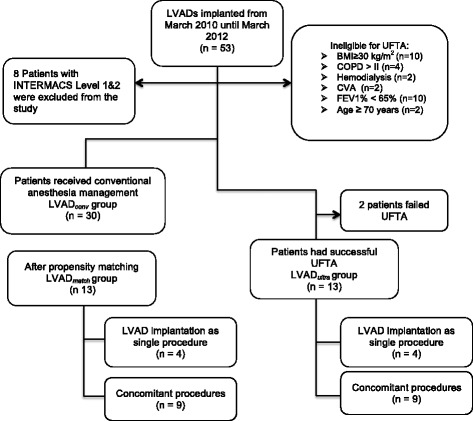
Patients groups and study design. BMI: Body mass index kg/m2; COPD: Chronic obstructive lung disease; CVA: Cerebrovascular accident; FEV1%: Ratio of forced expiratory volume in 1 s (FEV1)/ Forced vital capacity (FVC); INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; LVAD: Left ventricular assist device; LVADconv: All LVAD patients, who received conventional anesthesia; LVADmatch: LVAD patients, who received conventional anesthesia and were matched with the 13 patients who received ultra-fast-track anesthesia; LVADultra: Patients, who had ultra-fast-track anesthesia
Anesthesia protocol
All patients scheduled for LVAD implantation received no premedication prior to surgery. Cardiac medication was continued until the morning of surgery. In both groups, anesthesia was induced with sufentanil 0.25–0.5 μg/kg, propofol 1 to 1.5 mg/kg and rocuronium 1 mg/kg. Muscle relaxants were not repeated during the operation. Anesthesia was maintained with propofol 2–4 mg/kg/h and sufentanil 0.5–2.0 μg/kg/h. When the surgeon started the actual LVAD implantation procedure, sufentanil was stopped, and remifentanyl (continuous infusion 0.2 μg/kg/min) was used for analgesia in the LVADultra group. At the end of surgery, before skin closure, remifentanyl application was stopped, and the patients received piritramid 0.1 mg/kg. Propofol was discontinued, and patients were put in a beach-chair position. A remaining neuromuscular block was excluded. On arousal, the patients were asked to obey simple commands and tasks, e.g., move arms and legs, swallow and lift head. Finally, after negotiation of pain, the patient’s trachea was extubated. Oxygen was given via a facemask (target SpO2 94–100%), and carbon dioxide retention was excluded.
In the conventional group, both sufentanil and propofol were continued in the ICU. These patients were ventilated and weaned from ventilation according to clinical standards, including lung protective ventilation. Extubation criteria included: 1. Normothermia and normovolemia; 2. Absence of surgical bleeding with adequate hemostasis with normal activated coagulation time; 3. Complete reversal of the neuromuscular blockade assessed by limb movements and spontaneous ventilation sufficient to maintain arterial oxygen saturation over 95% with 40% FiO2 and end-tidal carbon dioxide under 50 mmHg; 4. Hemodynamic stability without significant inotropic support; and 5. A conscious patient obeying simple verbal commands.
Hemodynamic monitoring in the OR and ICU
In addition to basic monitoring (ECG, pulse oximetry, invasive blood pressure measurements, temperature measurements and arterial and central venous blood gas analysis with a sampling frequency of 30 min or as determined by clinical protocol), all patients received an additional pulmonary artery catheter (PAC) to control cardiac index (CI) and central venous oxygen saturation (ScvO2). Transesophageal echocardiography (TEE) was routinely used in all procedures.
Definition of RVF
With no universally accepted definition of RVF after LVAD placement, we used the following definition: 1. ≥ 48 h. nitric oxide (NO) (or other pulmonary vasodilator, such as iloprost); 2. Multi-organ failure from persistent hypotension without evidence of sepsis; 3. Positive inotropic agents for ≥14 days post-LVAD or late re-institution of inotropes (>14 days post-LVAD); or 4. Needing right ventricular assist device. This model was used by Kalogeropoulos et al. [19] and is consistent with the Kormos et al. model [14].
Diagnostic criteria of postoperative delirium
The definition of delirium is based on the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) from the American Psychiatric Association [20]. We use the Confusion Assessment Method (CAM) for the ICU (CAM-ICU) [21, 22]. The CAM-ICU was estimated for each patient in the ICU at least twice a day during both day and night shift rounds.
Statistical analyses
Continuous variables are expressed as the means ± standard deviation (SD) and categorical variables as absolute numbers and percentages. Due to non-normally distributed data the comparisons between groups before matching were performed with the Mann-Whitney-U-test for continuous variables and Fisher’s exact test or χ2 test, where appropriate, for categorical variables. Due to the small group of patients who had successful UFTA (LVADultra) and to reduce selection bias, we performed propensity score matching to match all 13 patients in the LVADultra group with the appropriate patients in the LVADconv group after excluding patients classified as INTERMACS level 1–2 from LVADconv group. Propensity scores were calculated for each patient using multivariate logistic regression based on the following preoperative covariates: Age, BMI, COPD ≤ grade II, FEV1%, peripheral arterial disease (PAD), preoperative creatinine, Re-do procedures, European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), left ventricular ejection fraction (EF), pulmonary artery mean pressure (PAMP), pulmonary capillary wedge pressure (PCWP), CI, and right ventricular end-diastolic basal-diameter from a four chamber view (RVEDD1), measured according to the American Society of Echocardiography guidelines [23]. Variables were chosen for the propensity matching according to known preoperative risk-factors, which promote prolonged mechanical ventilation, prolonged ICU stay after open heart cardiac surgery [10, 24] and right heart failure after LVAD implantation [14, 18, 19]. LVADultra patients were matched to LVADconv patients with the closest propensity score with the nearest-neighbor algorithm without replacement and with a 0.2 matching tolerance. The LVADconv patients who could be matched formed the matched group LVADmatch. Figure 1 describes the design of the study and the patient groups. Kaplan–Meier analyses were used to estimate the survival functions for patients in both groups. Differences in survival were evaluated using the log-rank test. Patients were censored for transplantation. After matching, categorical outcomes were compared with the McNemar’s test, and continuous outcomes were compared with Wilcoxon signed-rank test. For the comparisons of continuous variables with repeated measurements (CI, ScvO2, CVP, MPAP) a One-Way ANOVA test with Sidak’s correction were performed. All statistical analyses were performed using SPSS software, version 23.0 (Chicago, IL, USA). Propensity matching was performed with the extension package of the statistical program R version 3.1. A two-tailed p-value of < 0.05 was considered significant. All p-values were reported as three digit numbers.
Results
A total of 53 patients (16.9% female, mean age 62 ± 7.9) received LVAD implantation (50 Heart Mate II; HMII, Thoratec, Pleasanton, CA, USA and 3 HeartWare HVAD, HeartWare Inc., Framingham, MA, USA). 8 patients, who were categorized in INTERMACS level 1 or 2, were excluded from the study (Fig. 1). 15 patients were eligible for UFTA. UFTA was successfully performed in 13 patients and failed in 2 patients. The two patients, who were not able to be extubated within the first 4 h postoperatively, required high doses of inotropic support at the end of surgery and were hemodynamically unstable, possibly due to systemic inflammatory response syndrome. Demographics and preoperative data are listed in Table 1. Combined surgery was performed in 29 patients; details of procedures and intraoperative data are described in Table 2. Six patients in LVADultra group and 10 patients in the LVADmatch group had LVAD implantation as destination therapy (DT). No differences in preoperative risk factors and demographics were detected between LVADultra and LVADmatch groups (Table 1). The FEV1% was significantly lower in the LVADconv group compared to the LVADultra group (LVADconv vs. LVADultra: 64.8 ± 7.1 vs. 74.4 ± 8.5, p = 0.001). All patients survived surgery. Patient in LVADconv group had significantly higher body mass index compared to the LVADultra group (29.1 ± 4.2 Kg/m2 vs. 26.1 ± 3.1 Kg/m2, p = 0.009) and higher preoperative creatinine values (1.3 ± 0.2 mg/dL vs. 1.1 ± 0.3 mg/dL, p = 0.032, respectively).
Table 1.
Demographic and preoperative data
| Unmatched | Matched | ||||||
|---|---|---|---|---|---|---|---|
| LVADconv | LVADultra | p-values | LVADmatch | LVADultra | p-values | Patients failed UFTA (n = 2) | |
| n = 30 | n = 13 | n = 13 | n = 13 | ||||
| Age | 65.2 ± 8.4 | 61.1 ± 7.9 | 0.108 | 64.1 ± 4.8 | 61.1 ± 7.9 | 0.485 | 63.5 ± 0.5 |
| Female n (%) | 6 (20) | 2 (15.4) | 1.000 | 1 (7.8) | 2 (15.4) | 0.500 | 1(50) |
| BMI kg/m2 | 29.5 ± 4.2 | 26.1 ± 3.1 | 0.009 | 26.3 ± 3.0 | 26.1 ± 3.1 | 0.735 | 25.8 ± 1.2 |
| PAD n (%) | 7 (23.3) | 5 (38.5) | 0.460 | 5 (38.5) | 5 (38.5) | 1.000 | 1(50) |
| creatinine (mg/dl) | 1.3 ± 0.2 | 1.1 ± 0.3 | 0.032 | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.907 | 1.1 ± 0.2 |
| GFR mL/min. | 65.6 ± 6.4 | 66.5 ± 6.5 | 0.673 | 65.3 ± 6.8 | 66.5 ± 6.5 | 0.693 | 65.5 ± 0.5 |
| Prior HD | 2 (6.7) | 0 | 1.000 | 0 | 0 | - | 0 |
| COPD ≤ II n (%) | 8 (26.7) | 5 (38.5) | 0.485 | 3 (23.1) | 5 (38.5) | 0.727 | 1(50) |
| COPD > II n (%) | 4 (6.7) | 0 | 0.297 | 0 | 0 | - | 0 |
| FEV1 % | 64.8 ± 7.1 | 74.4 ± 8.5 | 0.001 | 68.5 ± 6.2 | 74.4 ± 8.5 | 0.068 | 69.5 ± 1.5 |
| CVA | 2 (6.7) | 0 | 1.000 | 0 | 0 | - | 0 |
| DM n (%) | 12 (40) | 2 (15.3) | 0.163 | 4 (30.8) | 2 (15.3) | 0.625 | 2(100) |
| Nicotine | 20 (66.7) | 8 (61.5) | 0.742 | 11 (84.6) | 8 (61.5) | 0.453 | 2(100) |
| DCM n (%) | 6 (20) | 2 (15.4) | 1.000 | 3 (23.1) | 2 (15.4) | 1.000 | 0 |
| ICM n (%) | 24 (80) | 11 (84.6) | 1.000 | 10 (76.7) | 11 (84.6) | 1.000 | 2(100) |
| CI L/min/m2 | 2.4 ± 0.7 | 3.0 ± 1.6 | 0.651 | 2.3 ± 1 | 3.0 ± 1.6 | 0.251 | 2.9 ± 1 |
| MPAP mmHg | 28.9 ± 11.4 | 30.6 ± 11.6 | 0.758 | 34.5 ± 9.1 | 30.6 ± 11.6 | 0.198 | 30.5 ± 0.5 |
| PCWP mmHg | 19.3 ± 9.8 | 21.3 ± 9.4 | 0.614 | 23.8 ± 8.1 | 21.3 ± 9.4 | 0.330 | 24.5 ± 1.5 |
| LVEF % | 21.4 ± 6.7 | 17.7 ± 4.1 | 0.088 | 19.1 ± 4.7 | 17.7 ± 4.1 | 0.715 | 20 ± 2 |
| RVEDD1 mm | 38.9 ± 5.7 | 37.4 ± 6.1 | 0.389 | 37.5 ± 4.9 | 37.4 ± 6.1 | 0.984 | 38.5 ± 0.5 |
| TAPSE mm | 15.7 ± 3.1 | 14.7 ± 5.6 | 0.212 | 15.8 ± 4.1 | 14.7 ± 5.6 | 0.552 | 16.5 ± 0.5 |
| DT n (%) | 18 (60) | 6 (46.2) | 0.509 | 10 (76.9.6) | 6 (46.1) | 0.453 | 0 |
| BTC/BTT n (%) | 12 (40) | 7 (53.8) | 0.509 | 3 (23.1) | 7 (53.8) | 0.453 | 2(100) |
| EuroSCORE II % | 14.1 ± 11.6 | 13.4 ± 8.1 | 0.820 | 14.2 ± 7.8 | 13.4 ± 8.1 | 0.786 | 15.5 ± 0.5 |
| INTERMACS Level n (%): | |||||||
| Level 3 | 14 (46.7) | 4(30.8) | 0.502 | 2 (15.4) | 4 (30.8) | 0.431 | 0 |
| Level 4 | 16 (53.3) | 9(69.2) | 0.502 | 11 (84.6) | 9 (69.2) | 0.431 | 2(100) |
Continuous variables are expressed as means ± standard deviation (SD). Categorical variables are expressed as percentages and absolute numbers. Bold writing indicates significance
BMI body-mass-index, BTC bridge to candidacy, BTT bridge to transplantation, CI cardiac index, CPB time cardiopulmonary bypass time (min.), COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, DCM dilated cardiomyopathy, DM diabetes mellitus, DT destination therapy, EuroSCORE II European System for Cardiac Operative Risk Evaluation II, FEV1% ratio of forced expiratory volume in 1 s (FEV1)/ Forced vital capacity (FVC), GFR glomerular filtration rate (mL/min.), HD hemodialysis, ICM ischemic cardiomyopathy, INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support, LVEF left ventricular ejection fraction, MPAP mean pulmonary artery pressure, PAD peripheral artery disease, PCWP pulmonary capillary wedge pressure (mmHg), RVEDD1 right ventricular end-diastolic basal diameter (mm), TAPSE tricuspid annular plane systolic excursion (mm)
Table 2.
Surgical procedures and intraoperative data
| Procedures | LVADmatch | LVADultra | p-values | LVADconv. | p-values |
|---|---|---|---|---|---|
| n = 13 | n = 13 | n = 30 | LVADconv vs. LVADultra | ||
| Re-do OP | 0 | 0 | - | 4 (13.3) | 0.297 |
| LVAD alone n (%) | 4 (30.7) | 4 (30.7) | 1.000 | 10 (33.3) | 1.000 |
| LVAD + CABG n (%) | 4 (30.8) | 4 (30.7) | 1.000 | 8 (26.7) | 1.000 |
| LVAD + TVR n (%) | 3 (23) | 2 (15.3) | 0.984 | 5 (16.7) | 1.000 |
| LVAD + CABG + TVR n (%) | 2 (15.4) | 2 (15.3) | 1.000 | 1 (3.3) | 0.518 |
| LVAD + AVR n (%) | 0 | 1 (7.7) | 0.988 | 4 (13.3) | 1.000 |
| LVAD + AVR + CABG n (%) | 0 | 0 | - | 2 (6.7) | 1.000 |
| CPB time min. | 140.5 ± 34.8 | 118.6 ± 29.3 | 0.381 | 156.7 ± 56.1 | 0.032 |
| PRBC | 2.5 ± 2.5 | 2.4 ± 2.9 | 0.945 | 3.1 ± 2.9 | 0.459 |
Bold writing indicates significance
CABG coronary artery bypass graft, LVAD left ventricular assist device, TVR tricuspid valve repair, AVR aortic valve replacement, CPB cardiopulmonary bypass, PRBC packed red blood cells
Time to extubation and intensive care unit stay
The mean time to extubation differed significantly between the LVADultra and LVADmatch groups (1.2 ± 1.3 h. vs. 42.3 ± 32.1 h., respectively, p = 0.002). Five LVADultra patients (38.5%) were immediately extubated in the OR (Fig. 2). Three LVADmatch patients were re-intubated due to respiratory failure compared with one LVADultra patient (p = 0.125). Eight patients in LVADconv group required re-intubation (p = 0.236).
Fig. 2.
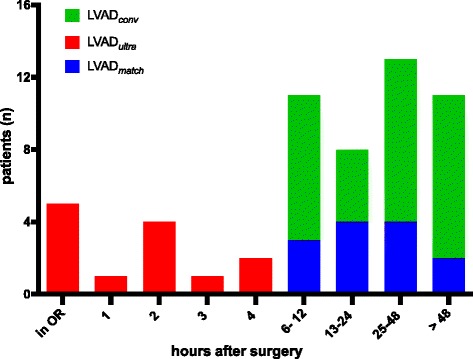
Cumulative number of patients extubated by postoperative hour. OR: Operating room
LVADultra patients had significantly shorter ICU stays than LVADmatch patients (LVADultra: 60.2 ± 43.4 h. vs. LVADmatch: 153.1 ± 95.9 h., p = 0.016) and required significantly shorter periods of inotropic support (LVADultra: 15.9 ± 19.5 h. vs. LVADmatch: 88.5 ± 108 h., p = 0.001). There was a tendency for shorter hospital length (LOS) of stay for the LVADultra patients (LVADultra: 22.1 ± 9.5 days vs. LVADmatch: 26.3 ± 14.9 days, p = 0.055). The LOS of LVADultra patients was significantly shorter compared with the LVADconv (22.1 ± 9.5 days vs. 37.8 ± 23.6, p = 0.026).
Postoperative complications
Postoperative data are described in Table 3. LVADultra patients had lower incidence of pneumonia (7.7% vs. 46.5%, p = 0.031) compared to LVADmatch patients. There was also a tendency for lower incidence of postoperative sepsis in the LVADultra group compared with LVADmatch group (0 vs. 23.1%. p = 0.250). None of the LVADultra patients developed postoperative delirium, while six patients in LVADmatch group developed postoperative delirium (p = 0.031). The glomerular filtration rate (GFR) measured 24 h. postoperatively was higher in the LVADultra group but did not differ significantly compared to the matched group (LVADultra vs. LVADmatch: 62.8 ± 10.2 vs. 58.1 ± 11.1 mL/min., p = 0.331).
Table 3.
Postoperative data
| LVADmatch | LVADultra | p-values | LVADconv | p-values | |
|---|---|---|---|---|---|
| n = 13 | n = 13 | LVADmatch vs. LVADultra | n = 30 | LVADconv vs. LVADultra | |
| Time to extubation in hr. | 42.3 ± 32.1 | 1.2 ± 1.3 | 0.0002 | 56.5 ± 67.7 | 0.0001 |
| Re-intubation n (%) | 3 (23.1) | 1(7.7) | 0.125 | 8(26.7) | 0.236 |
| ICU stays in hrs n (%) | 153.1 ± 95.9 | 60.2 ± 43.3 | 0.016 | 186.1 ± 163.1 | 0.0008 |
| Hospital LOS in days after Implantation | 26.3 ± 14.9 | 22.1 ± 9.5 | 0.055 | 37.8 ± 23.6 | 0.026 |
| Pneumonia n (%) | 7 (46.5) | 1 (7.7) | 0.031 | 10 (33.3) | 0.129 |
| Sepsis n (%) | 3 (23.1) | 0 | 0.250 | 7 (23.3) | 0.082 |
| Delirium n (%) | 6 (46.5) | 0 | 0.031 | 14 (46.7) | 0.003 |
| Hemodialysis n (%) | 3 (23.1) | 0 | 0.250 | 8 (26.7) | 0.081 |
| GFR 24 h. post-op mL/min. | 58.1 ± 11.1 | 62.8 ± 10.2 | 0.331 | 51.9 ± 16.5 | 0.033 |
| Re-thoracotomy n (%) | 0 | 0 | - | 3 (10) | 0.541 |
| Inotropic support in hr. | 88.5 ± 108 | 15.9 ± 19.5 | 0.001 | 113.3 ± 120.2 | 0.001 |
| RVF n (%) | 6 (46.5) | 0 | 0.031 | 8 (26.7) | 0.081 |
| RV-ECMO n (%) | 0 | 0 | - | 5 (16.7) | 0.300 |
Bold writing indicates significance
GFR glomerular filtration rate (mL/min.), ICU intensive care unit, LOS length of stay, Post-op postoperative, RV-ECMO extracorporeal membrane oxygenation as a right ventricular assist device, RVF right ventricular failure
Interestingly, none of the LVADultra patients developed RVF in the first 30 postoperative days (POD), whereas six LVADmatch patients developed RVF (p = 0.031). Of the six patients, who developed RVF in the LVADmatch group; One required implantation of extracorporeal membrane oxygenation as a temporary right ventricular assist device (RV-ECMO); Two patients required prolonged use of pulmonary vasodilator (NO) > 48 h.; One patient required prolonged use of positive inotropic agents ≥14 postoperative days; Two patients needed ICU re-admission with requirement of late positive inotropic support.
Hemodynamic parameters in the first 24 h. after surgery
An overview of the hemodynamic parameters is listed in Table 4 and in Fig. 3. At ICU admission, CI and central venous saturation (ScvO2) of the LVADultra group were significantly higher than those of the LVADmatch group, (LVADultra: 3.7 ± 1.1 vs. LVADmatch: 2.6 ± 0.4 L/min/m2, p = 0.013 and LVADultra: 73.4 ± 4.7% vs. LVADmatch: 63.7 ± 8.6%, p = 0.028). The difference in CI and ScvO2 between the two matched groups was still significant at 12 h. but at 24 h postoperatively no significant difference could be detected in CI and ScvO2 between the matched group (LVADultra: 3.4 ± 0.7 vs. LVADmatch: 2.8 ± 0.3 L/min/m2, p = 0.017 and LVADultra: 72.2 ± 6.5 vs. LVADmatch: 65.5 ± 5.6%, p = 0.034). CVP and MPAP did not differ significantly at ICU admission; however, at 12 h. and 24 h. postoperatively, LVADultra patients had significantly lower CVP and MPAP compared to LVADmatch patients (Table 4 and Fig. 3).
Table 4.
Postoperative Hemodynamic parameter
| LVADmatch | LVADultra | p-values | |
|---|---|---|---|
| Admission CI L/min/m2 | 2.6 ± 0.4 | 3.7 ± 1.1 | 0.013 |
| 12 h CI L/min/m2 | 2.8 ± 0.3 | 3.4 ± 0.7 | 0.017 |
| 24 h CI L/min/m2 | 2.9 ± 0.3 | 3.5 ± 0.7 | 0.078 |
| Admission ScvO2 % | 63.7 ± 8.6 | 73.4 ± 4.7 | 0.028 |
| 12 h ScvO2 % | 65.5 ± 5.6 | 72.2 ± 6.5 | 0.034 |
| 24 h ScvO2 % | 62.6 ± 15.4 | 68.9 ± 4.3 | 0.759 |
| Admission CVP mmHg | 14.2 ± 2.3 | 11.6 ± 2.7 | 0.071 |
| 12 h CVP mmHg | 13.9 ± 1.9 | 11.2 ± 1.2 | 0.049 |
| 24 h CVP mmHg | 13.6 ± 1.9 | 9.4 ± 2.1 | 0.005 |
| Admission MPAP mmHg | 29.2 ± 5.2 | 28.8 ± 6.5 | 0.996 |
| 12 h MPAP mmHg | 28.7 ± 3.2 | 24.2 ± 5.3 | 0.048 |
| 24 h MPAP mmHg | 26.6 ± 4.3 | 22.7 ± 2.9 | 0.003 |
P-values were carried out with one-way ANOVA test with Sidak’s correction; Bold writing indicates significance
CI cardiac index L/min/m2, CVP central venous pressure mmHg, ScvO 2 central venous saturation %, MPAP mean pulmonary artery pressure mmHg
Fig. 3.
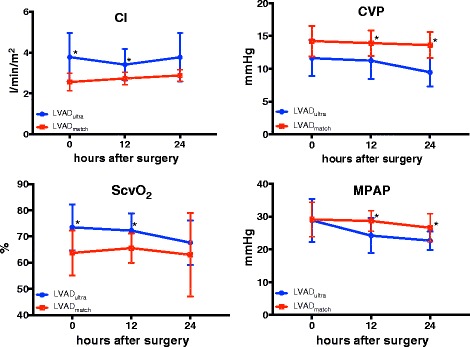
Hemodynamic parameters during 24 h postoperative. *: indicates significance; CI: cardiac index L/min/m2; CVP: central venous pressure mmHg, ScvO2: central venous saturation %; MPAP: mean pulmonary artery pressure mmHg. P-values were carried out with one-way ANOVA test with Sidak’s correction
Survival after surgery
There was no significant difference in the mean survival months after implantation between the LVADultra and LVADmatch groups (37.9 ± 20.7 and 49.5 ± 12.8, respectively, p = 0.150). The 30-day mortality was 7.7% in the LVADmatch group (n = 1) vs. 0 in the LVADultra group. The patient who died during the first 30 POD had RVF and was treated with RV-ECMO, but the clinical situation was then complicated by an additional septic shock and the patient died from multi-organ failure. There was no significant difference in the one-year and three-year survival after implantation between LVADultra and LVADmatch; 85% survived in each group after 1 year of implantation, while 69% of LVADultra patients and 64% of LVADmatch patients survived after 3 years of LVAD implantation.
Kaplan-Meier survival analysis for the follow-up period from March, 2010 until March, 2016 did not reveal any significant difference in survival between the LVADultra and LVADmatch groups (log-rank p = 0.776, Fig. 4).
Fig. 4.
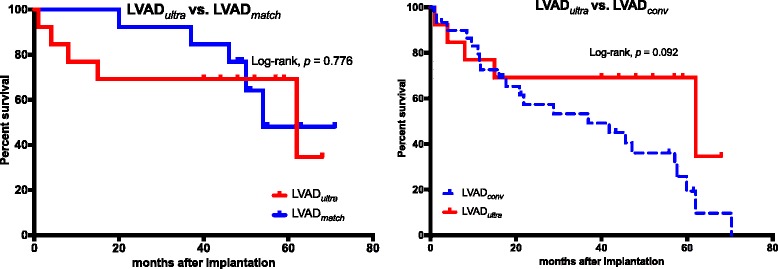
Survival proportions. CI: cardiac index L/min/m2; CVP: central venous pressure mmHg, ScvO2: central venous saturation %; MPAP: mean pulmonary artery pressure mmHg. Bold writing indicates significance
The Kaplan-Meier plots between LVADultra and LVADconv revealed no difference in survival between the LVADconv and LVADultra groups (log-rank p = 0.092), Fig. 4.
Discussion
This pilot study demonstrate that UFTA after LVAD implantation in INTERMACS level 3–4 patients is feasible and results in a lower incidence of postoperative complications and shorter ICU stay in selected patients. Therefore, our findings agree with other studies examining the feasibility of UFTA in cardiac surgery and heart transplantation albeit preoperative risk factors [5–8].
Postoperative complications
Previous studies did demonstrate that prolonged mechanical ventilation is associated with worse outcomes and higher mortality [9, 10, 25]. Cheng et al. found in a large randomized trial that prolonged mechanical ventilation results in worse physiologic outcomes as a result of atelectasis and intrapulmonary shunting [26]. In our study the incidences of pneumonia was significantly lower in the LVADultra group versus the LVADmatch group. These findings support those of Kurihara et al. and Kradzalic et al. [27, 28], who demonstrated a lower incidence of ventilator-associated pneumonia after FTA. Despite that the incidence of sepsis did not differ significantly between the two matched groups, a tendency for lower incidence of sepsis in the LVADultra could be detected (p = 0.055). Most importantly we could not detect any cases of postoperative delirium in the LVADultra group, while six patients in the LVADmatch group had postoperative delirium, which is a risk factor for prolonged ICU stay, especially in cardiac surgery patients. This is consistent with Cheng et al.’s results showing that patients had better results in mini-mental state testing after FTA and returned faster to baseline performance [29]. Previous studies did demonstrate that mechanical ventilation increases the risk of acute kidney failure [30, 31]. Despite the fact that the postoperatively GFR did not differ significantly between the two matched groups, there was a tendency for higher values in the LVADultra group with none of the patients in the LVADultra group requiring hemodialysis in the postoperative course.
Patients with end-stage heart failure, who require LVAD implantation, already have a limited tolerance of activity and loss of functional ability preoperatively. These patients had a high risk of morbidity and mortality when developing postoperative complications such as respiratory failure requiring prolonged mechanical support. Our results clearly demonstrate that LVADs patients had lower incidence of postoperative complication after UFTA. UFTA patients could be mobilized and discharged earlier from the ICU. Taken together, these factors have a markedly beneficial impact on the outcome of these severely ill patients accelerating the rehabilitation process [16, 32].
Incidence of RVF and hemodynamic performance
None of the LVADultra patients developed RVF, while six LVADmatch patients did. Early extubation and significantly shorter mechanical ventilation time are considered protective for the right ventricle; prolonged mechanical ventilation is a risk factor for RVF following LVAD implantation [33]. In the nineties, Jardin et al. showed a significant reduction in right ventricular stroke volume (RVS) during mechanical ventilation due to an increase in right ventricular (RV) afterload [34]. Studies of patients with acute respiratory distress syndrome (ARDS) revealed that mechanical ventilation affects RV function due to changes in RV impedance, preload and afterload, significantly affecting mortality [35–37]. RVF after LVAD implantation occurs in 10 to 40% of cases, and RVF results in higher mortality rates [15, 17, 38]. Our results demonstrate that selected patients with end-stage heart failure electively scheduled for LVAD implantation benefit from UFTA due to shorter cardiopulmonary impairment during mechanical ventilation preserving right ventricular function.
Indeed, CI differed significantly between the groups directly after LVAD implantation, but this effect vanished 24 h. after surgery. Most importantly, CVP values were significantly lower in the LVADultra group at 12 and 24 h. after surgery. These findings contrast with Meissner et al., who did not record any significant hemodynamic differences in either CI or CVP between fast-track-anesthesia (FTA) and conventional anesthesia (CA) following cardiac surgery in children [7]. Similarly, Djaiani et al. found no significant differences in cardiac output between UFTA and CA after adult cardiac surgery [8]. In accordance with our results, Morales et al. found significant improvement of hemodynamic performance after UFTA in children after Fontan’s procedure [39], and Kurihara et al. mentioned significantly lower CVPs after FTA compared to CA following cardiac surgery in children [28]. Our results clearly show that UFTA improves hemodynamics and reduces CVP after LVAD implantation in selected patients. This may help preserve RV function as many previous studies found that CVP ≥ 14 mmHg is a risk factor for RVF [14, 18, 19, 40].
UFTA failure
Previous studies revealed that reduced renal function, hypertension, age, EuroSCORE, cardiopulmonary bypass time, and cross-clamp time are risk factors predicting failure of fast track anesthesia [41, 42]. In our study the two patients, who failed to be extubated within the first 4 h. postoperatively were 64 and 63 years old, both of them had concomitant procedures (LVAD+ TKR and LVAD+ CABG) but the CPB time did not differ significantly compared to the LVADultra patients (127.6 ± 3.1 vs. 118.6 ± 29.3, p = 0.680). Also EuroSCORE II was not significantly higher compared to the LVADultra group, FEV1% was 68% and 71% in the same range of the LVADultra patients and GFR did not differ between the two patients and the rest of LVADultra patients (Table 1). Due to hemodynamic instability and requirement of high doses inotropic support, possibly due to systemic inflammatory response syndrome, the two patients were not able to be extubated within 4 h. postoperatively. These 2 patients were extubated 16 and 22 h. postoperatively. Due to the small group of patients, who failed UFTA in our study, multivariate regression analyzes could not be performed to detect risk factors predicting the failure of UFTA.
Limitations of the study
Deliberate selection of patients remains a strong source of bias that cannot be controlled by propensity matching. Careful selection of patients will remain necessary for UFTA procedure and future studies will have to determine coherent selection criteria. The retrospective nature of our study prevented standardization between the two groups. Accordingly, multivariate regression analyses to determine risk factors contributing to the failure of UFTA could not be performed. Adequate patient selection is vital when implementing fast-track regimens in perioperative LVAD protocols. The small number of matched pairs between both groups may limit generalization of the results. However, given the results of our study, prospective trials are encouraged to support broader application of UFTA in LVAD therapy.
Conclusion
In this pilot study, we demonstrated the feasibility of ultra-fast-track anesthesia in LVAD implantation in patients with INTERMACS level 3–4. Patients had a lower incidence of postoperative complications, better hemodynamic performance, shorter length of ICU stay and lower incidence of RVF after UFTA. Prospective investigations are encouraged to evaluate the capability of UFTA for sustainable protection of right ventricular function, and these studies should aim to identify useful criteria for adequate patient stratification.
Acknowledgements
Not applicable.
Funding
This study was performed by own departmental resources. No funding or grants were received.
Availability of data and material
All data generated or analysed during this study are included in this published article and its Additional file 1.
Authors’ contributions
AKM and RZ designed the study and developed the database. AKM and RZ wrote the manuscript. AG performed the statistical analysis, critically revised the design of the Study. AM, AKM and LT performed LVAD implantation. GS performed ultra-fast-track-anesthesia and developed the anesthesia protocol. CS, TPS made the clinical attendance on the ICU, collected the hemodynamic parameters and performed extubation on the ICU. RA as the department chair supported this study, participated in designing the study and critically revised the manuscript. AM, AG, AKM, LT and RZ made the follow-up in our outpatient department and collected the patient’s data. AM and RZ performed echocardiography. AM critically revised the manuscript in cooperation with the co-authors and interpreted the data. All authors read and approved the final manuscript.
Competing interests
AKM is an employee of Berlin Heart GmbH, Wiesenweg 10, 12247 Berlin. All other authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Due to the retrospective nature of the study, the need for approval was waived by the ethic commission (Ethik Kommission RWTH University, Pauwelsstr. 30, 52074, Aachen, Germany).
Abbreviations
- ACT
Activated clotting time
- AVR
Aortic valve replacement
- BMI
Body mass index Kg/m2
- CABG
Coronary artery bypass graft
- CI
Cardiac index L/min/m2
- COPD
Chronic obstructive pulmonary disease
- CPB
Cardiopulmonary bypass
- CVA
Cerebrovascular accident
- CVP
Central venous pressure
- ECG
Electro cardiogram
- ECMO
Extracorporeal membrane oxygenation
- EF
Ejection fraction %
- EuroSCORE II
European System for Cardiac Operative Risk Evaluation II
- FEV1
Forced expiratory volume in 1 s
- FTA
Fast-track anesthesia
- FVC
Forced vital capacity
- GFR
Glomerular filtration rate
- ICU
Intensive care unit
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LVAD
Left ventricular assist device
- NO
Nitric oxide
- OR
Operating room
- PAC
Pulmonary artery catheter
- PAD
Peripheral arterial disease
- PAMP
Pulmonary artery mean pressure mmHg
- PCWP
Pulmonary capillary wedge pressure mmHg
- POD
Postoperative day
- PRBCs
Packed red blood cells
- RVEDD1
Right ventricular end-diastolic basal-diameter mm
- RVF
Right ventricular failure
- ScvO2
Central venous saturation
- TEE
Transesophageal echocardiography
- TVR
Tricuspid valve repair
- UFTA
Ultra-fast-track anesthesia
Additional file
Detailed information of patient's data. (XLSX 56 kb)
Contributor Information
Rashad Zayat, Phone: +49 241 8089221, Email: rzayat@ukaachen.de.
Ares K. Menon, Email: ares.menon@berlinheart.de
Andreas Goetzenich, Email: agoetzenich@ukaachen.de.
Gereon Schaelte, Email: gschaelte@ukaachen.de.
Ruediger Autschbach, Email: rautschbach@ukaachen.de.
Christian Stoppe, Email: cstoppe@ukaachen.de.
Tim-Philipp Simon, Email: tsimon@ukaachen.de.
Lachmandath Tewarie, Email: ltewarie@ukaachen.de.
Ajay Moza, Email: amoza@ukaachen.de.
References
- 1.Zhu F, Lee A, Chee YE. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. 2012;10:CD003587. doi: 10.1002/14651858.CD003587.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Svircevic V, Nierich AP, Moons KG, Brandon Bravo Bruinsma GJ, Kalkman CJ, van Dijk D. Fast-track anesthesia and cardiac surgery: a retrospective cohort study of 7989 patients. Anesth Analg. 2009;108(3):727–33. doi: 10.1213/ane.0b013e318193c423. [DOI] [PubMed] [Google Scholar]
- 3.Cheng DCH, Wall C, Djaiani G, Peragallo RA, Carroll J, Li C, et al. Randomized assessment of resource use in fast-track cardiac surgery 1-year after hospital discharge. Anesthesiology. 2003;98(3):651–7. doi: 10.1097/00000542-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Plumer H, Markewitz A, Marohl K, Bernutz C, Weinhold C. Early extubation after cardiac surgery: a prospective clinical trial including patients at risk. Thorac Cardiovasc Surg. 1998;46(5):275–80. doi: 10.1055/s-2007-1010238. [DOI] [PubMed] [Google Scholar]
- 5.Kianfar AA, Ahmadi ZH, Mirhossein SM, Jamaati H, Kashani BS, Mohajerani SA, et al. Ultra fast-track extubation in heart transplant surgery patients. Int J Crit Illn Inj Sci. 2015;5(2):89–92. doi: 10.4103/2229-5151.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borracci RA, Ochoa G, Ingino CA, Lebus JM, Grimaldi SV, Gambetta MX. Routine operation theatre extubation after cardiac surgery in the elderly. Interact Cardiovasc Thorac Surg. 2016. doi:10.1093/icvts/ivv409. [DOI] [PMC free article] [PubMed]
- 7.Meissner U, Scharf J, Dotsch J, Schroth M. Very early extubation after open-heart surgery in children does not influence cardiac function. Pediatr Cardiol. 2008;29(2):317–20. doi: 10.1007/s00246-007-9023-0. [DOI] [PubMed] [Google Scholar]
- 8.Djaiani GN, Ali M, Heinrich L, Bruce J, Carroll J, Karski J, et al. Ultra-fast-track anesthetic technique facilitates operating room extubation in patients undergoing off-pump coronary revascularization surgery. J Cardiothorac Vasc Anesth. 2001;15(2):152–7. doi: 10.1053/jcan.2001.21936. [DOI] [PubMed] [Google Scholar]
- 9.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(23):e652–735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 10.Reddy SL, Grayson AD, Griffiths EM, Pullan DM, Rashid A. Logistic risk model for prolonged ventilation after adult cardiac surgery. Ann Thorac Surg. 2007;84(2):528–36. doi: 10.1016/j.athoracsur.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Cherpanath TG, Lagrand WK, Schultz MJ, Groeneveld AB. Cardiopulmonary interactions during mechanical ventilation in critically ill patients. Neth Heart J. 2013;21(4):166–72. doi: 10.1007/s12471-013-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cournand A, Motley HL, et al. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol. 1948;152(1):162–74. doi: 10.1152/ajplegacy.1947.152.1.162. [DOI] [PubMed] [Google Scholar]
- 13.Luecke T, Pelosi P. Clinical review: positive end-expiratory pressure and cardiac output. Crit Care. 2005;9(6):607–21. doi: 10.1186/cc3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139(5):1316–24. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 15.MacGowan GA, Schueler S. Right heart failure after left ventricular assist device implantation: early and late. Curr Opin Cardiol. 2012;27(3):296–300. doi: 10.1097/HCO.0b013e3283511e60. [DOI] [PubMed] [Google Scholar]
- 16.Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012;27(5):630–8. doi: 10.1111/j.1540-8191.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 17.Argiriou M, Kolokotron SM, Sakellaridis T, Argiriou O, Charitos C, Zarogoulidis P, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014;6(Suppl 1):S52–9. doi: 10.3978/j.issn.2072-1439.2013.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant.34(9):1123–30. doi:10.1016/j.healun.2015.06.015. [DOI] [PubMed]
- 19.Kalogeropoulos AP, Kelkar A, Weinberger JF, Morris AA, Georgiopoulou VV, Markham DW, et al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34(12):1595–603. doi: 10.1016/j.healun.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association . American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington: American Psychiatric Publishing; 2013. [Google Scholar]
- 21.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Ettema RG, Peelen LM, Schuurmans MJ, Nierich AP, Kalkman CJ, Moons KG. Prediction models for prolonged intensive care unit stay after cardiac surgery: systematic review and validation study. Circulation. 2010;122(7):682–9. doi: 10.1161/CIRCULATIONAHA.109.926808. [DOI] [PubMed] [Google Scholar]
- 25.Rashid A, Sattar KA, Dar MI, Khan AB. Analyzing the outcome of early versus prolonged extubation following cardiac surgery. Ann Thorac Cardiovasc Surg. 2008;14(4):218–23. [PubMed] [Google Scholar]
- 26.Cheng DC, Karski J, Peniston C, Asokumar B, Raveendran G, Carroll J, et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg. 1996;112(3):755–64. doi: 10.1016/S0022-5223(96)70062-4. [DOI] [PubMed] [Google Scholar]
- 27.Krdzalic A, Kosjerina A, Jahic E, Rifatbegovic Z, Krdzalic G. Influence of Remifentanil/Propofol Anesthesia on Ventilator-associated Pneumonia Occurence After Major Cardiac Surgery. Med Arch. 2013;67(6):407–9. doi: 10.5455/medarh.2013.67.407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurihara Y, Shime N, Miyazaki T, Hashimoto S, Tanaka Y. Clinical and hemodynamic factors associated with the outcome of early extubation attempts after right heart bypass surgery. Interact Cardiovasc Thorac Surg. 2009;8(6):624–8. doi: 10.1510/icvts.2008.189431. [DOI] [PubMed] [Google Scholar]
- 29.Cheng DC. Fast-track cardiac surgery: economic implications in postoperative care. J Cardiothorac Vasc Anesth. 1998;12(1):72–9. doi: 10.1016/S1053-0770(98)90061-1. [DOI] [PubMed] [Google Scholar]
- 30.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17(3):R98. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med. 2002;28(10):1426–33. doi: 10.1007/s00134-002-1442-z. [DOI] [PubMed] [Google Scholar]
- 32.Perme CS, Southard RE, Joyce DL, Noon GP, Loebe M. Early mobilization of LVAD recipients who require prolonged mechanical ventilation. Tex Heart Inst J. 2006;33(2):130–3. [PMC free article] [PubMed] [Google Scholar]
- 33.Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106(12 Suppl 1):I198–202. [PubMed] [Google Scholar]
- 34.Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology. 1990;72(6):966–70. doi: 10.1097/00000542-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Bouferrache K, Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care. 2011;17(1):30–5. doi: 10.1097/MCC.0b013e328342722b. [DOI] [PubMed] [Google Scholar]
- 36.Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985) 1999;87(5):1644–50. doi: 10.1152/jappl.1999.87.5.1644. [DOI] [PubMed] [Google Scholar]
- 37.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444–7. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 38.Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS et al. Right Heart Failure After Left Ventricular Assist Device Implantation in Patients With Chronic Congestive Heart Failure. J Heart Lung Transplant. 25(1):1–6. doi:10.1016/j.healun.2005.07.008. [DOI] [PubMed]
- 39.Morales DLS, Carberry KE, Heinle JS, McKenzie ED, Fraser CD, Jr., Diaz LK. Extubation in the Operating Room After Fontan's Procedure: Effect on Practice and Outcomes. Ann Thorac Surg. 86(2):576–82. doi:10.1016/j.athoracsur.2008.02.010. [DOI] [PubMed]
- 40.Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013;96(3):857–63. doi: 10.1016/j.athoracsur.2013.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youssefi P, Timbrell D, Valencia O, Gregory P, Vlachou C, Jahangiri M, et al. Predictors of failure in fast-track cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29(6):1466–71. doi: 10.1053/j.jvca.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Widyastuti Y, Stenseth R, Pleym H, Wahba A, Videm V. Pre-operative and intraoperative determinants for prolonged ventilation following adult cardiac surgery. Acta Anaesthesiol Scand. 2012;56(2):190–9. doi: 10.1111/j.1399-6576.2011.02538.x. [DOI] [PubMed] [Google Scholar]


