Abstract
Purpose
The aims of the present study were (i) to investigate the impact of great age on pharmacokinetics of capecitabine and its metabolites and (ii) to evaluate the exposure/effect relationship of capecitabine in elderly patients.
Methods
Data collected from 20 elderly patients (75–92 years old) with breast or colorectal cancer, who received oral capecitabine were analyzed. In order to study the old age effect on pharmacokinetics, data collected from two phase I studies involving 40 younger adults (<75 years old) with metastatic cancer who received oral capecitabine, were added in the database. The population pharmacokinetic analysis was based on a four compartment model describing the sequence of capecitabine and three of its metabolites.
Results
The absorption rate constant was found lower in the oldest patient group (≥75 y) compared to the youngest group, and the constant rate elimination of the 5-fluorouracil metabolite was found decreased over time (i.e. after 2 consecutive weeks of capecitabine administration). This time effect was not found different between the two age groups. In elderly patients, the exposure-safety analysis showed, from the second cycle of chemotherapy, significantly higher median exposures of capecitabine and its metabolites (5′-deoxy-5-fluorocytidine,5′-deoxy-5-fluorouridine and 5-fluorouracil) in patients who experienced hand-foot syndrome compared to patients who did not.
Conclusion
This study puts forward new arguments for the treatment of elderly cancer patients who could benefit from capecitabine chemotherapy with acceptable toxicity.
Keywords: Age Factors; Aged; Humans; Male; Aged, 80 and over; Antimetabolites, Antineoplastic; Antineoplastic Combined Chemotherapy Protocols; Breast Neoplasms; Colorectal Neoplasms; Deoxycytidine; Female; Fluorouracil
INTRODUCTION
Cancer is a major cause of death in developed countries, particularly in the elderly population. Most cancers occur after the age of 65. Colorectal and breast cancers are the most common cancers in the elderly population, in addition to prostate and lung cancers [1]. The risk of colorectal cancer increases with age and the incidence is higher in the seventh and eighth decades of life [2]. Breast cancer is the leading cause of cancer mortality in women worldwide, and nearly a third of breast cancer cases occurs in patients aged over 65 years old [3].
Despite the increasing risk of cancer in the elderly population, this age group is underrepresented in clinical trials [4,5]. Data on dose-concentration and dose-response relationships are therefore scant in such patients for whom the optimal treatment strategy is poorly defined so far. However, advancement of age is associated with significant physiological and morphological changes which may alter the different stages of the journey of a drug through the body: absorption, distribution, metabolism and elimination [6,7]. Decline of renal function is common in the elderly [6,7], thus a significant change in the pharmacokinetics (PK) of drugs in this population is the reduction in renal elimination. Capecitabine, an oral prodrug of the cytototoxic agent 5-fluorouracil (5-FU), has demonstrated considerable single-agent activity in metastatic breast or colorectal cancers [8]. After oral administration, capecitabine is rapidly converted into 5′-deoxy-5-fluorocytidine (5′-DFCR) mainly in liver via hepatic carboxyl esterase. 5′-DFCR is then metabolized to 5′-deoxy-5-fluorouridine (5′-DFUR) via cytidine deaminase, which is principally located in the liver and tumour tissues. Finally, 5′-DFUR is converted to the active cytotoxic agent 5-FU mainly via thymidine phosphorylase which is present at higher concentrations in tumour tissues [9]. 5-FU is further metabolized to an active phosphate analogue or is catabolized to alpha-fluoro-beta-alanine (FBAL) [10]. Capecitabine and its metabolites are mainly excreted in urine [11]; more than 70% of the administered dose is recovered in urine, of which 50 % as FBAL.
The PK of capecitabine and its metabolites have been mainly described with non-compartmental methods [12,13]. Population PK (popPK) models were developed to analyze the two sequences: 5′-DFUR>5-FU>FBAL [14] and capecitabine>5′-DFCR>5′-DFUR>5-FU [15].
In the elderly patients, some studies focused on efficacy/safety responses or cognitive changes related to capecitabine [16–18] but few studies have investigated the PK of capecitabine. In most of these PK studies, the proportion of elderly patients (> 70 years) was very low (<10%) or null [10,15,19]. Louie et al [13] analyzed, with a non-compartmental method, the impact of age on capecitabine and its metabolites disposition using a greater proportion of elderly patients, but the very small number of patients in the younger group (5 patients <60 years vs 24 ≥70 years) was a limitation of the study. Therefore, new studies are needed to investigate the influence of great age on PK and systemic exposure of capecitabine and its metabolites.
The present study aims (i) to report the results of the clinical trial CAPAGEC (NCT00812864) involving elderly patients with breast or colorectal cancer who received oral capecitabine, and (ii) to investigate the impact of age on PK of capecitabine and its metabolites. A secondary objective was to evaluate the response (tolerability and efficacy) of capecitabine in elderly patients, in particular with regard to the exposure/effect relationship.
MATERIAL AND METHODS
Patients and treatment
The mono-center CAPAGEC trial recruited 20 patients aged 75 years or more with breast or colorectal cancer in the University Hospital of Limoges (France). The study complied with legal requirements and the Declaration of Helsinki, and was approved by the regional Ethics Committee. Each patient had provided informed consent to participate in the study. Patients received 1250 mg/m2 of oral capecitabine twice daily for 14 consecutive days as anticancer monotherapy at each cycle. Cycles were repeated every 3 weeks (14 days treatment, 7 days break) for a total of six cycles. Pharmacokinetic evaluations were performed on day 1 of cycle 1 and day 14 of cycle 2. Blood samples were collected at pre-dose time and 0.5, 1, 1.5, 2, 4, 6 and 8h after drug intake. Capecitabine, 5′-DFUR, 5-FU and FBAL concentrations were measured with two validated, specific, selective reverse-phase high performance liquid chromatography–tandem mass spectrometry methods in positive (capecitabine and 5′-DFUR) and negativeion modes (5-FU and FBAL), following two ionic transitions per compound. The calibration curves were linear from 77 nmol/L up to 7688 nmol/L (5-FU), 41 nmol/L to 20309 nmol/L (5′-DFUR), 6 nmol/L to 27828 nmol/L (capecitabine) and 19 nmol/L to 93385 nmol/L (FBAL). The within-day and between-day coefficients of variation and bias were less than 15% over these ranges.
In order to study the old age effect on pharmacokinetics, data collected from two phase I studies including 40 younger adults (<75 years old) were added to the CAPAGEC database. The details of these two phase I studies were previously described elsewhere [15]. Briefly, patients had been diagnosed with metastatic cancer and were receiving second or third line chemotherapy. Capecitabine was orally administered every 12 hours at a dose of 1400, 1700, 2000 or 2300 mg/m2/day and was combined to either irinotecan or to irofulven. For most patients, two pharmacokinetic evaluations took place on days 1 and 15.
Population Pharmacokinetic analysis
Concentration-time data of capecitabine and its metabolites were analyzed via a population approach using NONMEM® (version 7.2.0, ICON Development Solutions, Hanover, MD, USA) [20] executed using Wings for NONMEM version 703 (developed by N. Holford, Auckland, New Zealand, available from http://wfn.sourceforge.net).
As a basis for this work, we used the PK model developed by Urien et al [15]. This model included four compartments, the first one for capecitabine, the three following compartments describing the sequence of metabolites 5′-DFCR, 5′-DFUR, and 5-FU. The possibility to add a fifth compartment for the final metabolite FBAL was tested using the PK data collected in the CAPAGEC trial only (as FBAL concentration data were not available in the other patients) (Figure 1). Concentration data of the first metabolite, 5′-DFCR was available only for the patients enrolled in the two phase I studies and not for the patients included in the CAPAGEC trial. The first-order estimation method was used. Improvement of the model by inclusion of inter-subject and inter-occasion variabilities (ISV and IOV, respectively) described using an exponential error model was tested for all PK parameters.
Figure 1.
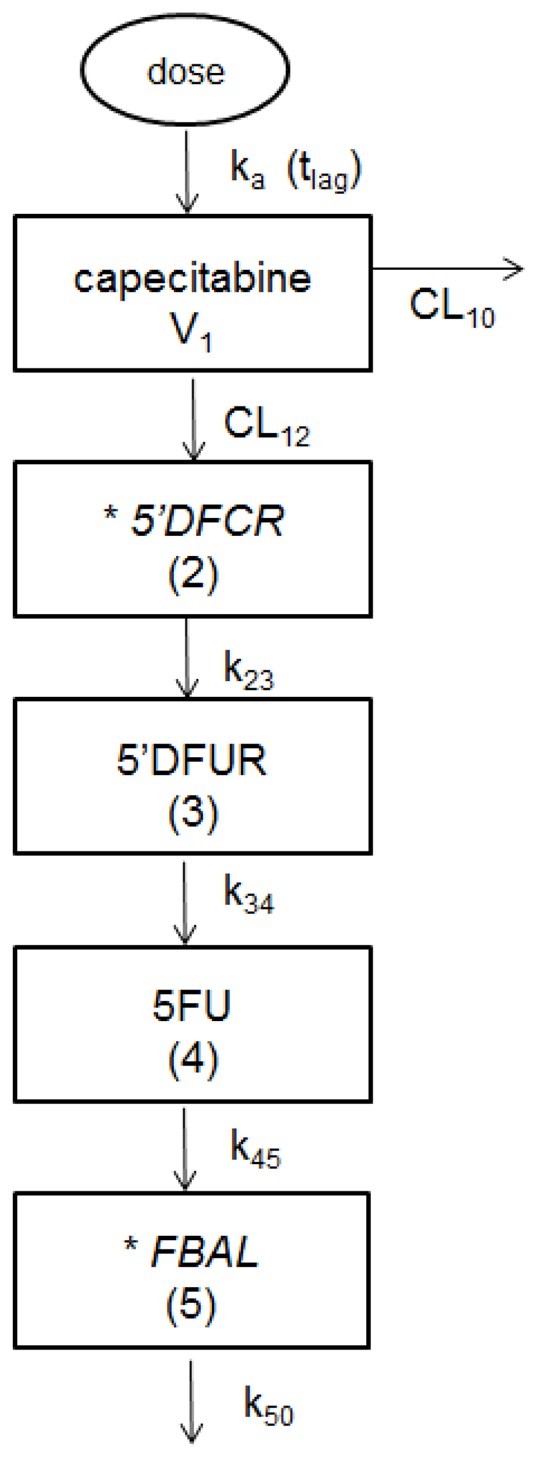
Compartmental model describing the pharmacokinetics of capecitabine and its four metabolites.
Abbreviations: ka: absorption rate constant; tlag: lag-time; V1: apparent distribution volume of capecitabine; CL10 and CL12: apparent capecitabine clearances; k23: intercompartmental rate constant of 5′-DFCR; k34: intercompartmental rate constant of 5′-DFUR; k45: intercompartmental rate constant of 5-FU; k50: elimination rate constant of FBAL.
*These two metabolites were measured only in one subgroup of patients; 5′-DFCR was measured only in patients of the two phase I study (<75 years) and FBAL was measured only in patients of the CAPAGEC trial (≥75 years).
In a second step, the influence on PK parameters (apparent clearance -CL- and k terms) of age (coded either as continuous or categorical - <75 years group versus≥75 years group - covariate), gender, body weight, body surface area, clearance of creatinine calculated according to the Cockcroft and Gault formula [21], total bilirubin were examined. Additionally, to investigate the modification of pharmacokinetic parameters over time, the parameters were allowed to vary between the two pharmacokinetic evaluations and this effect could be different between the two age groups because (i) the day of the second PK evaluation was different for these two groups and (ii) the chemotherapy protocols were different. Continuous covariates were investigated as shown in equation 1. Categorical covariates (gender, age group and day of PK evaluation were tested using equation 2:
| Eq.1 |
| Eq.2 |
where P is the mean PK parameter (also called typical value) of interest, θ is the parameter estimate and Cov is the value of the covariate tested.
The model was built stepwise [22]. A specific assumption was tested at each step. The relevant covariates were selected by taking into account the statistical significance, scientific plausibility and clinical relevance.
The covariates were first tested in univariate analysis using forward inclusion to build-up the full covariate model. The final model was then developed by backward exclusion of covariates that were not significant. Differences in objective function values (ΔOFV) were used for structural model selection and testing of covariates. The statistical significance was set to p<0.01 for the forward inclusion and p<0.001 for the backward exclusion.
Internal evaluation of the population PK model
The bootstrap resampling method [23] using 1000 samples was used for internal evaluation of the final model. Median and non-parametric 95% confidence interval based on the 2.5th–97.5thpercentiles were calculated on the bootstrap samples and compared to the final model parameters. The bootstrap procedure was performed using Wings for NONMEM.
The final model was used to study the relationship between capecitabine AUC and dosage.
Exposure-effect relationships
The individual area under the concentration-time curve (AUC) values were obtained from the individual pharmacokinetic parameters provided by the POSTHOC option using the final population PK model. In case of the model failing to describe the PK of some metabolite(s), the observed trapezoidal AUC was taken into account.
Toxicity and response data were available for the elderly patients only (i.e. patients included in the CAPAGEC trial).
Toxicity was evaluated after each cycle of chemotherapy according to the National Cancer Institute common toxicity criteria. The dependent toxicity variables were defined as binary (yes/no) variables and were identified in the analyses as grade 2–3 of hand–foot syndrome (HFS) and grade ≥ 2 of diarrhea. The AUCs of capecitabine and its metabolites at the first cycle of treatment were used to analyze the association with events which occurred between the two first cycles, and the exposures of the second cycle of treatment were used to analyze the association with events which occurred after the second cycle. Efficacy was measured at cycle 3 and cycle 6 using RECIST (Response Evaluation Criteria in Solid Tumors). These criteria are used in oncology studies to evaluate tumor burden and define when cancer patients improve (“respond”), stay the same (“stabilize”) or worsen (“progress”) in response to treatments. When disease progression occurred, the treatment was stopped. In the intent-to-treat analysis of efficacy, patients were classified into 2 categories: (i) patients who were “stable” or with “response”; (ii) patients with treatment failure including clinical progression of the disease and disruption of the treatment because of severe toxicity, comorbidities, or decision of the patient to stop the treatment. The dependent efficacy variable was defined in the analysis as binary variable for “response or stable” (yes) and treatment failure (no). The relationships between exposures of capecitabine and its metabolites at the first cycle of treatment and the tumor response evaluated at cycle 3 and cycle 6 were investigated.
Statistical analysis
Quantitative variables were expressed as median (range). To compare the groups of patients included in the two phase I studies (≤73 years) and the patients included in CAPAGEC (≥75 years) the Mann-Whitney non parametric test was used for continuous variables whereas the Chi square or exact Fisher test were used for categorical variables. Pearson correlation coefficient was used to study the dose/exposure relationship. Statistical analysis was performed using the MEDCALC 9.0 software (Medcalc Software, Mariakerke, Belgium).
RESULTS
Patients
The patients’ characteristics are summarized in Table 1. A total of 20 patients (5 with breast cancer and 15 with colorectal cancer) with a median age of 80.5 years (range: 75–92) were enrolled in CAPAGEC. All were metastatic and had comprehensive geriatric assessment. Data on patient ethnicity were not available as collection of such data is not legal in France.
Table 1.
Characteristics of the pharmacokinetic study population
| Characteristics | Patients of the two phase I studies median [range] |
Patients of CAPAGEC median [range] |
P |
|---|---|---|---|
| Number of patients | 40 | 20 | |
| Gender (Female/Male) | 15/25 | 12/8 | 0.098 |
| Age (years) | 54.5 [30–73] | 80.5 [75–92] | 3. 10−10 |
| Body weight (kg) | 68 [41–95] | 74 [48–113] | 0.055 |
| Body surface area (m2) | 1.80 [1.40–2.10] | 1.82 [1.41–2] | 0.52 |
| Total Bilirubin (μmol/L) | 8.8 [3–22] | 5.1 [2–13.3] | 0.001 |
| ClCR (ml/min) | 71 [28–115] | 66 [23–137] | 0.13 |
In the CAPAGEC trial, 9 patients received the total of the 6 cycles of chemotherapy. The remaining 11 patients received 1 cycle (n=3), 2 cycles (n=3), 3 cycles (n=3) or 4 cycles (n=2).
Population pharmacokinetic model
A total of 2213 concentration data were analyzed (i.e. 584 for capecitabine, 354 for 5′-DFCR, 577 for 5′-DFUR, 476 for 5-FU and 222 for FBAL). The model with the first 4 compartments adequately described the PK of capecitabine and three of its metabolites (5′-DFCR, 5′-DFUR, and 5-FU) in the studied population. The model including a fifth compartment for FBAL (concentrations available only in the 20 elderly patients) did not fit the FBAL data well. So the model with 4 compartments was retained. Introduction of ISV on tlag, V1, CL10, k23, k34 and k40 and of IOV on ka significantly improved the fit of the model. So it was used to test covariate effect.
In the univariate analysis, five covariates were selected: total bilirubin, body surface area, gender, day of pharmacokinetic evaluation (i.e. day 1 for all patients and day 15 in the <75 years patients or cycle 2 day 14 in the ≥ 75 years patients) and age group. Total bilirubin had a negative effect on k34(intercompartmental rate constant from 5′-DFCR to 5′-DFUR). The capecitabine absorption rate constant (ka) was lower in the ≥75 years patients group. The capecitabine apparent clearance (CL10) increased with body surface area and decreased with elapsed time, i.e. from the first to the second PK evaluation. The apparent clearance CL12 was lower in the elderly population and at the second PK evaluation compared to the first one. k40 was lower in women than in men and also decreased over time. As a result of the forward inclusion and backward exclusion procedure, only the age group and the day of PK evaluation covariates were retained on ka and k40, respectively in the final model. Thereby, an OFV decrease of 50 units in comparison with the free-covariate model was obtained. The mean parameter estimates of the final model were similar to the median estimates resulting from the bootstrap procedure (Table 3).
Table 3.
Population PK parameters of capecitabine and bootstrap results
| Parameter | Final model estimate (SD%) | Bootstrap results (n=1000 samples) | |
|---|---|---|---|
| Median | 2.5th – 97.5th percentiles | ||
| ka (h−1) = θ1. θ2agegroup* | |||
| θ1 | 1.86 (6.0) | 1.94 | 0.72 – 2.57 |
| θ2 | 0.45 (11.2) | 0.53 | 0.31 – 2.42 |
| tlag (h) | 0.31 (4.5) | 0.31 | 0.17 – 0.39 |
| V1 (L) | 292 (9.2) | 276 | 117 – 358 |
| CL10 (L/h) | 214 (8.2) | 220 | 186 – 264 |
| CL12 (L/h) | 13.6 (10.5) | 13.0 | 9.3 – 18.3 |
| k23 (h−1) | 10.9 (8.9) | 10.5 | 7.6 – 13.9 |
| k34 (h−1) | 6.0 (10.6) | 5.6 | 4.0 – 7.9 |
| k40 (h−1)=θ3.θ4day** | |||
| θ3 | 77.1 (11.9) | 76.8 | 47.2 – 84.8 |
| θ4 | 0.77 (4.4) | 0.81 | 0.56 – 1.15 |
| IOV ka (%) | 129 (36) | 134 | 78 – 213 |
| ISV tlag (%) | 148 (38) | 138 | 55 – 291 |
| ISV V1 (%) | 107 (44) | 112 | 79 – 176 |
| ISV CL10 (%) | 27 (97) | 28 | 12 – 44 |
| ISV k23 (%) | 49 (51) | 49 | 28 – 72 |
| ISV k34 (%) | 35 (69) | 33 | 20 – 47 |
| ISV k40 (%) | 45 (60) | 45 | 24 – 60 |
| Residualvariabilities (SD) | |||
| Capecitabine (μM) | 5.2 (3.7) | 5.0 | 3.2 – 7.2 |
| 5′ DFCR (mu;M) | 3.7 (16.9) | 3.6 | 2.6 – 4.5 |
| 5′ DFUR (μM) | 6.1 (6.2) | 5.8 | 4.7 – 6.9 |
| 5 FU (μM) | 0.7 (7.2) | 0.7 | 0.5 – 0.9 |
Abbreviations: ka: absorption rate constant; V1: apparent distribution volume of capecitabine; tlag: lag time; k23:intercompartmental rate constant of 5′-DFCR; k34:intercompartmental rate constant of 5′-DFUR; k40: elimination rate constant of 5-FU;CL10 and CL12: apparent capecitabine clearances; ISV: intersubject variability; IOV: inter-occasion variability; SD: standard deviation.
age group=0 for <75 years patients and 1 for ≥75 years patients
day=0 for first administration of capecitabine) and day=1 for both the 15th day of treatment and the 14thday of treatment of cycle 2
A significant positive linear correlation between predicted capecitabine AUC and administered dose was found in the elderly patients (≥ 75 years, r2=0.53, p<10−4) as well as in the younger patients. (r2= 0.41, p<10−3). These two coefficients were not significantly different (p=0.59). A similar correlation (r2=0.35) was observed between capecitabine AUC and administered dose expressed as mg/m2 (from 1400 to 2300 mg/m2/day).
Exposure-effect relationships
Capecitabine administration had to be stopped before completion of the study in 11 patients because of disease progression (n=6, including one death), severe toxicities (n=2, one grade 3 diarrhea and one grade 4 fatigue), comorbidities (n=1), patient’s decision to stop the treatment (n=1) and unknown reason (n=1). At the end of the treatment period, among the 9 patients who received the 6 cycles, 5 patients had stable disease, 1 patient was partial responder, 1 patient had progressive disease and 2 patients had missing or insufficient response information (one of these two patients continued the treatment after the end of the clinical trial).
Table 2 summarizes the treatment-related adverse events reported during the CAPAGEC trial. All the adverse events except one were in grade ≤3. Fatigue of grade 4 was reported in one patient leading to disruption of the treatment after the first cycle of treatment. The most frequently reported adverse events were HFS, fatigue and diarrhea, observed in 55%, 40% and 30% of patients, respectively. Almost all the 21 HFS events were reversible, only one was persistent and the reversibility of two was not determined. Eighteen of the HFS events reported were grade 1 and grade 2.
Table 2.
Summary of the most common reported treatment-related adverse events in the CAPAGEC trial
| Number of adverse events | Number of patients (%) | Grade of events | |
|---|---|---|---|
| Non hematologic adverse event | |||
| Hand-foot syndrome | 21 | 11 (55) | 1, 2, 3 |
| Diarrhea | 9 | 6 (30) | 1, 2, 3 |
| Fatigue | 8 | 8 (40) | 1, 2, 3, 4 |
| Nausea | 4 | 4 (20) | 1, 2 |
| Vomiting | 3 | 3 (15) | 1, 2 |
| Abdominal pain | 3 | 3 (15) | 1, 2 |
| Mucositis | 3 | 2 (10) | 1, 3 |
| Dysgeusia | 2 | 2 (10) | 1 |
| Paresthesia | 3 | 1 (5) | 2, 3 |
| Anorexia | 1 | 1 (5) | 2 |
|
| |||
| Hematologic adverse event | |||
| Anemia | 5 | 3 (15) | 1, 2, 3 |
| Neutropenia | 1 | 1 (5) | 1 |
| Thrombopenia | 3 | 1 (5) | 1, 2 |
The exposure-effect relationships were studied in the elderly patients enrolled in CAPAGEC: 20 patients for cycle 1, and 16 patients for cycle 2 (PK data were not available for 4 patients). The median AUCs of capecitabine and its metabolites were not statistically different between patients who experienced grade ≥ 2 of diarrhea and those who did not whatever the cycle of treatment (cycle 1 or 2). At the first cycle of treatment, only two patients experiences HFS (grade 1 and grade 2). These events did not seem associated with high AUC of capecitabine or of its metabolites. At cycle 2, AUCs of capecitabine, 5′-DFCR, 5′DFUR and 5-FU were significantly higher (p=0.01243 for capecitabine; p=0.03086 for 5′-DFCR; p=0.006392 for 5′-DFUR and p=0.008967 for 5-FU) for patients who experienced HFS compared to those who did not. The difference was not statistically different (p=0.57) for the observed AUCs of FBAL (Figure 2).
Figure 2.
Comparisons of the area under the concentration-time curve (AUC; μmol.h/L) of (a) capecitabine, (b) 5′DFCR, (c) 5′DFUR,(d) 5 FU and (e) FBAL in the second cycle of treatment, between patients who experienced hand-foot syndrome (HFS+) and who did not experience (HFS−).
No difference in median AUCs of capecitabine and its metabolites obtained at cycle 1 was observed between “responders or stable” and “treatment-failure” patients.
DISCUSSION
In a population including one third of elderly patients (≥75 years), the capecitabine absorption rate constant was found lower in the oldest patient group, while the constant rate elimination of the 5-FU metabolite (k40) decreased significantly over time (i.e. after 2 consecutive weeks of capecitabine administration).
Furthermore, from the second cycle of treatment, significantly higher median exposures of capecitabine and its metabolites (5′-DFCR, 5′-DFCR and 5-FU) were observed in patients who experienced HFS compared to those who did not.
The pharmacokinetics of capecitabine and its first three metabolites (5′-DFCR, 5′-DFCR and 5-FU) were satisfactorily described by a four compartment model. The population pharmacokinetic analysis failed to describe FBAL concentrations, available for only 20 patients over 60. The FBAL compartment was the last one in the tested five compartment model so no concentration downstream could help to describe the FBAL amounts eliminated. Herein, mean capecitabine absorption constant rate (ka) values of 1.86 and 0.84 h−1 (i.e. 1.86*0.4) were obtained in the <75 years group and in the ≥75 years group, respectively. Interestingly, these two typical values of ka were close together and similar to those reported in other studies with mean age lower than 65 years old [10,15]. Thereby, it seemed difficult to discriminate between an age effect or a “study” effect. Of note, the schedule of capecitabine administration and the chemotherapy regimen (dosage and combination of chemotherapies) differed between the analyzed trials. Magnitude of this effect was rather small, so it could be ignored for individual dose adjustment.
Elimination rate constant of 5-FU was found decreased over time. This time effect was not found different between (i) the <75 years group for which the second PK evaluation took place after 15 days of treatment and (ii) the ≥75 years group for which the second PK evaluation took place on day 14 of cycle 2. This suggests an increase of 5-FU exposure after two weeks of treatment whatever the cycle of chemotherapy. A time-dependency was previously shown in continuous 5-FU infusion [22,24,25].
In the univariate analysis, an association between BILT and k34 and between gender and elimination rate constant of 5-FU were also found, but this was not confirmed in the multivariate analysis. Similar conclusions were previously reported [15]. Interestingly, other studies reported gender effect on 5-FU elimination in populations receiving 5-FU chemotherapy [22,24]. No effect of age was found on elimination parameters of capecitabine and its metabolites. In the present population analysis, the only effect of age was the questionable relationship between age group and ka. This advocates for a negligible effect of great age which would not be associated with accumulation of capecitabine and its metabolites. It is noteworthy that the monograph of oral capecitabine claims that age does not affect the pharmacokinetic disposition of 5′-DFUR or 5-FU.
Louie et al.[13] reported a significant increase in capecitabine AUC (p<0.05) associated with a reduction in capecitabine apparent clearance in elderly patients (≥70 years). The elderly group also presented lower estimated clearance of creatinine (CLCR, estimated with the Cockcroft and Gault formula) than the younger control group. However, this control group included only 5 patients aged less than 60 years. In the present study, CLCR was not identified as a significant covariate in the population PK model for capecitabine, 5′DFCR, 5′DFUR and 5-FU. These results were in accordance with those reported by Poole et al.[26] and by Gieschke et al.[10] who did not find significant relationship between CLCR and systemic exposure to capecitabine or 5-FU.
This study confirmed a linear increase in capecitabine AUC with dosage increases, taking into account either the dose actually administered (in mg) or the dose level based on surface area (mg/m2) [12].
Five of the 20 elderly patients (25%) achieved stable disease and one patient (5%) was partial responder. Therefore, the response rate obtained was very encouraging in these old patients. A response rate of 20% (complete or partial responders) was previously reported in a population aged between 26 and 78 years with metastatic breast cancer and treated with a similar dose of capecitabine (2510 mg/m2/day of capecitabine) [27].
Treatment-related adverse events reported in CAPAGEC were almost all in grade ≤3 and only two caused disruption of the treatment before completion of the study (diarrhea grade 3 and fatigue grade 4). HFS was the most frequently reported adverse event, in addition to diarrhea and fatigue. Fifty percent of patients (n=11) experienced HFS at least once over the study period, and most of the HFS were rated as grade 1 or 2 in intensity. Out of these 11 patients, 6 received the entire 6 cycles. Similar or higher frequencies of HFS were reported in two studies performed in patients aged between 26 and 78 years [27], and between 25 and 79 years [28], and treated with similar doses of capecitabine than our study (2510 mg/m2/day and 2500 mg/m2/day, respectively). In the first study, HFS occurred in 56.2% of patients and most of them was graded as mild or moderate (grade 1 or 2) [27]. In the second study, the proportion of patients with HFS was 68.3%, with most of them occurring within the two first cycles and classed as grade 1 or 2 [28]. Thus, elderly patients did not seem to present any more HFS toxicity than the general population.
In CAPAGEC, the median AUCs of capecitabine, 5′-DFCR, 5′-DFUR and 5-FU (but not FBAL) observed at the last day of the second cycle of treatment were found to be significantly higher in patients who experienced grade 2–3 of HFS compared to those who did not. Interestingly, it was previously reported that both peak drug concentration and total cumulative dose determine HFS occurrence [28]. However, in a large population dataset (n=481 patients) Gieschke et al.[10] found no relationship between grade 3 of HFS and Cmax or AUC of 5′DFUR, 5-FU and FBAL. The exposure-efficacy analysis performed in the current study did not find a significant difference in the median AUC of capecitabine and its metabolites at the first cycle between “responders or stable” and “treatment-failure” patients. Similarly, Cmax and AUC of capecitabine and its metabolites were found poorly predictive of efficacy variables, defined as tumor response/non response, time to disease progression and duration of survival [10]. Only AUCs measured at the first cycle only were taken into account in this latter analysis. Of note, for docetaxel, a significant relationship was showed between first course AUC and time to progression, in non-small-cell-lung-cancer [30].
In conclusion, the current study has not demonstrated a major effect of great age on pharmacokinetics of capecitabine and its metabolites. 5-FU constant rate elimination was found decreased over time, but this effect was similar before and after 75 years old. Additionally no major difference in treatment tolerability and response rate in elderly patients as compared to reported data in younger subjects has been observed. This study puts forward therefore new arguments for the treatment of elderly cancer patients who could benefit from capecitabine chemotherapy. Additionally, the present exposure-effect analysis showed a relationship between exposure of capecitabine and some of its metabolites (5′-DFCR, 5′-DFUR and 5-FU) and the onset of hand-foot syndrome. Further studies with a larger number of elderly patients may be needed to confirm these results.
Acknowledgments
The CAPAGEC study was funded by the Limoges University Hospital.
We thank Fabrice Béavogui, Karine Bariller, Franck Giraudie and Jean Louis Dupuy for their technical assistance and Karen for her complete English and grammatical review of this paper.
Pierre Marquethas received research grants and honoraria from ROCHE.
Footnotes
These authors equally contributed to this work as the second authors (S.L.V. and A.P.).
These authors equally contributed to this work as the last authors (N.T.M. and A.R.).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wydra J, Kruszewski W, Jasiński W, Szajewski M, Ciesielski M, Szefel J, et al. Is Age a Risk Factor of Postoperative Complications in Colorectal Cancer? Pol Przegl Chir. 2013;85:491–495. doi: 10.2478/pjs-2013-0076. [DOI] [PubMed] [Google Scholar]
- 3.Wildiers H, Kunkler I, Biganzoli L, Fracheboud J, Vlastos G, Bernard-Marty C, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse KW. Pharmacokinetics of drugs in the elderly. J R Soc Med. 1994;87:2–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol. 2003;38:843–853. doi: 10.1016/s0531-5565(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 8.Twelves C. Vision of the future: capecitabine. Oncologist. 2001;6:35–39. doi: 10.1634/theoncologist.6-suppl_4-35. [DOI] [PubMed] [Google Scholar]
- 9.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 10.Gieschke R, Burger H-U, Reigner B, Blesch KS, Steimer J-L. Population pharmacokinetics and concentration-effect relationships of capecitabine metabolites in colorectal cancer patients. Br J Clin Pharmacol. 2003;55:252–263. doi: 10.1046/j.1365-2125.2003.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judson IR, Beale PJ, Trigo JM, Aherne W, Crompton T, Jones D, et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C-labelled drug. Invest New Drugs. 1999;17:49–56. doi: 10.1023/a:1006263400888. [DOI] [PubMed] [Google Scholar]
- 12.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Louie SG, Ely B, Lenz H-J, Albain KS, Gotay C, Coleman D, et al. Higher capecitabine AUC in elderly patients with advanced colorectal cancer (SWOGS0030) Br J Cancer. 2013;109:1744–1749. doi: 10.1038/bjc.2013.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gieschke R, Reigner B, Blesch KS, Steimer JL. Population pharmacokinetic analysis of the major metabolites of capecitabine. J Pharmacokinet Pharmacodyn. 2002;29:25–47. doi: 10.1023/a:1015716617967. [DOI] [PubMed] [Google Scholar]
- 15.Urien S, Rezaí K, Lokiec F. Pharmacokinetic modelling of 5-FU production from capecitabine--a population study in 40 adult patients with metastatic cancer. J Pharmacokinet Pharmacodyn. 2005;32:817–833. doi: 10.1007/s10928-005-0018-2. [DOI] [PubMed] [Google Scholar]
- 16.Grande C, Quintero G, Candamio S, París Bouzas L, Villanueva MJ, Campos B, et al. Biweekly XELOX (capecitabine and oxaliplatin) as first-line treatment in elderly patients with metastatic colorectal cancer. J Geriatr Oncol. 2013;4:114–121. doi: 10.1016/j.jgo.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RA, Pitcher B, Keating NL, Ballman KV, Mandelblatt J, Kornblith AB, et al. Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Res Treat. 2013;139:607–616. doi: 10.1007/s10549-013-2562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandvliet AS, Siegel-Lakhai WS, Beijnen JH, Copalu W, Etienne-Grimaldi M-C, Milano G, et al. PK/PD model of indisulam and capecitabine: interaction causes excessive myelosuppression. Clin Pharmacol Ther. 2008;83:829–839. doi: 10.1038/sj.clpt.6100344. [DOI] [PubMed] [Google Scholar]
- 20.Beal SL, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s Guide. NONMEM project group, University of California; San Francisco, CA: 1998. [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Etienne MC, Chatelut E, Pivot X, Lavit M, Pujol A, Canal P, et al. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion. A NONMEM analysis. Eur J Cancer. 1998;34:92–97. doi: 10.1016/s0959-8049(97)00345-6. [DOI] [PubMed] [Google Scholar]
- 23.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29. doi: 10.1016/s0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 24.Terret C, Erdociain E, Guimbaud R, Boisdron-Celle M, McLeod HL, Féty-Deporte R, et al. Dose and time dependencies of 5-fluorouracil pharmacokinetics. Clin Pharmacol Ther. 2000;68:270–279. doi: 10.1067/mcp.2000.109352. [DOI] [PubMed] [Google Scholar]
- 25.Fety R, Rolland F, Barberi-Heyob M, Hardoin A, Campion L, Conroy T, et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinoma. Clin Cancer Res. 1998;4:2039–2045. [PubMed] [Google Scholar]
- 26.Poole C, Gardiner J, Twelves C, Johnston P, Harper P, Cassidy J, et al. Effect of renal impairment on the pharmacokinetics and tolerability of capecitabine (Xeloda) in cancer patients. Cancer Chemother Pharmacol. 2002;49:225–234. doi: 10.1007/s00280-001-0408-0. [DOI] [PubMed] [Google Scholar]
- 27.Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;7:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 28.Abushullaih S, Saad ED, Munsell M, Hoff PM. Incidence and severity of hand-foot syndrome in colorectal cancer patients treated with capecitabine: a single-institution experience. Cancer Invest. 2002;20:3–10. doi: 10.1081/cnv-120000360. [DOI] [PubMed] [Google Scholar]
- 29.Kara IO, Sahin B, Erkisi M. Palmar-plantar erythrodysesthesia due to docetaxel-capecitabine therapy is treated with vitamin E without dose reduction. Breast. 2006;15:414–424. doi: 10.1016/j.breast.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]



