Abstract
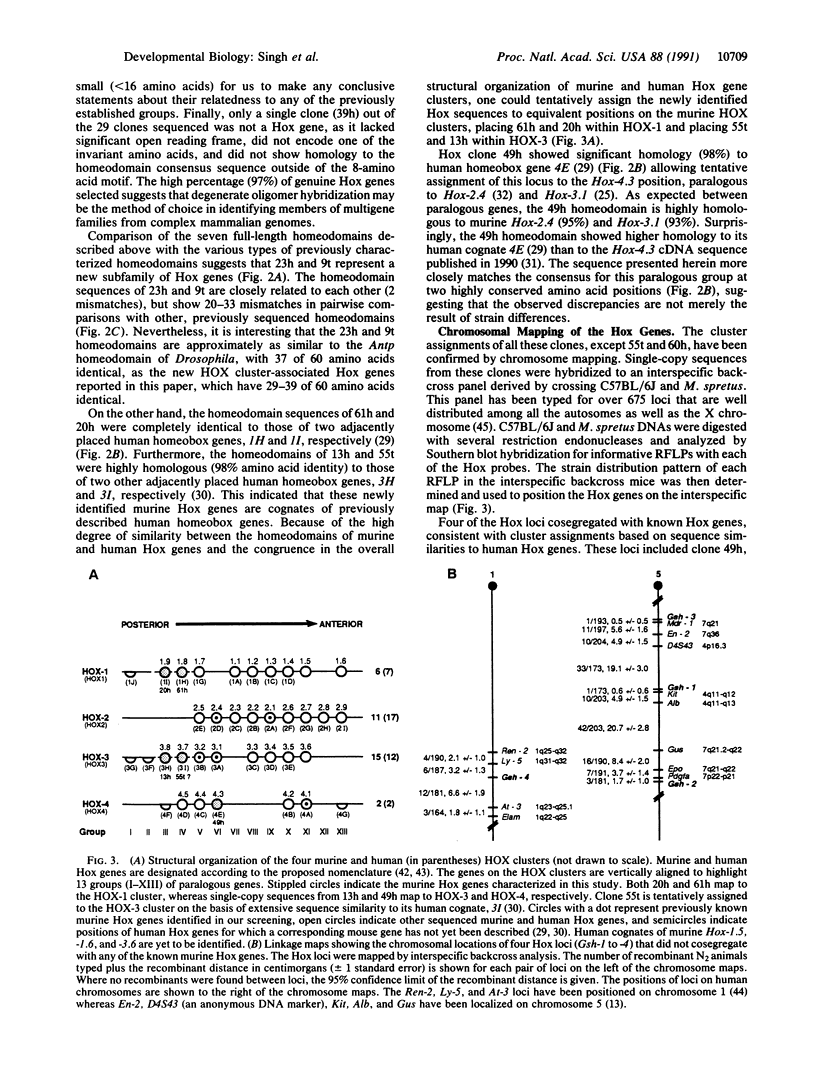
In Drosophila a number of genes important in establishing segmentation patterns and in determining segment identities have been shown to carry the homeobox sequence. Over 30 murine homeobox genes have been cloned, many on the basis of sequence homology to Drosophila prototypes. Here we report the cloning and sequencing of 10 new and 6 previously known homeobox genes by screening a murine genomic library with a 768-fold degenerate oligonucleotide corresponding to the most conserved 8-amino acid motif in the recognition helix of the homeodomain. Eight of these new homeobox genes have been chromosomally mapped. Four genes do not belong to any of the known homeobox gene clusters but instead map to new locations on chromosome 1 (single gene) and chromosome 5 (three genes). Sequence comparisons indicate that two of these are very closely related and represent a distinct new category of homeobox genes. The remaining four mapped genes reside in previously established murine homeobox gene clusters. Specifically, two map to the cluster HOX-1 on chromosome 6 and one each to HOX-3 and HOX-4 on chromosome 15 and 2, respectively. The ratio of newly identified homeobox genes to the previously characterized murine homeobox genes suggests that there remain several uncharacterized homeobox genes in the murine genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian H., Gruss P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 1990 Jun;9(6):1839–1852. doi: 10.1002/j.1460-2075.1990.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Breier G., Dressler G. R., Gruss P. Primary structure and developmental expression pattern of Hox 3.1, a member of the murine Hox 3 homeobox gene cluster. EMBO J. 1988 May;7(5):1329–1336. doi: 10.1002/j.1460-2075.1988.tb02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürglin T. R., Finney M., Coulson A., Ruvkun G. Caenorhabditis elegans has scores of homoeobox-containing genes. Nature. 1989 Sep 21;341(6239):239–243. doi: 10.1038/341239a0. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Keelan D. J., Lewis E. B. The molecular genetics of the bithorax complex of Drosophila: characterization of the products of the Abdominal-B domain. Genes Dev. 1989 Sep;3(9):1424–1436. doi: 10.1101/gad.3.9.1424. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Brönner G., Küttner F., Jürgens G., Jäckle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989 Mar 30;338(6214):432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Deng T. L., Li D. W., Jenh C. H., Johnson L. F. Structure of the gene for mouse thymidylate synthase. Locations of introns and multiple transcriptional start sites. J Biol Chem. 1986 Dec 5;261(34):16000–16005. [PubMed] [Google Scholar]
- Dickinson M. E., Kobrin M. S., Silan C. M., Kingsley D. M., Justice M. J., Miller D. A., Ceci J. D., Lock L. F., Lee A., Buchberg A. M. Chromosomal localization of seven members of the murine TGF-beta superfamily suggests close linkage to several morphogenetic mutant loci. Genomics. 1990 Mar;6(3):505–520. doi: 10.1016/0888-7543(90)90480-i. [DOI] [PubMed] [Google Scholar]
- Duboule D., Boncinelli E., DeRobertis E., Featherstone M., Lonai P., Oliver G., Ruddle F. H. An update of mouse and human HOX gene nomenclature. Genomics. 1990 Jul;7(3):458–459. doi: 10.1016/0888-7543(90)90185-w. [DOI] [PubMed] [Google Scholar]
- Duprey P., Chowdhury K., Dressler G. R., Balling R., Simon D., Guenet J. L., Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988 Dec;2(12A):1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hart C. P., Fainsod A., Ruddle F. H. Sequence analysis of the murine Hox-2.2, -2.3, and -2.4 homeo boxes: evolutionary and structural comparisons. Genomics. 1987 Oct;1(2):182–195. doi: 10.1016/0888-7543(87)90011-5. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Jones P. F., Rees A. R., Sime C. M., Justice M. J., Copeland N. G., Jenkins N. A., Graham E., Davidson D. R. A new family of mouse homeo box-containing genes: molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev. 1989 Jan;3(1):26–37. doi: 10.1101/gad.3.1.26. [DOI] [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Izpisùa-Belmonte J. C., Dollé P., Renucci A., Zappavigna V., Falkenstein H., Duboule D. Primary structure and embryonic expression pattern of the mouse Hox-4.3 homeobox gene. Development. 1990 Nov;110(3):733–745. [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Kappen C., Schughart K., Ruddle F. H. Two steps in the evolution of Antennapedia-class vertebrate homeobox genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5459–5463. doi: 10.1073/pnas.86.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Kim Y., Nirenberg M. Drosophila NK-homeobox genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7716–7720. doi: 10.1073/pnas.86.20.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine P. J., Shows T. B. Nomenclature for human homeobox genes. Genomics. 1990 Jul;7(3):460–460. doi: 10.1016/0888-7543(90)90186-x. [DOI] [PubMed] [Google Scholar]
- McDonald J. D., Lin F. K., Goldwasser E. Cloning, sequencing, and evolutionary analysis of the mouse erythropoietin gene. Mol Cell Biol. 1986 Mar;6(3):842–848. doi: 10.1128/mcb.6.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Hart C. P., Gehring W. J., Ruddle F. H. Molecular cloning and chromosome mapping of a mouse DNA sequence homologous to homeotic genes of Drosophila. Cell. 1984 Oct;38(3):675–680. doi: 10.1016/0092-8674(84)90262-9. [DOI] [PubMed] [Google Scholar]
- McGinnis W. Homeo box sequences of the Antennapedia class are conserved only in higher animal genomes. Cold Spring Harb Symp Quant Biol. 1985;50:263–270. doi: 10.1101/sqb.1985.050.01.033. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Fjose A., Gehring W. J. Molecular structure and spatial expression of a homeobox gene from the labial region of the Antennapedia-complex. EMBO J. 1988 Aug;7(8):2569–2578. doi: 10.1002/j.1460-2075.1988.tb03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting G., Qian Y. Q., Billeter M., Müller M., Affolter M., Gehring W. J., Wüthrich K. Protein--DNA contacts in the structure of a homeodomain--DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J. 1990 Oct;9(10):3085–3092. doi: 10.1002/j.1460-2075.1990.tb07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M. R., Toth L. E., Patel M. D., D'Eustachio P., Nguyen-Huu M. C. A mouse homeo box gene is expressed in spermatocytes and embryos. Science. 1986 Aug 8;233(4764):663–667. doi: 10.1126/science.3726554. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Bimodal and graded expression of the Xenopus homeobox gene Xhox3 during embryonic development. Development. 1989 May;106(1):173–183. doi: 10.1242/dev.106.1.173. [DOI] [PubMed] [Google Scholar]
- Schughart K., Kappen C., Ruddle F. H. Duplication of large genomic regions during the evolution of vertebrate homeobox genes. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7067–7071. doi: 10.1073/pnas.86.18.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornaiuolo A., Acampora D., Pannese M., D'Esposito M., Morelli F., Migliaccio E., Rambaldi M., Faiella A., Nigro V., Simeone A. Human HOX genes are differentially activated by retinoic acid in embryonal carcinoma cells according to their position within the four loci. Cell Differ Dev. 1990 Aug;31(2):119–127. doi: 10.1016/0922-3371(90)90015-o. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E., Veit B., Sinha N., Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991 Mar 21;350(6315):241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- Wedeen C. J., Kostriken R. G., Matsumura I., Weisblat D. A. Evidence for a new family of evolutionarily conserved homeobox genes. Nucleic Acids Res. 1990 Apr 11;18(7):1908–1908. doi: 10.1093/nar/18.7.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow G. M., Hayashi S., Krasnow M., Hogness D. S., Scott M. P. Transcriptional activation by the Antennapedia and fushi tarazu proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1017–1030. doi: 10.1016/0092-8674(89)90340-1. [DOI] [PubMed] [Google Scholar]
- Woo S. L. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. doi: 10.1016/0076-6879(79)68028-x. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]