Abstract
Sinus of Valsalva aneurysm accounts for only 1% of congenital cardiac anomalies. Sinus of Valsalva aneurysm can cause aortic insufficiency, coronary artery flow compromise, cardiac arrhythmia, or aneurysm rupture. Three-dimensional transesophageal echocardiography (3DTEE) represents an adjunctive tool to demonstrate the ruptured sinus of Valsalva with better delineation. We present an adult patient with rupture of noncoronary sinus of Valsalva aneurysm into the right atrium (RA). 3DTEE accurately delineated the site of rupture into the RA and showed the exact size and shape of the defect, which helped in the successful transcatheter closure of the defect with a duct occluder device.
Keywords: Non coronary sinus, ruptured sinus of Valsalva, three-dimensional transesophageal echocardiography
Introduction
Aneurysms of the sinus of Valsalva account for <1% of congenital cardiac anomalies. The rupture of sinus of Valsalva is an uncommon complication of infective endocarditis of the aortic valve. The right coronary sinus is the commonest site of aneurysm formation.[1] Ninety-five percent of these congenital aneurysms originate in the right or noncoronary sinus and project into the right ventricle (RV) or into the right atrium (RA). Aneurysm arising in the noncoronary sinus almost all rupture into the RA and those arising in the right coronary sinus generally communicate with the RV.[2]
Case Report
A 44-year-old male presented with a 3-month history of atypical chest pain, palpitation, and exertional dyspnea. Cardiac auscultation revealed a Grade III/IV to-and-fro murmur. Electrocardiography showed normal sinus rhythm.
Transthoracic echocardiography (TTE) revealed an aneurysm of the noncoronary cusp of sinus of Valsalva rupturing into the RA. Transesophageal echocardiography (TEE) and three-dimensional (3D) images were taken before the procedure. In the midesophageal right ventricle (RV) inflow view, they clearly showed ruptured sinus of Valsalva [RSOV] into RA.
The procedure was performed under general anesthesia with TEE guidance. Seldinger technique was used to achieve percutaneous access to the right femoral artery (RFA) and vein so that two hemostatic sheaths were inserted. Intravenous heparin (200 IU/kg) and cefazolin were given.
From RFA approach, right and left heart pressures and saturations were obtained, and aortic root angiography was performed. The RSOV was measured at its aortic end as well as at the rupture site on TEE. RSOV was tried crossing with terumo wire under real time-TEE guidance. However, we were not able to pass through RSOV. Hence, we took use of 3D imaging available in our TEE machine and tried passing terumo wire across RSOV and position in SVC under the guidance of midesophageal aortic valve long axis at 120° and other real time-3D video images and Aortography, and we succeeded in passing the guide wire across RSOV. It was then snared from venous end, and an arteriovenous loop was established. The defect was measured 5.5 mm at its aortic end as measured by TEE and 8 mm when measured in 3D images. According to the measurement in the 3DTEE and aortogram, a patent ductus arteriosus (PDA) occluder 2–4 mm larger than the narrowest diameter of the ruptured site was chosen to close most of the lesions. An 8F PDA delivery sheath then placed in the descending aorta and 10 mm × 8 mm RSOV PDA occluder device (life tech) was placed in the sac of RSOV noncoronary cusp after checking for aortic regurgitation (AR). The entire assembly was pulled back until the disk blocked the aortic end of the ruptured site without slipping into the aneurysm.
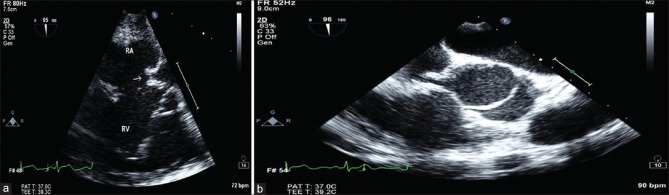
TEE images and aortogram after releasing of the device showed minimal residual shunt across RSOV and no AR. Subsequent echocardiography (echo) next day showed no residual flow [Figure 1 and Video 1a–e].
Figure 1.
(a) Midesophageal aortic valve short axis view showing ruptured noncoronary sinus of Valsalva into the right atrium. (b) Mid esophageal aortic valve short axis at 95° clearly showing RSOV with difficulty in passing the guide wire across RSOV in 2D TEE
Discussion
Causes of sinus of Valsalva aneurysm are classified into congenital or acquired. Congenital type occurs where a lack of fusion exists between the aortic media and the annulus fibrosis of the aortic valve.[3] Acquired sinus of Valsalva aneurysms can exist in patients with endocarditis,[4] syphilis,[1] injury, Behcet's disease, and Marfan's syndrome. Deficiency of the aortic media at the attachments on the aortic annulus portion may progressively lead to dilation of aortic sinus, usually over many years. Therefore, sinus of Valsalva aneurysm can remain subclinical for several years until it eventually ruptures between the third and fourth decades of life.
The most common sites of aneurysm ruptures are cardiac chambers, interventricular septum,[5] or pericardial space. Dilated sinus of Valsalva aneurysm also can lead to distortion of aortic valve and AR. Un-RSOV aneurysms usually do not cause any symptom and are often found during echocardiogram examination and cardiac catheterization. However, un-RSOV aneurysm may cause right ventricular outflow tract obstruction[6] and myocardial ischemia due to the mass compression effect of aneurysms. The RSOV aneurysms most often involve with the right coronary sinus (76.8%–83.3%) or with other noncoronary sinus, while an aneurysm of the left sinus is rare.[2] Most right sinus of Valsalva aneurysms rupture into the RV (86.7%),[2] or into the RA. Patients may remain asymptomatic for several years even if the sinus of Valsalva aneurysms has ruptured. However, dyspnea and exercise intolerance may develop because of the increased shunting and volume overloading.
The traditional treatment involves surgical repair with a patch closure at both ends under cardiopulmonary bypass. Percutaneous device closure of RSOV is an alternative with the merits of smaller trauma since Cullen reported the first case of transcatheter closure of RSOV.[7]
TTE and TEE have played an important role in the diagnostic confirmation of Valsalva aneurysm sinus.[8] Doppler findings may identify the exact shunt location. The “windsock” dilatation of sinus of Valsalva aneurysm is the feature of sinus of Valsalva aneurysm. Doppler features include a continuous high-velocity flow from the aorta to the heart chamber. The diagnosis usually is confirmed by color Doppler flow mapping. The color Doppler flow mapping may reveal a continuous mosaic flow jet from an aneurysm to the heart chamber. Continuous rotation of a multiplane TEE may be particularly helpful to identify the exact point of rupture.
However, there are certain limitations of two-dimensional (2D) TEE which include its limited ability to detect catheter position or a device to its relative surroundings due to only two spatial dimensions; it necessitates several planes to reconstruct the anatomical settings. Although several 3D reconstruction methods are done offline, so they will not be available during the intervention and rendered useless.
Visualization of the defect in 3D from different angles and flow acceleration across the defect demonstrated by volumetric color flow helps in accurate localization of the anatomic defect.[9]
3D echo helps in optimal deployment of the device and provides superior guidance, as compared to 2D echo. There are reports of successful percutaneous closure of RSOV aneurysms, utilizing real-time 3D echo guidance.[10]
The advantages of 3D TEE over 2D TEE which includes it provides a novel imaging modality for the guidance of RSOV closures, giving fast, and complete information about the appropriate position of the device in its surrounding environment, the size and shape of the defect including the rim of the defects are displayed on-line, allowing the assessment of the complete circumference. After unfolding the device, exact positioning and spatial orientation of the device as well as distance and relation of disc size to defect size can easily be assessed with 3D TEE, and it prevents mismatch of the device with the defect to avoid residual leak, device embolization.
There are other uses of 3D TEE in percutaneous procedures which include its use in device closure of interatrial communications, mitral valve interventions, aortic valve interventions, and electrophysiological procedures.
Conclusion
3D TEE represents an important adjunctive tool to demonstrate the RSOV with better delineation of its characteristics such as the site of rupture into the cardiac chambers, the size, shape of the defect, associated defects such as ventricular septal defect; however, it has lesser frame rate compared with 2DTEE. We recommend more usage of 3DTEE in catheterization laboratory for the guidance of RSOV device closure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.annals.in
References
- 1.Küçükoglu S, Ural E, Mutlu H, Ural D, Sönmez B, Uner S. Ruptured aneurysm of the sinus of valsalva into the left ventricle: A case report and review of the literature. J Am Soc Echocardiogr. 1997;10:862–5. doi: 10.1016/s0894-7317(97)70046-4. [DOI] [PubMed] [Google Scholar]
- 2.Shah RP, Ding ZP, Ng AS, Quek SS. A ten-year review of ruptured sinus of valsalva: Clinico-pathological and echo-Doppler features. Singapore Med J. 2001;42:473–6. [PubMed] [Google Scholar]
- 3.Henze A, Huttunen H, Björk VO. Ruptured sinus of valsalva aneurysms. Scand J Thorac Cardiovasc Surg. 1983;17:249–53. doi: 10.3109/14017438309099360. [DOI] [PubMed] [Google Scholar]
- 4.Shumacker HB., Jr Aneurysms of the aortic sinuses of valsalva due to bacterial endocarditis, with special reference to their operative management. J Thorac Cardiovasc Surg. 1972;63:896–902. [PubMed] [Google Scholar]
- 5.Choudhary SK, Bhan A, Reddy SC, Sharma R, Murari V, Airan B, et al. Aneurysm of sinus of valsalva dissecting into interventricular septum. Ann Thorac Surg. 1998;65:735–40. doi: 10.1016/s0003-4975(97)01432-x. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm I. Unruptured aneurysm of the sinus of valsalva. Can J Cardiol. 1996;12:783–5. [PubMed] [Google Scholar]
- 7.Cullen S, Somerville J, Redington A. Transcatheter closure of a ruptured aneurysm of the sinus of valsalva. Br Heart J. 1994;71:479–80. doi: 10.1136/hrt.71.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang KY, St. John Sutton M, Ho HY, Ting CT. Congenital sinus of valsalva aneurysm: A multiplane transesophageal echocardiographic experience. J Am Soc Echocardiogr. 1997;10:956–63. doi: 10.1016/s0894-7317(97)80012-0. [DOI] [PubMed] [Google Scholar]
- 9.Hansalia S, Manda J, Pothineni KR, Nanda NC. Usefulness of live/real time three-dimensional transthoracic echocardiography in diagnosing acquired left ventricular-right atrial communication misdiagnosed as severe pulmonary hypertension by two-dimensional transthoracic echocardiography. Echocardiography. 2009;26:224–7. doi: 10.1111/j.1540-8175.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 10.Oh-Icí D, Malergue MC, Garot J, Piéchaud JF. Sinus of valsalva rupture percutaneous closure with real-time 3-dimensional echocardiography. J Am Coll Cardiol. 2010;56:e31. doi: 10.1016/j.jacc.2010.01.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.