Abstract
Following watershed amendments in Schedule Y India's star rose rather rapidly on the clinical research (especially clinical trials) horizon. Just as dramatic was the fall of this empire. At the centre of these events has been the participant and indirectly, the Ethics Committee (EC) that is established primarily to protect this individual. This paper traces the evolution of the concept of ECs in India, examines the current state of these committees in the country and suggests the way forward.
The Past:
The requirement for an EC to oversee clinical research was first made in the ICMR Policy Statement for Ethics published in 1980 and then again in the Schedule Y (1988). Later, both the Amended Schedule Y (2005) assigned regulatory responsibility on the EC and the ICMR Guidelines (2006) described the functioning of ECs. Several challenges including inadequate formal training, contribution from non-technical members, administrative support as well no SOPs and a heavy workload were identified. In the absence of regulatory oversight of ECs, the introduction of the Clinical Trial Registry - India (CTRI) and self-regulation through voluntary accreditation programs brought a measure of accountability and transparency.
The Present:
A slew of regulatory reforms led to more than 1000 ECs to be registered with CDSCO although the actual impact on participants' protection and safety of these new regulations still remains to be seen.
Way Forward:
A method to oversee all ECs, improved functioning of ECs including on site monitoring, central ECs for multicentric studies, the development of metrics to assess the ability of ECs to protect the participant are other ideas for the future.
Conclusions:
Although ECs in India have evolved from being mere rubber stamps for approval of protocols to efficiently functioning accredited ECs, yet there is much to be done for and by Ethics Committees.
Keywords: Accreditation of Ethics Committee, Ethics Committee Registration, research participant, Schedule Y
INTRODUCTION
With a population of over 1.3 billion and a high disease burden,[1] India must engage in clinical research relevant to its health-care needs to build evidence that drives policy. Yet, this was not so in the past. The National Health Policy of India observed that “In our country, where the aggregate annual health expenditure is of the order of Rs. 80,000 crores, the expenditure in 1998–99 on research, both public and private sectors, was only of the order of Rs. 1150 crores. It would be reasonable to infer that with such low research expenditure, it is virtually impossible to make any dramatic breakthrough within the country, by way of new molecules and vaccines.”[2]
However, the last decade saw a marked and dramatic change in the landscape. Following watershed amendments in Schedule Y, India's star rose rather rapidly on the clinical research (especially clinical trials) horizon. The country's contribution to global clinical trials grew from 0.9% in 2008 to 5% in 2013.[3] This led to both optimistic forecasts that India could attract up to 10% of the global market of clinical trials,[4] and morose, sometimes crusty observations that India was being colonized again.[5] Reality was somewhere in between as always.
Just as dramatic as the rise was the fall of this empire. At its peak in 2010, the clinical trial industry started floundering with the global economic meltdown[3] and then more perilously in 2013 following the slew of regulatory changes brought about following Supreme Court orders and observations of the Parliamentary Standing Committee Reports.[6,7]
Against this background, it is pertinent to review the status of Ethics committees (EC) that are established primarily to protect a research participant's rights, dignity and ensure her/his well-being. This paper traces the evolution of the concept of ECs in India, examines the current state of these committees in the country, and suggests the way forward. For the purpose of this article, we have divided the decade (2005–2015) into three sections: The past which covers 2005–2013, the present running from 2013 to 2015, and the future after that.
THE PAST (2005–2013)
It was as far back as 1975 when the second revision of the Declaration of Helsinki adopted at the 29th World Medical Association General Assembly in Tokyo recommended, “The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol which should be transmitted to a specially appointed independent committee for consideration, comment, and guidance.”[8] The Belmont Report issued in 1979 further emphasized the need for review of all clinical research by ECs.[9]
In India, the requirement for an EC to oversee clinical research was first made in the Indian Council of Medical Research (ICMR) Policy Statement for Ethics published in 1980[10] and then again in the original version of the Schedule Y issued in 1988.[11] Schedule Y (1988) mentioned said, “It is desirable that protocols for clinical trials be reviewed and approved by the Institution's Ethical Committee. Since such committees at present do not exist in all institutions, the approval granted to a protocol by the Ethical Committee of one institution will be applicable to the use of that protocol in other institution, which do not have an Ethical Committee. In case none of the trial centres/institutions has an Ethical Committee, the acceptance of the protocol by the investigator and its approval by the Drugs Controller (India) or any officer as authorized by him to do so will be adequate to initiate the trials.”
Then, came the Indian good clinical practices (GCP)[12] in 2001, the watershed Amended Schedule Y[13] in 2005, and the ICMR's Ethical Guidelines For Biomedical Research On Human Participants in 2006.[14] Schedule Y (2005) detailed for the first time regulatory requirements of an EC. The ICMR guidelines released by the same Ministry (Health and Family Welfare) a year later described in more detail the composition, roles, and responsibilities of an EC. Some salient differences between these two documents are highlighted in Table 1.
Table 1.
Salient differences in Schedule Y (2005) and Indian Council of Medical Research Ethical Guidelines (2006) in the Ethics Committee constitution and functioning
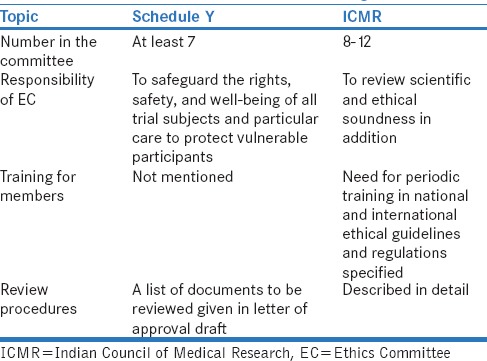
It is interesting to note that both the Amended Schedule Y (2005) and the ICMR Guidelines (2006) referred to “independent ECs (IEC[Ind])” favorably. The Schedule Y clearly stated, “The trial site(s) may accept the approval granted to the protocol by the ethics committee of another trial site or the approval granted by an independent ethics committee (constituted as per Appendix VIII), provided that the approving ethics committee(s) is/are willing to accept their responsibilities for the study at such trial site(s) and the trial site(s) is/are willing to accept such an arrangement and that the protocol version is same at all trial sites”[13] while the ICMR Guidelines said, “There are also independent ethics committees (IEC[Ind]) functioning outside institutions for those researchers who have no institutional attachments or work in institutions with no ethics committee.”[14] At that time, therefore, there was no limitation to the “IEC(Ind) s” overseeing clinical trials. This aspect bears relevance to the current change in thought at regulatory level about what an IEC(Ind) may oversee.
Functioning of Ethics Committees
Several papers addressed conditions under which ECs functioned in the time before 2013 when the clinical research was booming in the country. Basic issues such as lack of formally trained manpower (leading to mere scientific review), a heavy workload, inadequate space allocated for EC operations, and lack of administrative support were identified as important challenges leading to concerns whether the country was ready to take on a load of research. Infrequent meetings (at times only twice a year!) led to delays in approvals and constrained ECs to initial review and approval of protocols. Continued monitoring was often not undertaken, except for cursory review of serious adverse events (SAEs) and annual reports. The lack of active participation by the nonmedical members in the discussions at the meetings and the absence of standard operating procedures (SOPs) were other issues identified.[15,16] There was no requirement for members to declare conflict of interest which could compromise the functioning of the EC.[17]
In a questionnaire-based study conducted by the ICMR[10] (in which 32 medical colleges and ICMR institutes participated out of 147 approached), it was found that no legal expert was appointed to most committees and appointment procedures were unethical and included lobbying. This survey found that committees reviewed up to sixty projects per meeting and minutes and record keeping was poor.
In 2005, an editorial by Dr. Vasantha Muthuswamy, then the Senior Deputy Director General, ICMR, said, “Many IECs lack the expertise to adequately fulfill the mission of protecting the research participants. In most instances, the ethics approval can be regarded only as a minimal ethical standard for research.”[18] This editorial went on to say, “There are no SOPs for these committees which may or may not meet regularly and have varied methods of evaluation and decision-taking process. They end up doing scientific review of the research proposals and scrutinize the consent forms as the only proof of ethical requirement.”[18] It was recommended that ethics review mechanisms needed drastic improvement, for India to face the challenge of becoming a “global hub” for clinical trials.
At that time, investigators and sponsors alike considered ECs as obstacles, and in turn, ECs had inadequate or no “teeth” to do what they were supposed to do, which was, protect the research participant!
This did not change even when the number of clinical trials done in India peaked. A survey of 11 ECs revealed a lack of knowledge of Schedule Y among the EC members, coupled with inadequate training in GCP, inability to enlist the essential documents for EC review, and failure to realize the important role of EC approval in clinical research.[19] A suboptimal understanding of ethical issues among members and a need of formal training in ethics along with the need of networking among various ECs were reiterated in a study from Pune.[20]
The observations of the Parliamentary Standing Committee in their report on the human papillomavirus (HPV) vaccine[7] noted that the ECs that oversaw the HPV trials did not meet periodically to assess the progress of the project and review SAE reports. They appeared to exist only as a formality said the report. The Committee strongly recommended that there should be a mechanism in place to take appropriate action against such dereliction of duty on the part of the ECs and that the functioning of ECs should be regularly monitored.
The nontechnical members of an EC are central to its functioning and their contribution is of immense value. A survey among laypersons serving on ECs revealed several of them did not review informed consent documents (ICDs), those who said they did not understand technical terms in the ICDs, and one-third were unaware that their presence was vital for quorum to be met.[21] Other papers have also identified the lack of active participation of nonmedical members in the Committee's deliberations, as well as the fact that they lack training and are diffident.[22,23]
A review[24] identified 31 articles published between 2004 and 2012 related to the topic of the challenges faced while establishing Institutional ECs (IECs) and their functioning. The most common challenge mentioned in these articles was inappropriate functioning of IECs (n = 17), followed by inappropriate structure (n = 14). Other challenges included lack of oversight by regulatory bodies (n = 14) and inadequate training of EC members and investigators (n = 13). The need for administrative and financial support to ECs has been a common cry[25,26,27] as has been the creation of a central registration system.[18]
An important function of an EC is continuing monitoring, which is often passive monitoring, for example, reviewing SAE reports, periodic status reports, and protocol deviations. Active site monitoring was not practiced in the past, and IEC members were not trained to conduct monitoring. The EC of KEM Hospital (Mumbai) conducted seven site visits during 2008–2009 using a standardized format[28] to monitor adherence to protocol and the informed consent process. The monitoring identified issues related to informed consent (6/7), protocol deviation (5/7), reporting of study progress to the IEC (3/7), recruiting additional participants without IEC approval (2/7), reporting of SAEs (1/7), investigator's lack of awareness of protocol and the ICD.[29]
Oversight of Ethics Committees
In the time before 2013, although the regulator issued inspection plans for sites and sponsors, nothing was forthcoming for ECs. There was no oversight of ECs. A study reported a high degree of noncompliance of study approval letters issued by ECs to the Schedule Y/ICMR guidelines. For example, the quorum was not met (no legal experts and social scientists) in the EC meetings, and the ECs did not review essential documents such as the clinical trial agreement and the insurance policy.[30]
During this time, one important development that brought some transparency to EC oversight was the introduction of the Clinical Trial Registry-India (CTRI).[31] The CTRI was set up in 2007 at the National Institute of Medical Statistics, ICMR, New Delhi, as a free and online system for registration of all clinical trials being conducted in India (http://ctri.nic.in/Clinicaltrials/login.phpd). Since June 15, 2009, the Drugs Controller General of India made registration in the CTRI mandatory for regulatory trials. Although this portal brought in some transparency, information on ECs was often incomplete and did not really achieve any real oversight of ECs.
In the absence of regulatory oversight, self-regulation through voluntary accreditation programs was undertaken by some ECs. Five ECs were recognized by the Forum for Ethics Review Committees in Asia-Pacific (FERCAP) under the Strategic Intiative for Developing Capacity in Ethical Review (SIDCER) Recognition Program in this period.[32] The SIDCER is an initiative by WHO to build capacity in ethics review globally. These included Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital's Institutional Review Board (IRB) (Mumbai, 2009, 2012), The IRB, Tata Memorial Centre (Mumbai, 2009, 2012), IEC of Sanjay Gandhi Postgraduate Institute of Medical Sciences (Lucknow, 2011), Institutional Human EC of PSG Institute of Medical Sciences and Research (Coimbatore City, 2012), and IEC, National Institute for Research in Tuberculosis (NIRT), ICMR (Chennai, 2013) and a couple of these were re-recognized 3 years later. The Forum for Ethics Review Committees in India (FERCI) assisted in this effort.
Similarly, three organizations were accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP). These included Jehangir Clinical Development Centre Pvt. Ltd., Pune (2012), Kasturba Hospital, Manipal, Karnataka, India (2011), and Manipal Hospitals, Bengaluru, Karnataka, India (2011).[33]
Training efforts
Several organizations have, over the last few years, conducted formal training for members of EC in India. The Indian Society for Clinical Research has established a Training Council which aims to help in capacity building of clinical research professionals (including EC members) on various topics related to clinical research since 2005.[34] Similarly, the Clinical Development Services Agency (CDSA) under the Department of Biotechnology, Government of India, has trained over 4500 EC members since 2009 in about 50 workshops.[35] FERCI[36] is a registered society set up as the national chapter of FERCAP, the latter being an initiative undertaken by WHO TDR (Tropical Diseases Division). FERCI has established a network of ECs in India and has been contributing to training and awareness regarding research ethics through various ventures- freely downloadable detailed model SOPs incorporating current regulations, and holding conferences every alternate year. Recently FERCI in collaboration with PATH (CREaTe – FERCI initiative) has created 5 softwares to facilitate the functions of ECs and trained 23 ECs, which are now named “Smart ECs”.[37]
What recommendations were made?
In this period, several articles were written passionately asking for reforms and several solutions[24] were suggested that would improve EC functioning and their oversight. Issues related to constitution of the EC were on the top of the list of challenges that ECs faced, and authors strongly urged institutions to lend the ECs administrative and financial support to enable them to make improvements in their structure.[25,26,27]
To improve functioning of ECs, the need to have and follow SOPs was stressed.[38] Other suggestions included the need to set up separate scientific review boards,[39] use of checklists for review of protocols,[38] mandatory CTRI registration, and regular monitoring of the study.[22,31] Establishment of EC consortia[40] or a state-level EC to address multicenter studies and minimize “EC shopping” were other suggestions.[15]
A large number of papers addressed the need for training investigators in ethics and research methods.[24] The need to give the ICMR guidelines greater legal authority has been stressed.[38] An interesting recommendation was to make the need for having an established IEC as a prerequisite for Medical Council of India recognition for medical colleges.[25]
THE PRESENT (2013–2015)
The years after 2013 brought much transparency and some control over the constitution and functioning of ECs which were also entrusted with increasing responsibility in regulatory trials. The major drivers for this are the regulatory reforms listed in Table 2.
Table 2.
List of regulations that impacted Ethics Committees
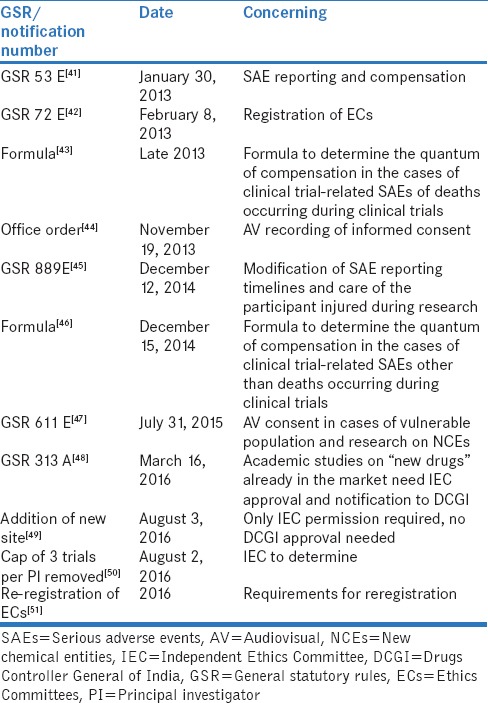
The major milestone in the history of ECs in India was the requirement of registration with Central Drugs Standard Control Organization (CDSCO).[42] As on August 1, 2016, there were 1083 registered ECs, of which 841 (77.7%) were institutional and 242 (22.3%) were independent.[52] The IECs(Ind) were not allowed to oversee clinical trials – only BA/BE studies were allowed. However, it is not clear whether IECs(Ind) can oversee nonregulatory clinical trials and other observational studies. Incidentally, the time to approval of an EC after application to the CDSCO had a median (range) of 77.5 (24–919) days with a significant difference (P = 0.002) between the Institutional 58 (5–919) and IECs(Ind) 165.5 (24–822) days.[53]
Maharashtra has the highest number of ECs registered (259/1083, 23.9%), followed by Gujarat (125/1083, 11.5%) and Karnataka and Tamil Nadu (112/841, 10.3%). The least number of registered ECs is in Himachal Pradesh (n = 2) and Jharkhand, Sikkim, and Jammu and Kashmir (n = 1 each). Some states and Union Territories, namely, Arunachal Pradesh, Manipur, Meghalaya, Tripura, Andaman and Nicobar, Dadra and Nagar Haveli, Daman, and Lakshadweep, do not have a single EC. A large number of ECs in a single state and lack of even a single one in several others reflect a skewed distribution of research in the country and needs to be addressed by policy makers and organizations such as the ICMR.[54] Another issue of concern is that of the medical colleges approved by the Medical Council of India to conduct postgraduate courses, several do not have registered ECs. Today with academic nonregulatory type of studies needing approval by registered ECs, this will need to change.
As has been said above, in the absence of all ECs registering with the CDSCO, the CTRI remains a very powerful source of information and gives a degree of transparency and some accountability to EC functioning. However, a cursory study of the CTRI website for EC information reveals several issues that need to be addressed by the organization. We examined 722 studies registered in the CTRI in 2016 (till August 10, 2016) and found as many as 67.6% (488/722) studies registered retrospectively. Around 488 ECs, 94% (454/488) of which were institutional were overseeing these studies. As many as 211/488 (43.2%) were not registered with the CDSCO. Interestingly, 153 (21%) studies were from the AYUSH sector (whose ECs are not registered with CDSCO). An important finding was that the name of the EC that investigators from the same institution registered in the CTRI varied for different studies. Thus, one institution would have as many as ten “names” for their Institutional Committee making it difficult to draw any conclusion.
On another note, we found that only about 82.4% EC registration approval letters could be downloaded from the CDSCO website.[53] This situation is likely to improve now as the re-registration of IECs is expected to be primarily online.[55] We assessed the effect of these regulations on our IEC and found that although the number of regulatory studies reviewed by our IEC remained the same, the number of studies actually approved decreased with an increase in the turnover time. The administrative workload rose with increased documentation and expenses.[54] We also found a significant effect on SAE reporting![56]
The new checklist for re-registration is stricter in requirements, and it is encouraging to note that training certifications of each member have to be submitted and monitoring has been made a requirement and data of monitoring has to be shown.[51]
The recent registration process of EC by the CDSCO required submission of SOPs (including the need for a separate SOP for handling projects that studied vulnerable populations). We studied SOPs of IECs of ten medical colleges/hospitals that were freely available (on the internet). We do not mention the names of the institutions as this is not an individual critique, but rather an exercise to assess the strengths and areas for improvement that ECs may address when making SOPs. We found that in general all SOPs mentioned a structure and composition that was in general as per the regulations and guidelines; however, only 1/10 stated that the composition would be multidisciplinary and multisectoral, a very important issue that must be adhered to. All mentioned how the EC would be constituted (always appointed by the Director/Principal, and none had these individuals as Chair), but none described the desirable qualities of each member in terms of their functions. Surprisingly, not one had a SOP for writing an SOP! And even more surprisingly, only some actually specified the effective dates (especially validity date). All the SOPs we downloaded were a single document describing the various activities. Only two had “appendices” which were templates for applications or approval letters. Interestingly, only 3/10 mentioned the need for confidentiality and conflict of interest declaration and none had a separate SOP for studies on vulnerable populations in spite of the regulatory requirement.[42] No SOP addressed the way complaints from patients/participants would be handled. In spite of regulatory requirement, only 4/10 mentioned the correct quorum (most said the number but not the expertise that was needed). The way the meeting was to be conducted, and minutes recorded were also poorly addressed. None had any timeline for EC procedures specified. This analysis indicates that although registration has achieved some transparency, there is a long way to go.
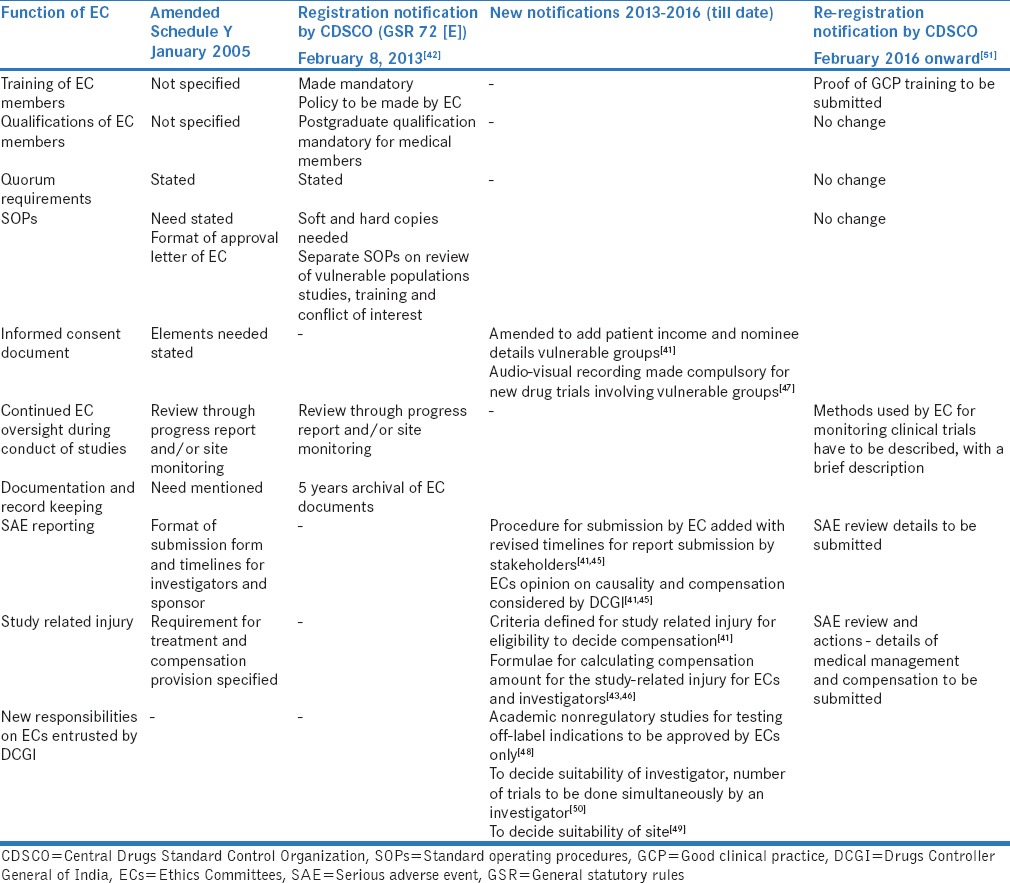
The actual impact on participants' protection and safety of these new regulations still remains to be seen although the impact of the regulations on the functioning of ECs is evident with a hugely increased responsibility and workload [Table 3].
Table 3.
Evolution of Ethics Committees in India in response to changes in regulatory guidelines (2005-2016)
Recognition/accreditation systems
While in the earlier years, some ECs did undergo accreditation processes, the numbers increased in the subsequent years. In 2014, the ECs of two ICMR Institutes (Institutional Human EC, National Institute of Epidemiology, Chennai, and the National Institute for Research in Reproductive Health EC for Clinical Studies, Mumbai), as well as IRB, Y.R. Gaitonde Centre for AIDS Research and Education, Chennai, were recognized by SIDCER. Further in 2015, the IEC Fortis Escorts Heart Institute, New Delhi, receieved SIDCER recognition.[32] In 2014, The Sahyadri Hospital Ltd., Pune; St. John's National Academy of Health Sciences, Bengaluru; and the Tata Memorial Centre, Mumbai, also obtained AAHRPP accreditation.[33] Some institutes such as the KEM Hospital, Mumbai, and Tata Hospital have been re-recognized twice more and the NIRT, Chennai, once more. These are encouraging observations that confirm the functioning of these committees along the international standards. The recognition processes have been described to have impacted the functioning of ECs favorably.[57] Each time, an EC gets re-recognized, the bar is set higher, and the quality of human research participant protection improves (personal communication).
WAY FORWARD
What then is the way forward for ECs in India? With increasing responsibility, there is a growing need for improved functioning so that research participants are protected and ethical clinical research, which is the need of the hour in our country, is promoted.
By far, the most important aspect that needs to be addressed is to have in place a method to oversee all ECs – either by voluntary accreditation (as described earlier) processes or by regulatory inspections. The National Accreditation Board for Hospitals and Healthcare Providers, Quality Council of India,[58] in consultation with various stakeholders, has formulated draft accreditation standards for clinical trial sites, ECs, and investigators, and inspectors have been trained. However, the process of mandatory accreditation has not yet started. Accreditation, which is a process meant to continuously improve working of the EC, is best left to be taken up voluntarily and concerns have been described the mandatory accreditation process.[59] The need of the hour is to initiate routine regulatory inspections of ECs. In the meantime, the re-registration process of ECs has begun and this time around the CDSCO is asking for evidence for statements (e.g., training of EC members, monitoring of studies, SAE review details)[51] made in the application form. This itself will impose certain discipline in ECs.
Although, in general, the composition and quorum are maintained in all registered ECs due to the regulatory requirements, the way projects reviewed, approved, and continuously monitored still need attention. The use of checklists is desirable with a better assessment of benefits and risks. This is especially true at continuing review where benefits and risks may change as new data emerges.[38,39]
Continuous monitoring is an activity that ECs do not undertake always. Apart from the review of continuing reports, protocol deviations,[60] and SAEs,[56] ECs must make efforts to conduct on-site monitoring.[29]
High-quality clinical research must be encouraged at postgraduate teaching institutes, and for this, an optimally functioning EC is needed. Institutes must encourage the setup of such ECs which have infrastructural and financial support from the Institute while having an internal audit mechanism in place for the EC.[54]
The concept of having a central EC for multicenter studies was suggested some years ago with cogent arguments in favor, to improve ethical oversight and minimize the variability that arises out of multiple ECs overseeing the same protocol.[61,62] This idea is supported by the US Food and Drug Administration and Office for Human Research Protections.[63,64] This is something India needs to consider. However, local sociocultural issues including review of consent forms in regional languages will have to be deliberated by local ECs at the site.[65] And finally, what is paramount is to see whether ECs function to achieve their primary objective – that of protecting the research participant. It has been said before that while we have tools to assess whether ECs adhere to policies and SOPs, there are not adequate tools to assess whether ECs actually protect participants.[66] The IRB Researcher's Assessment Tool (RAT) is one such tool,[67] which has been used in Gujarat to assess the functioning of the IECs at two medical colleges.[68]
CONCLUSIONS
We have seen in this paper that ECs in India have evolved from being mere rubber stamps for approval of protocols to efficiently functioning accredited ECs. Yet, considering the vastness of the country and the teeming population demanding quality research to generate evidence that will assist policy decisions, the need for unbiased, peer supervision of this research by efficient and regulated ECs is undoubted. Clearly, there is much to be done for and by Ethics committees.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1. [Last accessed on 2016 Aug 01]. Available from: http://www.who.int/gho/countries/ind.pdf?ua=1.
- 2.National Health Policy (India) 2002. [Last accessed on 2016 Aug 01]. Available from: http://www.apps.who.int/medicinedocs/documents/s18023en/s18023en.pdf.
- 3.Mondal S, Abrol D. Clinical Trials Industry in India: A Systematic Review. Working Paper 179, Institute for Studies in Industrial Development New Delhi. 2015. Mar, [Last accessed on 2016 Aug 01]. Available from: http://www.isid.org.in/pdf/WP179.pdf.
- 4.Singh S. Clinical Trials: New Horizon – India. [Last accessed on 2016 Aug 01]. Available from: http://www.pharmexcil.com/data/uploads/clinicaltrials.dr.surinder.ppt.
- 5.Nundy S, Gulhati CM. A new colonialism.– Conducting clinical trials in India? N Engl J Med. 2005;352:1633–6. doi: 10.1056/NEJMp048361. [DOI] [PubMed] [Google Scholar]
- 6.Department-Related Parliamentary Standing Committee on Health And Family Welfare Fifty.Ninth Report On The Functioning of The Central Drugs Standard Control Organization (CDSCO) (Presented to the Rajya Sabha on 8 May; 2012. Laid on the Table of the Lok Sabha on 8 May; 2012) [Last accessed on 2016 Aug 01]. Available from: http://www. 164.100.47.5/newcommittee/reports/EnglishCommittees/Committee%20on%20Health%20and%20Family%20Welfare/59.pdf.
- 7.Department-Related Parliamentary Standing Committee Rajya Sabha Report No. 72 On Health and Family Welfare August, 2013. Seventy Second Report Alleged Irregularities in the Conduct of Studies using Human Papilloma Virus (HPV) Vaccine by Path in India (Department of Health Research, Ministry of Health and Family Welfare) (Presented to the Rajya Sabha on 30th August; 2013) (Laid on the Table of Lok Sabha on 30th August; 2013) [Last accessed on 2016 Aug 01]. Available from: http://www.164.100.47.5/newcommittee/reports/EnglishCommittees/Committee%20on%20Health%20and%20Family%20Welfare/72.pdf.
- 8.The Declaration of Helsinki. 1975. [Last accessed on 2016 Aug 01]. Available from: http://www.ethics.iit.edu/ecodes/node/3931.
- 9.The Belmont Report, Office of the Secretary Ethical Principles and Guidelines for the Protection of Human Subjects of Research, The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research April 18; 1979. [Last accessed on 2016 Aug 02]. Available from: http://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/
- 10.Kumar NK. Bioethics Activities – Report from India. [Last accessed on 2016 Aug 01]. Available from: http://www.icmr.nic.in/bioethics/cc_biothics/presentations/int_chennai/CR_India.pdf.
- 11.Schedule Y. 8th Amendment of the Drugs and Cosmetics Rules. 1988. [Last accessed on 2016 Aug 01]. Available from: http://www.dbtbiosafety.nic.in/Files/CD_IBSC/Files/Cosmetic.PDF.
- 12.Indian GCP Guidelines. 2001. [Last accessed on 2016 Aug 04]. Available from: http://www.cdsco.nic.in/html/GCP1.html.
- 13.Amended Schedule Y. 2005. [Last accessed on 2016 Aug 04]. Available from: http://www.cdsco.nic.in/html/D&C_Rules_Schedule_Y.pdf.
- 14.Ethical Guidelines for Biomedical Research on Human Participants, Indian Council of Medical Research. 2006. [Last accessed on 2016 Aug 04]. Available from: http://www.icmr.nic.in/ethical_guidelines.pdf.
- 15.Thatte UM, Bavdekar SB. Clinical research in India: Great expectations? J Postgrad Med. 2008;54:318–23. doi: 10.4103/0022-3859.43517. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt A. Clinical trials in India: Pangs of globalization. Indian J Pharmacol. 2004;36:207–8. [Google Scholar]
- 17.Chatterjee P. Clinical trials in India: Ethical concerns. Bull World Health Organ. 2008;86:581–2. doi: 10.2471/BLT.08.010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthuswamy V. Status of ethical review and challenges in India. Indian Pediatr. 2005;42:1189–90. [PubMed] [Google Scholar]
- 19.Nadig P, Joshi M, Uthappa A. Competence of ethics committees in patient protection in clinical research. Indian J Med Ethics. 2011;8:151–4. doi: 10.20529/IJME.2011.061. [DOI] [PubMed] [Google Scholar]
- 20.Brahme R, Mehendale S. Profile and role of the members of ethics committees in hospitals and research organizations in Pune, India. Indian J Med Ethics. 2009;6:78–84. doi: 10.20529/IJME.2009.026. [DOI] [PubMed] [Google Scholar]
- 21.Kuyare MS, Marathe PA, Kuyare SS, Thatte UM. Perceptions and experiences of community members serving on institutional review boards: A questionnaire based study. HEC Forum. 2015;27:61–77. doi: 10.1007/s10730-014-9263-3. [DOI] [PubMed] [Google Scholar]
- 22.Nair YM, Martin DK. Concerns about ethical review of health research in India. Indian J Med Ethics. 2004;1:119–20. doi: 10.20529/IJME.2004.056. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G. Institutional ethics committees: Critical gaps. Indian J Med Ethics. 2011;8:200–1. doi: 10.20529/IJME.2011.080. [DOI] [PubMed] [Google Scholar]
- 24.Kuyare MS, Taur SR, Thatte UM. Establishing institutional ethics committees: Challenges and solutions – A review of the literature. Indian J Med Ethics. 2014;11:181–5. doi: 10.20529/IJME.2014.047. [DOI] [PubMed] [Google Scholar]
- 25.Singh S. Procedures & operations of Institutional Ethics Committees in public sector hospitals in Delhi, India. Indian J Med Res. 2009;130:568–9. [PubMed] [Google Scholar]
- 26.Bhat S, Hegde TT. The costs of institutional review boards. N Engl J Med. 2005;353:315–7. [PubMed] [Google Scholar]
- 27.Shrotri DS. Role of ethics committees in medical research. Indian J Med Ethics. 2004;1:121. doi: 10.20529/IJME.2004.057. [DOI] [PubMed] [Google Scholar]
- 28.Site Monitoring Visit SOP Code: SOP 15/V4 Dated 22nd August, 2013. [Last accessed on 2016 Aug 15]. Available from: http://www.kem.edu/wp-content/uploads/2014/04/SOP-15.pdf.
- 29.Shetty YC, Marathe P, Kamat S, Thatte U. Continuing oversight through site monitoring: Experiences of an institutional ethics committee in an Indian tertiary-care hospital. Indian J Med Ethics. 2012;9:22–6. doi: 10.20529/IJME.2012.006. [DOI] [PubMed] [Google Scholar]
- 30.Taur SR, Bavdekar SB, Thatte UM. Survey of ethics committee protocol approval letters: Compliance with Schedule Y/ICMR guidelines 2006. Indian J Med Ethics. 2011;8:214–6. doi: 10.20529/IJME.2011.083. [DOI] [PubMed] [Google Scholar]
- 31.Tharyan P. Ethics committees and clinical trials registration in India: Opportunities, obligations, challenges and solutions. Indian J Med Ethics. 2007;4:168–9. doi: 10.20529/IJME.2007.066. [DOI] [PubMed] [Google Scholar]
- 32.SIDCER Recognition Program. [Last accessed on 2016 Aug 31]. Available from: http://www.fercap.sidcer.org/recognized.php?Mem_Form=main.
- 33.Association for the Accreditation of Human Research Protection Programs. [Last accessed on 2016 Aug 31]. Available from: http://www.aahrpp.org/learn/find-an-accredited-organization.
- 34.Indian Society for Clinical Research. [Last accessed on 2016 Aug 04]. Available from: http://www.iscr.org/home/training.
- 35.Clinical Development Services Agency. [Last accessed on 2016 Aug 30]. Available from: http://www.cdsaindia.in/training-clinical-research.
- 36.Forum for Ethics Review Committees in India. [Last accessed on 2016 Aug 30]. Available from: http://www.ferci.org/
- 37.Thatte U, Ahuja V, Sharma V, Deshpande S. Conference Proceedings of “Accreditation of Ethics Committees, Clinical Investigators and Clinical Trial Sites” Held at Sri Ramachandra University, Chennai; 28th-29th October; 2015. p. 84. [Google Scholar]
- 38.Pandiya A. Quality of independent review board/ethics committee oversight in clinical trials in India. Perspect Clin Res. 2011;2:45–7. doi: 10.4103/2229-3485.80364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanmukhani J, Tripathi CB. Ethics in clinical research: The Indian perspective. Indian J Pharm Sci. 2011;73:125–30. doi: 10.4103/0250-474x.91564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadam R, Karandikar S. Ethics committees in India: Facing the challenges! Perspect Clin Res. 2012;3:50–6. doi: 10.4103/2229-3485.96444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SAE Reporting and Compensation. GSR 53E, January 30. 2013. [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/GSR%2053(E)%20dated%2030.01.2013.pdf.
- 42.Registration of Ethics Committees. GSR72E, February 8. 2013. [Last accessed on 2016 Aug 14]. Available from: http://www.cdsco.nic.in/writereaddata/G.S.R%2072(E)%20dated%2008.02.2013.pdf.
- 43.Compensation Formula (Clinical Trial) 2013. [Last accessed on 2016 Aug 14]. Available from: http://www.cdsco.nic.in/writereaddata/formula2013SAE.pdf.
- 44.Office Order, Audio-visual Recording of Informed Consent of Process, November 19. 2013. [Last accessed on 2016 Aug 14]. Available from: http://www.cdsco.nic.in/writereaddata/Office%20Order%20dated%2019.11.2013.pdf.
- 45.GSR 889(E) [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/Notificatiohn%20on%20Compensation%20on%20clincial%20trial%20 (1).pdf.
- 46.Non Death SAEs. [Last accessed Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/ORDER%20and%20Formula%20to%20Determine%20the%20quantum%20of%20compensation%20in%20the%20cases%20of%20Clinical%20Trial%20related%20serious%20Adverse%20Events(SAEs)%20of%20Injury%20other%20than%20Death.pdf.
- 47.AV Consent Requirements GSR 611 (E) [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/Gazette%20Notification%2031%20July%202015.pdf.
- 48.GSR 313 (E) Academic studies 16th March. 2016. [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/GSR%20313%20(E)%20dated%2016_03_2016.pdf.
- 49.Addition of New Site. [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/noc.pdf.
- 50.Number of Trials by PI. [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/restricion%20of%20conducting%20three.pdf.
- 51.Re-Registration of Ethics Committee Checklist. [Last accessed on 2016 Aug 14]. Available from: http://www.cdsco.nic.in/writereaddata/23_5_2016ecchecklist.pdf.
- 52.Ethics Committee Registration, Central Drugs Standard Control Organization, Government of India. [Last accessed on 2016 Aug 31]. Available from: http://www.cdsco.nic.in/forms/list.aspx?lid=1859&Id=1.
- 53.Bhide S, Katkar J, Maurya M, Gogtay N, Thatte U. An audit of the approval letters issued by Drugs Controller General of India to Ethics Committees in India. Accepted for publication. Perspect Clin Res. 2016;7:165–7. doi: 10.4103/2229-3485.192037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhide SS, Jalgaonkar SV, Katkar JV, Shetty YC, Tripathi RK, Marathe PA, Thatte UM. Impact of recent regulatory notifications on the institutional ethics committee of a tertiary-care teaching hospital in Mumbai. Indian J Med Ethics. 2016;1((4)NS):210–14. doi: 10.20529/IJME.2016.061. [DOI] [PubMed] [Google Scholar]
- 55.Circular Regarding Online Registration of Ethics Committees. [Last accessed on 2016 Aug 28]. Available from: http://www.cdsco.nic.in/writereaddata/NOTICE-%20COSECR.pdf.
- 56.Tripathi RK, Marathe PA, Kapse SV, Shetty YC, Kamat SK, Thatte UM. Serious Adverse Events Reports: Analysis and Outcome of Review by an Institutional Ethics Committee of a Tertiary Care Hospital in Mumbai, India. J Empir Res Hum Res Ethics. 2016;11:267–73. doi: 10.1177/1556264616654809. [DOI] [PubMed] [Google Scholar]
- 57.Kulkarni R, Saraiya U. Accreditation of ethics committees in India: Experience of an ethics committee. [Last accessed on 2016 Aug 30];Indian J Med Ethics. 2015 12:241–5. doi: 10.20529/IJME.2015.064. Available from: http://www.issuesinmedicalethics.org/index.php/ijme/article/view/2279/4838. [DOI] [PubMed] [Google Scholar]
- 58.Draft Accreditation Standards for Clinical Trial Sites, Ethics Committee and Investigators. [Last accessed on 2016 Aug 09]. Available from: http://www.cdsco.nic.in/writereaddata/finalAccreditation%20Standards.pdf and http://www.nabh.co/Notice_draft_accreditation_standards.aspx.
- 59.Ghooi RB. Accreditation – A solution for problems or a fresh problem? Perspect Clin Res. 2015;6:123–4. doi: 10.4103/2229-3485.159932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jalgaonkar SV, Bhide SS, Tripathi RK, Shetty YC, Marathe PA, Katkar J, et al. An audit of protocol deviations submitted to an Institutional Ethics Committee of a tertiary care hospital. PLoS One. 2016;11:e0146334. doi: 10.1371/journal.pone.0146334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menikoff J. The paradoxical problem with multiple-IRB review. N Engl J Med. 2010;363:1591–3. doi: 10.1056/NEJMp1005101. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald MH, Phillips PA. Centralized and non-centralized ethics review: A five nation study. Account Res. 2006;13:47–74. doi: 10.1080/08989620600588944. [DOI] [PubMed] [Google Scholar]
- 63.US Food and Drug Administration. Guidance for Industry: Using a Centralized IRB Review Process in Multicenter Clinical Trials. 2006. [Last accessed on 2016 Aug 31]. Available from: http://www.fda.gov/cber/gdlns/irbclintrial.pdf.
- 64.Department of Health and Human Services. Human subjects research protections: Enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. Fed Regist. 2011;76:44512–31. [Google Scholar]
- 65.Walanj AS. Research ethics committees: Need for harmonization at the national level, the global and Indian perspective. Perspect Clin Res. 2014;5:66–70. doi: 10.4103/2229-3485.128022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grady C. Do IRBs protect human research participants? JAMA. 2010;304:1122–3. doi: 10.1001/jama.2010.1304. [DOI] [PubMed] [Google Scholar]
- 67.Keith-Spiegel P, Koocher GP. Institutional review board researchers assessment tool. Boston Children's Hospital and Harvard Medical School. 2005. [Last accessed on 2016 Sept 02]. Available from: http://www.ethicsresearch.com/images/IRB_RAT_User_s_Guide.revised_3-11-05.pdf.
- 68.Chenneville T, Menezes L, Bylsma LM, Mann A, Kosambiya J, Baxi R. Assessing Institutional Ethics Committees in India Using the IRB-RAT. J Empir Res Hum Res Ethics. 2014;9:50–9. doi: 10.1177/1556264614544101. [DOI] [PMC free article] [PubMed] [Google Scholar]


