Abstract
Background
Photodynamic therapy (PDT) utilizes light to activate a photosensitizer in the presence of oxygen, and leads to local photodamage by the generation of highly reactive oxygen species (ROS). Liposomal delivery of photosensitizers is adaptable to the treatment of cancers. We examined the phototoxicity of free or liposome-embedded phthalocyanine photosensitizers using HeLa cervical carcinoma and HSC-3 oral squamous cell carcinoma cells.
Material/Methods
Liposomes were composed of palmitoyloleoyphosphatidylcholine (POPC): phosphatidylglycerol (PG), and contained either zinc phthalocyanine (ZnPc) or aluminum phthalocyanine chloride (AlPc). Free or liposomal ZnPc and AlPc were incubated with cells for 24 h at 37°C. Cells incubated with ZnPc were exposed to broadband visible light (350–800 nm; light dose 43.2 J/cm2), whereas cells treated with AlPc were exposed to light at 690 nm (light dose 3.6 J/cm2). The effect of folate receptor-targeted liposomal ZnPc was evaluated with HeLa cells. Cytotoxicity was analyzed by the Alamar Blue assay.
Results
Cell viability, expressed as a percentage of control cells, was calculated according to the formula [(A570–A600) of test cells]×100/[(A570–A600) of control cells]. The relative percentage changes then defined the phototoxic efficacy of the experimental conditions. In HeLa cells, 1 μM free ZnPc and AlPc, reduced cell viability to 52.7±2.1 and 15.4±8.0%, respectively. Liposomal phthalocyanines, at 0.1, 0.5, and 1.0 μM, reduced the viability to 68.0±8.6, 15.1±9.9 and 0% (ZnPc), and to 25.8±8.2, 0 and 0% (AlPc), respectively. In HSC-3 cells, 1 μM free ZnPc and AlPc, reduced cell viability to 22.1±2.8 and 56.6±8.6%, respectively. With 1 μM liposomal ZnPc and AlPc, the viability was reduced to 0 and 21.3±0.3%, respectively.
Conclusions
The embedding of phthalocyanines in liposomes enhanced their phototoxicity and this effect was dependent on cell type.
MeSH Keywords: Folate Receptor 1, Head and Neck Neoplasms, Liposomes, Photochemotherapy
Background
Oral cancer is a global health problem with considerably poor survival. Despite advances in current treatment modalities, there has been minimal improvement in survival rates, emphasizing the need for novel strategies for managing this disease. Head and neck cancers (HNCs) develop in the oral cavity, the pharynx, the nasal cavity, and the larynx. Most of these cancers are squamous cell carcinomas (SCCs) that originate from the mucosal epithelium. Photodynamic therapy (PDT) is a promising treatment modality for cancer [1,2]. PDT appears to be a viable alternative to conventional therapy for oral premalignant lesions [3,4]. Several clinical trials have found PDT to be highly effective in the treatment of early and recurrent of HNCs. Patients have been treated with PDT using Photofrin, hematoporphyrin derivative (HPD), aminolevulinic acid (ALA) and Foscan (Temoporfin) [5–10]. The FDA has not approved PDT for the treatment of head and neck SCCs. The use of conventional therapies does not preclude the use of PDT, and PDT does not compromise future surgical interventions or radiation therapy [11]. Originally, the aim of this study was to evaluate the photodynamic effects of phthalocyanines in oral cancer cells. However, because of the controversy regarding HeLa-contaminated KB cells, which have been wrongly identified as oral cancer cells, we compared HSC-3 oral cancer cells with HeLa cervical carcinoma cells.
PDT utilizes light to activate a photosensitizing agent (photosensitizer) in the presence of oxygen. The exposure of the photosensitizer to light results in the formation of highly reactive oxygen species (ROS) and free radicals, causing localized photodamage and cell death. The administration of the photosensitizer is followed by subsequent irradiation with red visible light to promote the excitation of the photosensitizer. PDT produces cytotoxic effects through damage to subcellular organelles and molecules. Mitochondria, lysosomes, cell membranes, and nuclei of tumor cells are considered potential targets. During light exposure, sensitizers that localize in mitochondria may induce apoptosis, while sensitizers that localize in lysosomes and cell membranes may cause necrosis [11].
Over the past few decades, liposomes have drawn considerable attention as carriers of therapeutic agents. Due to their capability to incorporate hydrophilic and hydrophobic drugs, biocompatibility, low toxicity, and lack of immune system activation, liposomes have also become attractive carriers for photosensitizers. Liposomal delivery may help to overcome certain limitations of photosensitizing agents, including aggregation [12,13]. This “passive targeting” mechanism is affected by the physicochemical properties of the photosensitizer. Active targeting of liposomes to tumor surface markers, e.g., receptors of growth factors, transferrin, integrin, insulin and folate, has been studied extensively [14,15].
The folate receptor (FR), a glycosylphosphatidylinositol-anchored cell membrane protein, is upregulated in a variety of epithelial cancer cells, including ovarian, breast, kidney, lung, and colon cancers, and is rarely present in normal cells [16]. FR binds extracellular folate with high affinity and delivers it to cells via endocytosis [17]. The enhanced phototoxicity of several photosensitizers conjugated with folate or encapsulated in folate-targeted liposomes has been reported mostly in FR-positive KB cells, which were thought to be oral cancer cells, and in HeLa cells [18–25].
In this study, we investigated the photodynamic effects of free and liposome-embedded zinc phthalocyanine (ZnPc) and aluminum phthalocyanine chloride (AlPc) on the viability of HeLa cervical cancer cells and HSC-3 oral squamous cell carcinoma cells. We also examined the effect of FR-targeted liposomal ZnPc using highly FR-positive HeLa cells [25,26]. We did not use KB cells, used often as a model for oral cancer cells and FR-positive cells [18,19,21,22,24,27–29]. The nasopharyngeal KB cell line, originally isolated in 1955 from a human epidermoid carcinoma of the mouth, was subsequently found to be contaminated by HeLa cells (cervical adenocarcinoma), based on isoenzyme analysis, HeLa marker chromosomes, and DNA fingerprinting [30,31]. Therefore, the use of KB cells in any study that claims to represent an investigation of oral cancer phenotype cells is incorrect. We have used HeLa cells as an appropriate comparison with previous results obtained with KB cells.
Material and Methods
Cell culture
HeLa cervical adenocarcinoma cells were purchased from ATCC (CRL-8798, Rockville, MD, USA). HSC-3 cells, derived from squamous cell carcinoma (SCC) of the tongue [32], were provided by Dr. R. Kramer (UCSF). Cells were incubated in tissue culture flasks (Falcon, Becton Dickinson Labware, UK) at 37°C in a humidified atmosphere containing 5% CO2, and were passaged 1: 6 twice a week. They were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), streptomycin (100 μg/ml), and L-glutamine (4 mM) (DMEM/10). All media, with or without phenol red, penicillin-streptomycin solution, L-glutamine, FBS, Trypsin-EDTA (ethylenediaminetetraacetic acid) and phosphate buffered saline (PBS), were obtained from the UCSF Cell Culture Facility (San Francisco, CA). Photosensitizers were solubilized in 100% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) to a final concentration of 10 mM, and subsequently diluted in DMEM (without FBS and phenol red) to a concentration of 50 μM (0.5% DMSO). The 50 μM solutions were diluted in DMEM to obtain the desirable concentration of photosensitizers used in the experiments. The cells were plated at a density of 1.8×105 cells per well, in 1 ml of DMEM/10 in 48-well plates (BD Falcon™, Franklin Lakes, NJ, USA), and used after a 24 h incubation at 37°C at approximately 80% confluence. Subsequently, the cells were pre-washed twice with PBS (0.5 ml) and 1 ml of medium without FBS and phenol red, containing photosensitizer at a given concentration, was added to each well except controls. The FBS-free media were used to avoid binding of photosensitizers to serum proteins. Directly after light exposure, medium without FBS and phenol red was replaced with 1 ml of complete medium (DMEM/10), and the cells were re-incubated for an additional 24 h at 37°C. Cell viability was quantified by the Alamar Blue assay.
Liposome preparation
Liposomes were prepared by hydration of dry lipid films in HEPES-buffered saline, pH 7.4, followed by extrusion through polycarbonate membranes. Negatively charged liposomes were composed of palmitoyloleoylphosphatidylcholine (POPC): phosphatidylglycerol (PG) (1: 1) (Avanti Polar Lipids, Alabaster, AL, USA). The lipids were dissolved in chloroform (Fisher Scientific, Fremont, CA, USA) and the solvent was evaporated to dryness in a rotatory evaporator (Labconco, Kansas City, MO, USA) to deposit a lipid film on the walls of a test tube. To remove final traces of the solvent, the films were kept in a vacuum desiccator (Thermo Scientific Napco, Fremont, CA, USA) for 12 h. Multilamellar vesicles were prepared by hydration of the dried lipid films under an argon atmosphere (achieved by flushing argon over the buffer) at a concentration of 10 μmol lipid/ml of 140 mM NaCl and 10 mM HEPES buffer (pH 7.4), followed by vortexing for 10 min. Liposomes were extruded 21 times through polycarbonate membranes of 100-nm pore diameter, using an Avanti Mini Extruder, to achieve a uniform size distribution (approx. 100 nm diameter). The liposomes were not subjected to further purification to avoid exchange of the photosensitizers with gel filtration material. Since the photosensitizers are hydrophobic and have very poor solubility in water, the hydrophobic interior of the liposome membrane provided an ideal “solvent” for these molecules. Folate-conjugated liposomes contained 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG(2000) Folate; Avanti Polar Lipids). Liposomes were stored at 4°C under an argon atmosphere.
Phthalocyanine zinc salt (ZnPc) (CAS Number 14320-04-8, C32H16N8Zn, MW 577.91) and aluminum phthalocyanine chloride (chloro(29H,31H-phthalocyaninato)aluminum (AlPc) (CAS Number 14154-42-8, C32H16AlClN8, MW 574.96) were purchased from Sigma and used without further purification. Liposomes were composed of POPC: PG and contained in their membranes either ZnPc or AlPc at a 5: 5: 0.1 molar ratio. Although we did not compare liposomes of different composition, previous studies have shown that negatively charged liposomes are taken up more efficiently by cells than neutral liposomes [33].
Photodynamic treatment
HeLa or HCS-3 cells were incubated with free or liposomal ZnPc or AlPc, in the range 0.1–1 μM, for 24 h at 37°C. Two light sources were used for irradiation, because of the differences in the photophysical properties of ZnPc and AlPc [34]. Cells incubated with free or liposomal ZnPc were exposed to broadband visible light (350–800 nm) from a Dura Max light bulb (75W Med 120V A19 Cl/LL 20W; Philips Electronics North America Corporation, Andover, MA, USA), at a distance of 10 cm to the plate, for 20 min at room temperature. The total spectral irradiance at the level of cells was 36 mW/cm2 (light dose of 43.2 J/cm2), as measured by an RD 0.2/2 radiometer with a TD probe (Optel Vision, Quebec City, Canada). These measurements indicated that the irradiance was constant over the small area occupied by the 48-well plates [35]. Infrared radiation was minimized using a 1 cm water filter between the cell plates and the light source. Cells incubated with free or liposomal AlPc were exposed to light (690 nm) from a high-power LED Multi Chip Emitter (9.8V; Roithner Lasertechnik, Vienna, Austria) for 20 min. The light intensity at the surface of the plate was 3.0 mW/cm2 according to a Thorlabs TM100A Optical Power Meter (Thorlabs Inc. Newton, NJ, USA). The total light dose was 3.6 J/cm2. Cells treated with the photosensitizer but shielded from light were used for the evaluation of dark toxicity. Cells incubated with medium alone, medium/liposomes, or medium/0.5% DMSO served as controls. Three independent experiments were performed in triplicate (3 wells) for each condition (dark and light toxicity of free or liposomal ZnPc or AlPc, in the range 0.1–1.0 μM), and controls. In the figures, each presented value is the mean ± standard deviation (SD) of 2 or 3 independent experiments performed in triplicate.
Flow cytometric analysis
The expression of the folate receptor 1 (FOLR1) on the surface of HeLa and HSC-3 cells was examined by flow cytometry. The cells were maintained in DMEM high-glucose medium (ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (ThermoFisher) and penicillin (100 units/ml)/streptomycin (100 μg/ml) (UCSF Cell Culture). To evaluate the effect of folate on the FOLR1 expression, cells were cultured also for 1 week in DMEM without folate (UCSF Cell Culture; Custom Made). The cells were harvested with StemPro® Accutase® Cell Dissociation Reagent (ThermoFisher). After centrifugation for 3 min at 6000 rpm in a microcentrifuge (Eppendorf 5418; USA Scientific, Ocala, FL, USA), the cells were washed twice in PBS containing 2% FBS and 0.05% sodium azide (Sigma) (FACS buffer) and stained at 4°C for 30 min with allophycocyanin (APC)-conjugated anti-FOLR1 or the corresponding APC-conjugated isotype-matched control monoclonal antibody (R&D Systems, Inc., Minneapolis, MN, USA). The cells were post-fixed with 1% paraformaldehyde in FACS buffer and analyzed using a Guava flow cytometer and InCyte 2.7 software (EMD Millipore, Hayward, CA, USA).
Cell viability
Cell morphology was evaluated by inverted phase contrast microscopy at 25x magnification. The number of viable cells used for the experiments was determined by the Trypan Blue exclusion assay. Cell viability was quantified by the modified Alamar Blue assay [36]. Briefly, 1.0 ml of 10% (v/v) Alamar Blue dye (Life Technologies, Carlsbad, CA, USA) in complete DMEM/10 was added to each well. After incubation at 37°C for 1.5–2 h, 200 μl of the supernatant was assayed by measuring the absorbance at 570 nm and 600 nm in a Molecular Devices Vmax microplate reader (Mountain View, CA, USA). Cell viability (as a percentage of control cells) was calculated according to the formula [(A570–A600) of test cells]×100/[(A570–A600) of control cells]. Statistical analysis was performed by the unpaired Student’s t-test, using StatView software (BrainPower, Inc., Calabasas, CA, USA). A probability value (p) of less than 0.05 was considered significant.
Results
Dark toxicity of free and liposomal phthalocyanines
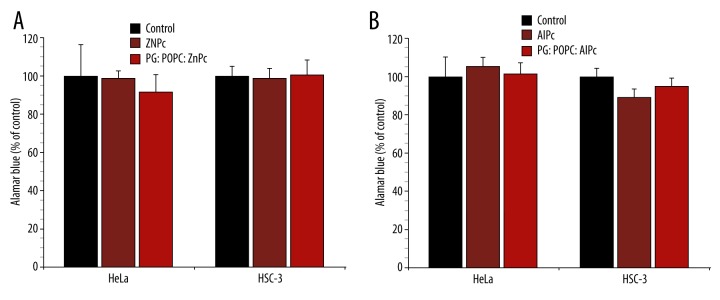
Cells treated with free or liposomal ZnPc and AlPc, in the range 0.1–1.0 μM, but shielded from light, were used for the evaluation of dark toxicity. The mean values of OD570–600 obtained for controls were considered as 100% and were used to calculate the percentage of control. The results obtained with 1.0 μM free and liposomal phthalocyanines are shown in Figure 1. The treatment without exposure to light did not significantly affect the metabolic activity of HeLa and HSC-3 cells. Thus, the effects of free and liposomal phthalocyanines (vide infra) were irradiation-dependent.
Figure 1.
Dark toxicity of 1 μM free and liposomal ZnPc (A) and AlPc (B) on the viability of HeLa and HSC-3 cells. The metabolic activity was measured by the Alamar Blue assay and expressed as a percentage of the control OD570–600 (control cells). Each value is a mean ± standard deviation (SD) of 2 or 3 independent experiments performed in triplicate.
Phototoxicity of free and liposome-embedded ZnPc
Figure 2 shows the percentage of cell viability of HeLa and HSC-3 cells incubated with free or liposomal ZnPc, in the range 0.1–1 μM, and exposed to broadband visible light (350–800 nm) at a light dose of 43.2 J/cm2. Liposome-embedded ZnPc was much more effective than free ZnPc in reducing cell viability in both cell lines. Free ZnPc, at 0.5 and 1 μM, reduced HeLa cell viability to 87.0±13.6 and 52.7±2.1%, respectively, and HSC-3 cell viability to 58.1±9.9 and 22.1±2.8%, respectively. Both cell lines were not sensitive to ZnPc-mediated PDT at a concentration of 0.1 μM. Liposomal ZnPc reduced HeLa cell viability to 68.0±8.6 and 15.1±9.9%, at 0.1 and 0.5 μM, respectively, and HCS-3 cell viability to 7.2±2.0% at 0.1 μM. A lethal photodynamic effect (100% of the cells are killed: LD100) of liposomal ZnPc was observed at 1 μM in HeLa cells and at 0.5 and 1 μM in HCS-3 cells. Thus, HSC-3 cells were significantly more sensitive to the phototoxic activity of both free and liposomal ZnPc (p<0.0005) than HeLa cells.
Figure 2.
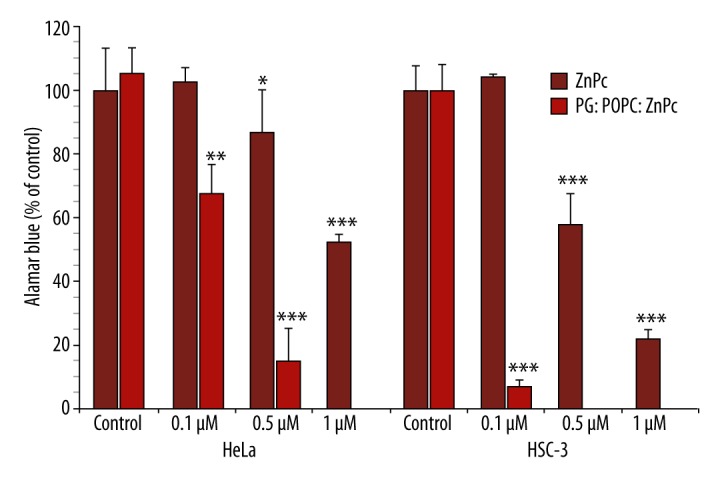
Phototoxicity of free and liposomal ZnPc against HeLa and HSC-3 cells. The metabolic activity was measured by the Alamar Blue assay and expressed as a percentage of the control OD570–600 (control cells). Each value is a mean ± standard deviation (SD) of 2 or 3 independent experiments performed in triplicate. Asterisks indicate significant differences compared with control (* p<0.05; ** p<0.01; *** p<0.0005).
Phototoxicity of free and liposome-embedded AlPc
Figure 3 shows the percentage of cell viability of HeLa and HSC-3 cells incubated with free or liposomal AlPc, in the range 0.1–1 μM, and exposed to light (690 nm) at a light dose of 3.6 J/cm2. Liposome-embedded AlPc was much more effective than free AlPc in reducing cell viability. At 0.1, 0.5, and 1 μM, free AlPc reduced HeLa cell viability to 78.2±9.9, 55.1±11.9, and 15.4±8.0%, respectively. HSC-3 cells were not sensitive to PDT mediated by free AlPc at 0.1 μM. At 0.5 and 1.0 μM, however, HSC-3 cell viability was reduced to 76.7±1.5 and 56.6±8.6%, respectively. Liposomal AlPc, at 0.1 μM, reduced the HeLa cell viability to 25.8±8.2%, while a lethal effect (LD100) was achieved at 0.5 and 1 μM. HSC-3 cells were significantly less susceptible to PDT mediated by liposomal AlPc (p<0.0005) than HeLa cells. At 0.1, 0.5, and 1 μM, HSC-3 cells viability was reduced to 72.8±18.0, 25.3±8.1, and 21.3±0.3%, respectively.
Figure 3.
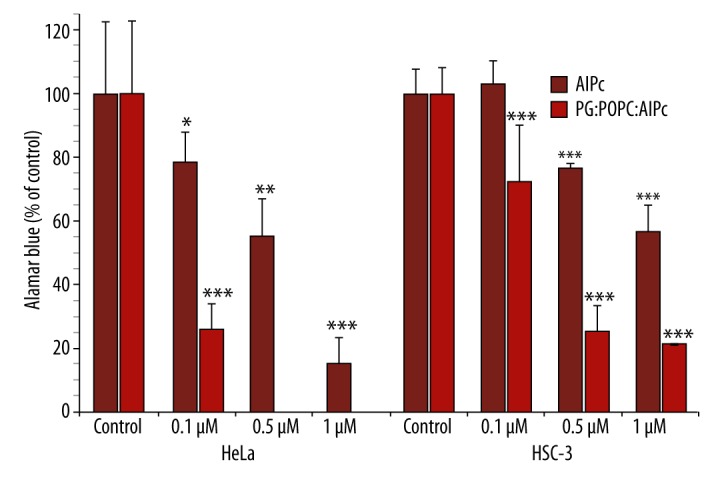
Phototoxicity of free and liposomal AlPc against HeLa and HSC-3 cells. The metabolic activity was measured by the Alamar Blue assay and expressed as a percentage of the control OD570–600 (control cells). Each value is a mean ± standard deviation (SD) of 2 or 3 independent experiments performed in triplicate. Asterisks indicate significant differences compared with control (* p<0.025; ** p<0.005; *** p<0.0005).
The expression of folate receptor 1 (FOLR1) on the surface of HeLa and HSC-3 cells
Flow cytometric analysis of folate receptor 1 (FOLR1) was performed for HeLa and HSC-3 cells cultured in complete DMEM medium. The flow cytometric data are provided in Figure 4. HeLa cells displayed a significant amount of binding of the anti-FOLR1 antibody. The cells had a heterogeneous level of expression of FOLR1. Deprivation of the cells from folate increased the percentage of cells displaying FOLR1 (solid lane) compared to cells cultured in medium containing folate. In HSC-3 cells however, there was essentially no expression of FOLR1.
Figure 4.
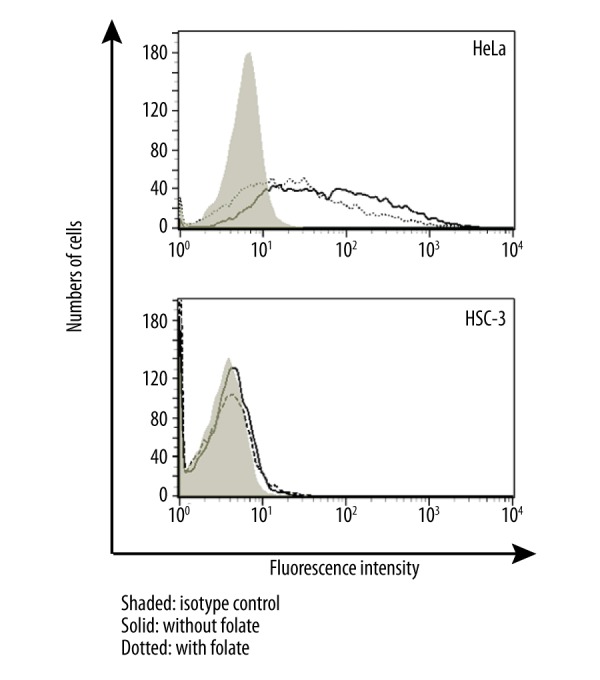
Flow cytometric analysis of folate receptor 1 (FOLR1) on HeLa and HSC-3 cells cultured in the DMEM/10 with folate (dotted histograms) or without folate (solid histograms). Shaded histograms represent isotype control.
Phototoxicity of liposomal ZnPc and folate-embedded liposomal ZnPc
The viability of highly FR-positive HeLa cells treated with liposomal ZnPc at 0.1 μM and irradiated decreased to ~70.0% (Figure 2). To check whether targeting liposomal ZnPc to FR would enhance phototoxicity at the lower concentrations, we investigated the photodynamic activity of FR-targeted vs. non-targeted liposomal ZnPc in the range of 0.01–0.1 μM. Non-targeted liposomal ZnPc reduced HeLa cell viability to 89.8±3.0, 88.9±6.4, and 77.0±3.7% at 0.01, 0.05, and 0.1 μM, respectively. However, targeting of liposomal ZnPc to FR was found to have no phototoxic effect on cell viability (Figure 5). We did not examine the effect of targeting of liposome-embedded ZnPc using FR-negative HSC-3 cells.
Figure 5.
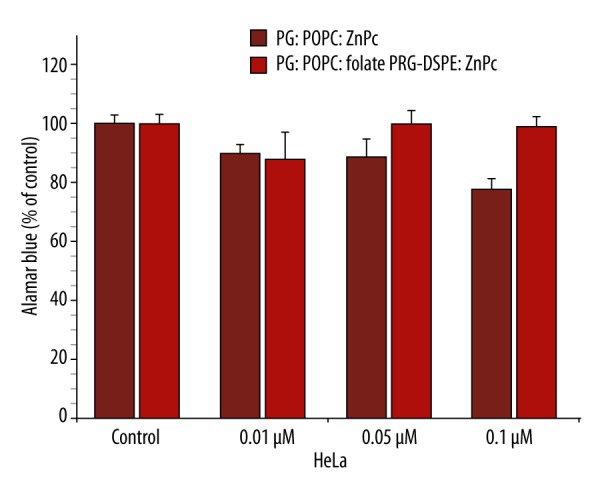
Phototoxicity of liposomal and folate-conjugated liposomal ZnPc against HeLa cells. The metabolic activity was measured by the Alamar Blue assay and expressed as a percentage of the control OD570–600 (control cells). Mean ±SD are shown (* p<0.025).
Discussion
Phthalocyanines have emerged as promising candidates for use as second-generation photosensitizers. They are activated by light at longer wavelengths (650–680 nm) and exhibit a greater depth of tissue penetration, leading to a better PDT response [2]. Most of the phthalocyanine derivatives are, however, insoluble in water and tend to form aggregates in a hydrophilic environment. They are strongly hydrophobic and lipophilic, and are usually administered in liposomes [13]. Among the metal phthalocyanines, Zn(II) and Al(III) complexes (ZnPc and AlPc) present the most favorable photophysical properties for application in PDT [37].
Oral squamous cell carcinoma (OSCC) is the most frequent cancer of the head and neck region and the sixth leading cancer by incidence worldwide. Despite advances in surgical, radiotherapeutic, and chemotherapeutic treatment, the 5-year survival rate of patients has not improved notably and is still about 40–50% [38] and searching for alternative treatment of these cancers is essential [9,39,40].
In this work, we examined the phototoxicity of free or liposome-embedded ZnPc and AlPc on the viability of HeLa cervical carcinoma cells and HSC-3 OSCC cells. Although it has been reported in 1967 [41] that the KB cell line is a sub-line of HeLa cells and is not derived from OSCC [30,31], KB cells have continued to be identified as being of oral cancer phenotype [18,21,22,24,27–29]. We have used HeLa cells (ATCC) as an appropriate comparison with previous results obtained with KB cells. As a model for OSCC cells, we used HSC-3 cells derived from SCC of the tongue [32] that were used recently to evaluate the phototoxicity of novel zinc and magnesium phthalocyanine derivatives [35,42], and novel porphyrazines and their liposomal formulations [43].
Our studies showed that both free and liposomal phthalocyanines had not effect on cell viability without exposure to light and that the embedding of phthalocyanines in liposomes enhanced their phototoxicity. The efficacy of PDT was dependent on cell type. HeLa cells were more sensitive to AlPc, whereas HSC-3 cells were more sensitive to ZnPc.
Only a few studies have evaluated free and liposomal phthalocyanine-mediated PDT against oral cancer cells. Ketabchi et al. [44] investigated the effect of aluminum disulfonated phthalocyanine (AlS2Pc) at 25 μg/ml (34 μM) on the viability of OSCC-derived H376 cells and human HPV16-transformed epidermal keratinocytes. The treatment reduces cell viability by ~73% and increases the number of apoptotic cells. Human dysplastic oral keratinocytes (premalignant DOK cells) established from oral SCC and standardized by the European Collection (ECACC No.94122104) were incubated with di- or tetra-sulfonated AlPc (AlS2–4Pc) at 2–4 μM for 24 h and irradiated with a He-Ne laser source (λ=632.8 nm). The cell viability was reduced by 70% by apoptosis, as determined by protein microarray analysis [45,46]. PDT with pyropheophorbide-a methyl ester (MPPa) enhanced apoptosis and reduced the mitochondrial membrane potential in CNE2 nasopharyngeal carcinoma cells [47]. In KB cells, PDT mediated by 5 μM AlPc encapsulated in dimyristoyl phosphatidylcholine (DMPC) liposomes reduced cell viability by 95%, causing morphologic alterations and necrosis [27]. In HeLa cells, PDT mediated by 1 μM ZnPc encapsulated in dipalmitoyl phosphatidylcholine (DPPC) liposomes showed a decrease in survival close to 100% [48]. The effect of non-liposomal AlPc and ZnPc was not reported in these studies. PDT efficacy of the symmetric derivative Zn(II)Pc 1 and asymmetrically substituted ZnPc(II) 2 have been investigated in mouse mammary carcinoma EMT6/P cells and HeLa cervical adenocarcinoma cells [49,50]. Zn(II)Pc 1 was photocytotoxic in EMT6/P cells (IC50 ~3.14 μM) but not in HeLa cells, whereas Zn(II)Pc 2 was not phototoxic in EMT6/P cells but was photoactive in HeLa cells (IC50 ~3.13 μM at 30 J/cm2). These results are in agreement with our observations that the phototoxicity of free and liposomal ZnPc and AlPc is dependent on cell type.
A variety of epithelial cancer cells overexpress FR [16]. The high affinity of folate for its receptor provides a novel approach for specific delivery of photosensitizers encapsulated in FR-targeted liposomes [16]. We evaluated the expression of FOLR1 on the surface of HeLa and HSC-3 cells used in our study. As reported previously [23,25,26], HeLa cervical carcinoma cells were highly positive for FOLR1. However, in HSC-3 OSCC cells there was essentially no expression of FOLR1. Our results showed that PDT mediated by FR-targeted liposomal ZnPc, in the range of 0.01–0.1 μM, did not reduced the viability of FR-positive HeLa cells. Garcia-Diaz et al. [23] investigated the phototoxicity of 5,10,15,20-tetraphenyl-21H,23H-porphine zinc (ZnTPP) encapsulated in non-targeted POPC: DOPS (1,2-dioleoyl-sn-glycero-3-phospho-L-serine) liposomes and FR-targeted liposomes POPC/DOPS/FA-PEG-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N [folate(polyethylene glycol)-2000]). In HeLa cells incubated with 1 μM ZnTPP for 24 h and irradiated with 10 J cm−2, non-targeted liposomes cause 65±5% cell death, while FR-targeted liposomes increase cell mortality to 94±5%. We did not perform experiments with FR-negative HSC-3 cells.
There has been some controversy about the FR expression in cells derived from OSCC. Ward et al. [51] screened 10 human OSCC cell lines and KB cells for folate binding protein-alfa (FBP-α), using the quantitative real-time polymerase chain reaction. Seven cell lines were obtained from the University of Michigan HNC SPORE (UM-SCC-1, 6, 11, 14A, 17B, 22B, 81B) and 4 cell lines from ATCC (FADU, SCC-25, SCC-15, KB). All HNSCC lines display low, variable expression patterns of FR. Compared to KB cells the expression of FBP-α in HNSCC cells is 1000-fold lower. Interestingly, already in 1995, Orr et al. [52] reported that UM-SCC-38 cells express limited amounts of FRα antigen, which does not bind folic acid or 5-methyltetrahydrofolic acid. A tissue microarray with tumor and tumor-free tissue from 22 patients with HNSCC demonstrated that SCC cells do not express FR [53]. FR was expressed in tissue samples obtained from nasopharyngeal and laryngeal carcinomas, but not in nasopharyngeal carcinoma cells [54]. Saba et al. [55] detected the FR in 45% of primary HNSCC and in 40% of corresponding lymph node metastases. These results emphasize that the KB cell line should not be regarded as a model for highly FR-positive oral cancer cells. Because of the confusing reports regarding the FR expression in oral cancer cells, alternative ligands could be explored, e.g., the epidermal growth factor receptor (EGFR), which is overexpressed on oral carcinomas [56]. Further studies are planned to evaluate liposomal phthalocyanine-mediated PDT against several OSCC cells (e.g., CAL27, FaDu, H357) and non-tumor gingival epithelial cells (GECs), and the effect of targeting liposomes encapsulating photosensitizers to oral cancer cells.
Conclusions
The present study shows that free and liposomal ZnPc and AlPc, in the range 0.1–1.0 μM, did not cause dark toxicity, the embedding of phthalocyanines in negatively charged POPC: PG (1: 1) liposomes enhance their phototoxicity, and that this effect is dependent on cell type. SCC HSC-3 cells were more sensitive to free and liposomal ZnPc, while HeLa cervical adenocarcinoma cells were more sensitive to AlPc. In HSC-3 cells, there was essentially no expression of folate receptor (FOLR1); therefore, alternative ligands should be explored for targeting OSCC cells. The possibility of using PDT selectively to stimulate phototoxicity in malignant oral epithelium remains. It is likely that targeted PDT will minimize the effects of the treatment on normal cells in the vicinity of the malignancy.
Acknowledgements
The authors thank Drs. Tomasz Goslinski and Jaroslaw Piskorz (Poznan University of Medical Sciences, Poland) for helpful discussions.
Footnotes
Source of support: This work was supported in part by a Research Pilot Project Award 03-Activity 095 (N. Düzgüneş, M. Yee and J. Young) from the Arthur A. Dugoni School of Dentistry
Conflict of interest statement
The authors do not have any conflict of interest related to the topic of this study.
References
- 1.Allison RR, Bagnato VS, Cuenca R, et al. The future of photodynamic therapy in oncology. Future Oncol. 2006;2:53–71. doi: 10.2217/14796694.2.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn Photodyn Ther. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Vohra F, Al-Kheraif AA, Qadri T, et al. Efficacy of photodynamic therapy in the management of oral premalignant lesions. A systematic review. Photodiagn Photodyn Ther. 2015;12:150–59. doi: 10.1016/j.pdpdt.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: A pilot study. J Can Res Ther. 2015;11:464–67. doi: 10.4103/0973-1482.147703. [DOI] [PubMed] [Google Scholar]
- 5.Allison RR, Cuenca RE, Downie GH, et al. Clinical photodynamic therapy of head and neck cancers – a review of applications and outcomes. Photodiagn Photodyn Ther. 2005;2:205–22. doi: 10.1016/S1572-1000(05)00092-X. [DOI] [PubMed] [Google Scholar]
- 6.Kvaal SI, Warloe T. Photodynamic treatment of oral lesions. J Environ Pathol Toxicol Oncol. 2007;26:127–33. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i2.70. [DOI] [PubMed] [Google Scholar]
- 7.Biel MA. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 2006;38:349–55. doi: 10.1002/lsm.20368. [DOI] [PubMed] [Google Scholar]
- 8.Wildeman MA, Nyst HJ, Karakullukcu B, et al. Photodynamic therapy in the therapy for recurrent/persistent nasopharyngeal cancer. Head Neck Oncol. 2009;1:40. doi: 10.1186/1758-3284-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini R, Poh CF. Photodynamic therapy: A review and its prospective role in the managements of oral potentially malignant disorders. Oral Dis. 2013;19:440–51. doi: 10.1111/odi.12003. [DOI] [PubMed] [Google Scholar]
- 10.Green B, Cobb ARM, Hopper C. Photodynamic therapy in the management of lesions of the head and neck. Br J Oral Maxillofac Surg. 2013;51:283–87. doi: 10.1016/j.bjoms.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 12.Huang YY, Sharma SK, Chung H, et al. Can nanotechnology potentiate photodynamic therapy? Nanotechnol Rev. 2012;1:111–46. doi: 10.1515/ntrev-2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skupin-Mrugalska P, Piskorz J, Goslinski T, et al. Current status of liposomal porphyrinoid photosensitizers. Drug Discov Today. 2013;18:776–84. doi: 10.1016/j.drudis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J Liposome Res. 2006;16:175–83. doi: 10.1080/08982100600848769. [DOI] [PubMed] [Google Scholar]
- 15.Stallivieri A, Baros F, Jetpisbayeva G, et al. The interest of folic acid in targeted photodynamic therapy. Curr Med Chem. 2015;22:3185–207. doi: 10.2174/0929867322666150729113912. [DOI] [PubMed] [Google Scholar]
- 16.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–93. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Vlahov IR, Leamon CP. Engineering folate-drug conjugates to target cancer: From chemistry to clinic. Bioconjug Chem. 2012;23:1357–69. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 18.Schneider R, Schmitt F, Frochot C, et al. Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorg Med Chem. 2005;13:2799–808. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Morosini V, Bastogne T, Frochot C, et al. Quantum dot-folic acid conjugates as potential photosensitizers in photodynamic therapy of cancer. Photochem Photobiol Sci. 2011;10:842–51. doi: 10.1039/c0pp00380h. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Li P, Lin H, et al. A novel chlorin-PEG-folate conjugate with higher water solubility, lower cytotoxicity, better tumor targeting and photodynamic activity. J Photochem Photobiol B. 2013;127:28–37. doi: 10.1016/j.jphotobiol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor mediated endocytosis. J Biol Chem. 1994;269:3198–204. [PubMed] [Google Scholar]
- 22.Qualls MM, Thompson DH. Chloroaluminum phthalocyanine tetrasulfonate delivered via acid-labile diplasmenylcholine-folate liposomes: Intracellular localization and synergistic phototoxicity. Int J Cancer. 2001;93:384–92. doi: 10.1002/ijc.1339. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Diaz M, Nonell S, Villanueva A, et al. Do folate-receptor targeted liposomal photosensitizers enhance photodynamic therapy selectivity? Biochim Biophys Acta. 2011;1808:1063–71. doi: 10.1016/j.bbamem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Moret F, Scheglmann D, Reddi E. Folate-targeted PEGylated liposomes improve the selectivity of PDT with meta-tetra(hydroxyphenyl)chlorin (m-THPC) Photochem Photobiol Sci. 2013;12:823–34. doi: 10.1039/c3pp25384h. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Xiang G, Zhang Y, et al. Increase of doxorubicin sensitivity for folate receptor positive cells when given as the prodrug N-(phenylacetyl)doxorubicin in combination with folate-conjugated PGA. J Pharm Sci. 2006;95:2266–75. doi: 10.1002/jps.20714. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Li L, Li P, et al. Apoptosis of HeLa cells induced by a new targeting photosensitizer-based PDT via a mitochondrial pathway and ER stress. OncoTargets Ther. 2015;8:703–11. doi: 10.2147/OTT.S76370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tapajos ECC, Longo JP, Simioni AR, et al. In vitro photodynamic therapy on human oral keratinocytes using chloroaluminum-phthalocyanine. Oral Oncol. 2008;44:1073–79. doi: 10.1016/j.oraloncology.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Jung H, Lim W, et al. Down-regulation of heat-shock protein 27-induced resistance to photodynamic therapy in oral cancer cells. J Oral Pathol Med. 2013;42:9–16. doi: 10.1111/j.1600-0714.2012.01155.x. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Vior MC, Marino J, Roguin LP, et al. Photodynamic effects of zinc(II) phthalocyanine-loaded polymeric micelles in human nasopharynx KB carcinoma cells. Photochem Photobiol. 2013;89:492–500. doi: 10.1111/j.1751-1097.2012.01229.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill ID. Continued misrepresentation of KB cells as being of oral cancer phenotype requires action. Oral Oncol. 2009;45:e117–18. doi: 10.1016/j.oraloncology.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Zen X, Wang Z, et al. Cell line cross-contamination: KB is not an oral squamous cell carcinoma cell line. Eur J Oral Sci. 2009;117:90–91. doi: 10.1111/j.1600-0722.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Horikoshi M, Rikimaru K, et al. A study of an in vitro model for invasion of oral squamous cell carcinoma. J Oral Pathol Med. 1989;18:498–501. doi: 10.1111/j.1600-0714.1989.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 33.Düzgüneş N, Nir S. Mechanisms and kinetics of liposome-cell interactions. Adv Drug Deliv Rev. 1999;40:3–18. doi: 10.1016/s0169-409x(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 34.Alonso L, Sampaio RN, Souza TFM, et al. Photodynamic evaluation of tetracarboxy-phthalocyanines in model systems. J Photochem Photobiol B. 2016;161:100–7. doi: 10.1016/j.jphotobiol.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Goslinski T, Osmalek T, Konopka K, et al. Photophysical properties and photocytotoxicity of novel phthalocyanines – potentially useful for their application in photodynamic therapy. Polyhedron. 2011;30:1538–46. [Google Scholar]
- 36.Konopka K, Pretzer E, Felgner PL, et al. Human immunodeficiency virus type-1 (HIV-1) infection increases the sensitivity of macrophages and THP-1 cells to cytotoxicity by cationic liposomes. Biochim Biophys Acta. 1996;1312:186–96. doi: 10.1016/0167-4889(96)00033-x. [DOI] [PubMed] [Google Scholar]
- 37.Nunes SMY, Sguilla FS, Tedesco AC. Photophysical studies of zinc phthalocyanine and chloroaluminum phthalocyanine incorporated into liposomes in the presence of additives. Braz J Med Biol Res. 2004;37:273–84. doi: 10.1590/s0100-879x2004000200016. [DOI] [PubMed] [Google Scholar]
- 38.Rousseau A, Badoual C. Head and neck: Squamous cell carcinoma: an overview. Atlas Genet Cytogenet Oncol Haematol. 2012;16:145–55. [Google Scholar]
- 39.Massano J, Regateiro FS, Januario G, et al. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Jeries W, Upile T, Akram S, et al. The surgical palliation of advanced head and neck cancer using photodynamic therapy. Clin Oncol. 2010;22:785–91. doi: 10.1016/j.clon.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Gartler SM. Genetic markers as tracers in cell culture. Natl Cancer Inst Monogr. 1967;26:167–95. [PubMed] [Google Scholar]
- 42.Wierzchowski M, Sobotta L, Skupin-Mrugalska P, et al. Phthalocyanines functionalized with 2-methyl-5-nitro-1H-imidazolylethoxy and 1,4,7-trioxanonyl moieties and the effect of metrinidazole substitution on phototoxicity. J Inorg Biochem. 2013;127:62–72. doi: 10.1016/j.jinorgbio.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Piskorz J, Konopka K, Düzgüneş N, et al. Diazepinoporphyrazines containing peripheral styryl substituents and their promising nanomolar photodynamic activity against oral cancer cells in liposomal formulations. Chem Med Chem. 2014;9:1775–82. doi: 10.1002/cmdc.201402085. [DOI] [PubMed] [Google Scholar]
- 44.Ketabchi A, MacRobert A, Speight PM, et al. Induction of apoptotic cell death by photodynamic therapy in human keratinocytes. Arch Oral Biol. 1998;43:143–49. doi: 10.1016/s0003-9969(97)00079-4. [DOI] [PubMed] [Google Scholar]
- 45.Matei C, Tampa M, Ion RM, et al. Photodynamic properties of aluminum sulphonated phthalocyanines in human dysplastic oral keratinocytes. Dig J Nanomater Bios. 2012;7:1535–47. [Google Scholar]
- 46.Matei C, Tampa M, Carantu C, et al. Protein microarray for complex apoptosis monitoring of dysplastic oral keratinocytes in experimental photodynamic therapy. Biol Res. 2014;47:33. doi: 10.1186/0717-6287-47-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu CS, Leung AWN. Photodynamic effects of pyropheophorbide – a methyl ester in nasopharyngeal carcinoma cells. Med Sci Monit. 2006;12:257–62. [PubMed] [Google Scholar]
- 48.Soriano J, Villanueva A, Stocker JC, et al. Regulated necrosis in HeLa cells induced by ZnPc photodynamic treatment: A new nuclear morphology. Int J Mol Sci. 2014;15:22772–85. doi: 10.3390/ijms151222772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ocakoglu K, Er O, Ersoz OA, et al. Evaluation of nuclear imaging potential and photodynamic therapy efficacy of symmetrical and asymmetrical zinc phthalocyanines. J Drug Deliver Sci Technology. 2016;33:164–69. [Google Scholar]
- 50.Ince M, Er O, Ocakoglu K, et al. Investigation of in vitro PDT activities and in vivo biopotential of Zn phthalocyanines using (131)I radioisotope. Chem Biol Drug Des. 2016;87:224–32. doi: 10.1111/cbdd.12659. [DOI] [PubMed] [Google Scholar]
- 51.Ward BB, Dunham T, Majors IJ, et al. Targeted dendrite chemotherapy in an animal model for head and neck squamous cell carcinoma. J Oral Maxillofac Surg. 2011;69:2452–59. doi: 10.1016/j.joms.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orr RB, Kaman BA. Identification of a point mutation in the folate receptor gene that confers a dominant negative phenotype. Cancer Res. 1995;55:847–52. [PubMed] [Google Scholar]
- 53.Sun JY, Shen J, Thibodeaux J, et al. In vivo optical imaging of folate receptor-β in head and neck squamous cell carcinoma. Laryngoscope. 2014;124:E312–19. doi: 10.1002/lary.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie M, Zhang H, Xu X, et al. Expression of folate receptors in nasopharyngeal and laryngeal carcinoma and folate receptor-mediated endocytosis by molecular targeted nanomedicine. Int J Nanomedicine. 2013;8:2443–51. doi: 10.2147/IJN.S46327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saba NF, Wang X, Müller S, et al. Examining expression of folate receptor in squamous cell carcinoma of the head and neck as a target for a novel nanotherapeutic drug. Head Neck. 2009;31:475–81. doi: 10.1002/hed.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ribeiro FAP, Noguti J, Oshima CTF, et al. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: A promising approach. Anticancer Res. 2014;34:1547–52. [PubMed] [Google Scholar]