Abstract
Background
Bilastine is a safe and effective commonly prescribed non-sedating H1-antihistamine approved for symptomatic treatment in patients with allergic disorders such as rhinoconjunctivitis and urticaria. It was evaluated in many patients throughout the clinical development required for its approval, but clinical trials generally exclude many patients who will benefit in everyday clinical practice (especially those with coexisting diseases and/or being treated with concomitant drugs). Following its introduction into clinical practice, the Medical Information Specialists at Faes Farma have received many practical queries regarding the optimal use of bilastine in different circumstances.
Data sources and methods
Queries received by the Medical Information Department and the responses provided to senders of these queries.
Results
The most frequent questions received by the Medical Information Department included the potential for drug-drug interactions with bilastine and commonly used agents such as anticoagulants (including the novel oral anticoagulants), antiretrovirals, antituberculosis regimens, corticosteroids, digoxin, oral contraceptives, and proton pump inhibitors. Most of these medicines are not usually allowed in clinical trials, and so advice needs to be based upon the pharmacological profiles of the drugs involved and expert opinion. The pharmacokinetic profile of bilastine appears favourable since it undergoes negligible metabolism and is almost exclusively eliminated via renal excretion, and it neither induces nor inhibits the activity of several isoenzymes from the CYP 450 system. Consequently, bilastine does not interact with cytochrome metabolic pathways. Other queries involved specific patient groups such as subjects with renal impairment, women who are breastfeeding or who are trying to become pregnant, and patients with other concomitant diseases. Interestingly, several questions related to topics that are well covered in the Summary of Product Characteristics (SmPC), which suggests that this resource is not being well used.
Conclusions
Overall, this analysis highlights gaps in our knowledge regarding the optimal use of bilastine. Expert opinion based upon an understanding of the science can help in the decision-making, but more research is needed to provide evidence-based answers in certain circumstances.
Keywords: antihistamines, bilastine, drug information, drug interactions, medical information services, pregnancy, renal disease
Introduction
A key function of the Medical Information Specialist in a pharmaceutical company is to respond to queries from patients, healthcare providers (doctors, nurses and pharmacists) and researchers regarding the medicinal products that are in their portfolio. They need to provide accurate and concise information based upon a balance of best evidence from the literature, practical experience from everyday usage, and experience and expert opinion relating to the scientific properties (both pharmacodynamic and pharmacokinetic) of the molecule in question. To perform this role the Medical Information Specialist requires a strong scientific/clinical background, a deep understanding of the product and the ability to use computer-based technologies that interface with internal and external databases that store much of the product information. Traditionally, responses to queries have been over the phone or by e-mail [1,2].
In this review, we present details of real-world enquiries for the non-sedating antihistamine bilastine received by Medical Information Specialist staff at Faes Farma (Spain) from General Practitioners (GPs), specialists, pharmacists (retail and hospital), and the medical departments of pharmaceutical companies that cross-license the product. In addition, the responses (with supporting data) to these queries are provided, and they highlight the practical nature of the services provided by the Medical Information Department. It should be noted that some queries relate to unapproved uses and/or doses of bilastine in some countries. The fact that the Medical Information Specialist responds to these queries based on the best available evidence should not be taken as an endorsement of such usage by Faes Farma (Spain).
Drug-drug interactions
Interactions between different drugs have the potential to interfere with the clinical activity and/or safety of the agents involved through changes in their pharmacodynamic or pharmacokinetic characteristics [3]. Enquiries concerning potential drug-drug interactions are one of the most common reasons for contacting Medical Information Specialists. The elderly are particularly prone to experiencing drug-drug interactions because of the high prescription rate in this population, and as a result of age-related changes in renal function, hepatic function, nutritional status and body weight; as well as individual variation and changes in pharmacokinetics/pharmacodynamics [4,5].
Can bilastine be administered with…?
The Medical Information Specialists at Faes Farma have fielded a number of queries relating to potential drug interactions involving bilastine and these are summarised in Table 1. An area of concern relates to drugs with a narrow therapeutic index such as anticoagulants (including the novel oral anticoagulants), digoxin, and antiretroviral and antituberculosis regimens.
Table 1.
Queries relating to potential drug interactions involving bilastine fielded by the Medical Information Specialists at Faes Farma.
| Drug | Potential interaction | Advice | |
|---|---|---|---|
| Conventional anticoagulants | |||
| Acenocoumarol (ACN) | Interaction mainly via CYP 450; through displacement of ACN from its strong binding (98.7%) to plasma proteins; or through a reduction in vitamin K bioavailability. | BIL does not inhibit or induce CYP 450. No information is available regarding effects on plasma binding or vitamin K bioavailability. | SmPC for ACN notes that there is only a small risk of clinically significant DIs, but caution is always advised. The SmPC for BIL does not specify any risk of an interaction. |
| Novel Oral Anticoagulants (NOACs) and Antiplatelet Drugs | |||
| Dabigatran (DAB) | DAB is a substrate for the efflux P-gp transporter, which can be inhibited/induced by concomitant drug therapy. | BIL does not inhibit or induce P-gp. Like digoxin it acts as a P-gp substrate. | As for digoxin it is not expected that BIL will be associated with any relevant changes in exposure to DAB. |
| Rivaroxaban (RIV) | RIV is metabolised via CYP 3A4 or P-gp pathways, and inhibition of these is expected to increase RIV plasma levels. | BIL is not metabolised and does not inhibit or induce CYP 450. | It is not expected that BIL will be associated with any relevant changes in exposure to RIV. |
| Apixaban (API) | API is metabolised via CYP 3A4 or P-gp pathways, and inhibition of these is expected to increase API plasma levels. | The SmPC recommends that API should not be coadministered with strong inhibitors of CYP 3A4 or P-gp, such as azole antimycotics and HIV protease inhibitors. Strong inducers of CYP 3A4 or P-gp should be coadministered with caution. | It is not expected that BIL will be associated with any relevant changes in exposure to API. |
| Clopidogrel (CLO) | CLO is metabolised via CYP 2C19 to its active metabolite. | Whilst the clinical significance of this interaction is uncertain, concomitant treatment with moderate to strong inhibitors of CYP 2C19 should be avoided in patients receiving CLO. | Since BIL does not affect CYP 450 pathways it will be associated with any relevant changes in exposure to CLO. |
| Drugs used in the treatment of asthma or rhinitis | |||
| Chlorphenamine (CHL) | CHL is a first-generation sedating antihistamine, and the SmPC has a specific warning that it should not be used with other antihistamines. | There is no scientific evidence to support combining CHL and BIL, and if greater antihistamine effects are required (e.g. urticaria), then increasing the dose of BIL is recommended. | |
| Steroids | Corticosteroids undergo very rapid metabolism in the liver. | BIL does not inhibit or induce CYP 450. | There are no apparent reasons to preclude the coadministration of BIL and corticosteroids if deemed appropriate by the physician. |
| Other drugs | |||
| Digoxin (DIG) | DIG is a cardiac glycoside used in the treatment of heart failure, and it has a narrow therapeutic window. It is a substrate for P-gp, the membrane-bound transporter enzyme. Drugs which inhibit P-gp will decrease the renal tubular elimination of DIG. | BIL is also a substrate for P-gp, but it does not inhibit its action. There is no scientific rationale why BIL would affect the bioavailability of DIG. | In the absence of clinical data, caution should be exercised when coadministering BIL and DIG, but the probability of an interaction seems low. |
| Antituberculosis drugs | Classically, drugs such as rifampicin, isoniazid, pyrazinamide, and ethambutol have been used in different combinations as first-line therapy. Second-and third-line treatments include aminoglycosides, quinolones, rifabutin and others. Many of these agents induce the efflux P-gp transporter and/or are eliminated via renal pathways. | The potential for these drugs to reduce the elimination of BIL and increase plasma levels cannot be ruled out. | Since pharmacokinetic data for BIL in combination with antitubercular drugs is not currently available, the doctor needs to carefully assess the overall risk-benefit if such treatment is being considered. |
| Antiretrovirals | There are a large number of available antiretroviral drugs, and they are used in different combinations. They have a narrow therapeutic window, and DIs may be important. | If metabolism is via CYP 450, then BIL will not usually have an effect. If combined with a P-gp inhibitor, there may be an increase in the bioavailability of bilastine, which is generally not clinically significant, but caution should be exercised in patients with renal impairment. | To make an informed choice based on a risk-benefit assessment, the doctor needs to know the precise antiretroviral regimen the patient is receiving. BIL should not be administered when they have renal impairment and are receiving a drug which is a P-gp inhibitor. |
| Proton pump inhibitors (PPIs) | PPIs are inhibitors of CYP 450, and this explains many of their drug-drug interactions. Furthermore, PPIs also inhibit P-gp, but this effect does not appear to be clinically relevant. | BIL is not metabolised and is unlikely to be affected by concomitant PPI therapy. | No interaction between BIL and PPIs is anticipated. |
| Oral contraceptives (OCs) | OCs are metabolised by CYP 450, and this explains many of their drug-drug interactions and the potential risk of an unwanted pregnancy. In addition, OCs inhibit CYP 450, and this might interfere with the metabolism of other drugs. | BIL does not inhibit or induce CYP 450. Furthermore, BIL is not metabolised, and so OCs cannot interfere with their elimination. | No interaction between BIL and OCs is anticipated. Women included in the clinical trials’ programme were required to use an effective contraceptive method, including OCs, and no interactions were observed. |
Abbreviations: ACN = acenocoumarol; API = apixaban; BIL = Bilastine; CHL = chlorphenamine; CLO = clopidogrel; CYP 450 = cytochrome 450; DAB = dabigatran; DI = drug interactions; DIG = digoxin; NOACs = Novel Oral Anticoagulants; OCs = oral contraceptives; PPIs = proton pump inhibitors; P-gp = p-glycoprotein; RIV = rivaroxaban; SmPC = summary of product characteristics.
Whilst direct experience regarding the concomitant use of many agents with bilastine is lacking, there are data available that can help us make an informed decision regarding the risk-benefit balance of certain drug combinations. In particular, the pharmacokinetic profile of bilastine appears favourable since it undergoes negligible metabolism and is almost exclusively eliminated via renal excretion. Bilastine neither induces nor inhibits the in vitro activity of several isoenzymes from CYP 450 system [6–8]. Consequently, bilastine does not interact with cytochrome metabolic pathways, and this explains the positive response given to the question, ‘Can bilastine be administered with drugs such as acenocoumarol, rivaroxaban, apixaban, clopidogrel, corticosteroids, proton pump inhibitors, and oral contraceptives?’ (Table 1). Like digoxin, bilastine is a substrate for the P-glycoprotein (P-gp) efflux transporter, and so it has the potential to interact with drugs that are eliminated via this pathway such as rivaroxaban, apixaban, digoxin, and a number of drugs that are used to treat tuberculosis (Table 1). However, since it does not inhibit the activity of the P-gp transporter, the risks with bilastine are considered to be minimal [6].
Pregnancy
For many pregnant women, pharmacotherapy is necessary to provide symptom control. Although medications are potentially hazardous, suboptimal treatment of the mother might be more harmful to the unborn child [9,10]. Any drug taken during the pregnancy poses a potential teratogenic risk to the foetus. For ethical reasons, product labelling for antihistamines generally states that they should be avoided during pregnancy due to a lack of foetal safety data [11,12]. However, in the USA, between 2001 and 2006, almost half of all pregnancies were unintended, and this could lead to a period of foetal exposure to antihistamines before the pregnancy is confirmed, and therefore greater awareness of the overall risk to the developing foetus is important [13].
Can bilastine be used during pregnancy?
At this point in time, we do not have sufficient information to make an informed decision about the safety of bilastine during pregnancy. Antihistamines are commonly used for the treatment of a number of common ailments in women of childbearing age with the most frequent being allergic rhinitis (AR) and urticaria. Indeed, it has been estimated that up to 20%–30% of women experience AR and 4%–7% suffer from asthma during pregnancy which, along with some dermatological complaints, make them some of the most common groups of medical conditions that complicate pregnancy [9,14,15]. These illnesses might affect the well-being and quality of life of the mother since they can be highly debilitating, presenting with a range of symptoms such as nasal discharge, itching and nasal blockage or congestion in patients with AR and an array of dermatological complaints in patients with urticaria.
The safety of antihistamines has not been fully established during pregnancy and they should only be used if, in the doctor’s assessment, the benefits outweigh the risks. Pregnancy labelling recommendations by the FDA are currently being revised. The old system used pregnancy safety categories A, B, C, D, and X (Table 2), which the FDA now consider confusing. These will be discontinued, and a new narrative-based system incorporating summaries of the risks of a drug during pregnancy and discussions of the data supporting the summaries will be required. The FDA believes that these changes in labelling will provide more meaningful information for clinicians [16]. Guidance from the EMA (European Medicines Agency) requires that all available knowledge both clinical and non-clinical should be taken into account when making recommendations for drug usage in pregnant or lactating women, and in women of childbearing potential. This should include an integrated evaluation of non-clinical and clinical data, which includes consideration of non-clinical pharmacological and pharmacokinetic properties of the medicinal product, as well as results from non-clinical toxicity studies and of clinical experience/knowledge about compounds within the same class [17].
Table 2.
FDA pregnancy categories [www.fda.gov].
| Category | Interpretation |
|---|---|
| A | Controlled studies show no risk. Adequate, well-controlled studies in pregnant woman have failed to demonstrate a risk to the foetus in any trimester of pregnancy. |
| B | No evidence of risk in humans. Adequate, well-controlled studies in pregnant woman have not shown in creased risk of metal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of metal harm is remote, but remains a possibility. |
| C | Risk cannot be ruled out. Adequate, well-controlled human studies are lacking, and animal studies have shown a risk to the foetus or are lacking as well. There is a chance of metal harm if the drug is administered during pregnancy, but the potential benefits may outweigh the potential risk. |
| D | Positive evidence of risk. Studies in humans, or investigational or postmarking data, have demonstrated metal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. |
| X | Contraindicated in pregnancy. Studies in animal or human, or investigational or postmarketing reports, have demonstrated positive evidence of metal abnormalities or risk that clearly outweighs any possible benefit to the patient. |
Psychomotor performance
First-generation H1-antihistamines have a much greater sedative effect than the second-generation H1-antihistamines, and this explains their negative impact on psychomotor performance [18]. This appears to relate to the interaction between second-generation H1-antihistamines and the P-gp transporter, which is expressed in the blood-brain barrier and acts as an efflux pump. The net result is decreased concentrations of second-generation H1-antihistamines in the brain which minimises possible central nervous system effects such as sedation.
Can bilastine be used by pilots and air traffic control specialists?
According to US Federal Aviation Administration (FAA) advice to pilots, some people are under the impression that if a drug is safe enough to be bought over the counter, without a prescription, then it must also be safe enough to pilot an aircraft whilst under its influence [19]. Statistics show that about 80% of all major aircraft accidents, both military and civilian, usually involve human factors [20–22].
For Air Traffic Control Specialists (ATCSs), therapeutic drug guidelines are available, and the recommendations for antihistamines are [20] ‘Antihistamines: Older, sedating type antihistamines (e.g., chlorpheniramine [chlorphenamine; Chlor-Trimeton®, Teldrin®], diphenhydramine [Benadryl®]) and the newer, but still sedating drugs like cetirizine (Zyrtec®), are not acceptable. The newer, non-sedating antihistamines (e.g., fexofenadine [Allegra®], loratadine [Claritin®], desloratadine [Clarinex®]) including decongestant combinations, are acceptable for use by working ATCSs after review by the Regional Flight Surgeon confirming the absence of adverse side effects during a brief trial of the drug. The condition must not adversely affect the ability of the ATCS to perform safely.’
In the case of pilots, and since the effects of a particular drug can be intensified with altitude, it is important that the individual is aware of the effects that any medication might have. Despite the recognised concerns with sedating first-generation antihistamines (e.g. diphenhydramine, chlorphenamine), evidence of inappropriate use was found in 4%–11% of pilot fatalities recorded in civil aviation accidents between 1990 and 2005. Moreover, the use of antihistamines, with or without other drugs and/or alcohol, was considered to be the principal cause in 13 cases, and as a concurrent factor in another 50 cases, out of 338 aviation accidents. Indeed, diphenhydramine is the most common drug found in pilots who have died in aviation accidents [21,22].
If the pilot has any concerns about a particular drug then they should consult their local Aviation Medical Examiner (AME) [19]. Importantly, the US FAA accepts the use of the second-generation non-sedating antihistamines such as loratadine, desloratadine and fexofenadine by airline pilots. The study of antihistamines in airline pilots is facilitated by using altitude chambers (such as the TNO altitude chamber at The Royal Netherlands Center for Man and Aviation facility) which are valuable training devices, providing aviators with the opportunity to experience many of the hazards of high-altitude flight whilst in a controlled and safe environment. Altitude chambers allow study participants to experience unpressurised flight conditions: gas expansion, rapid decompression, hypoxia, and the use of oxygen equipment.
In a study by Valk and colleagues, a single dose of desloratadine 5 mg had no detrimental effects, over a 6-hour period (Stanford Sleepiness Scale 1, 2, 3, 5 and 6 hours after treatment), on sleepiness and performance of tasks associated with flying ability under conditions of simulated cabin pressure (8000 feet [2500 metres]; hypobaric chamber 564 mmHg [75 kPa]) [23]. In contrast, patients receiving the first-generation H1-antihistamine diphenhydramine 50 mg showed significant sleepiness at each time point from 1 hour up to, and including, 5 hours [23].
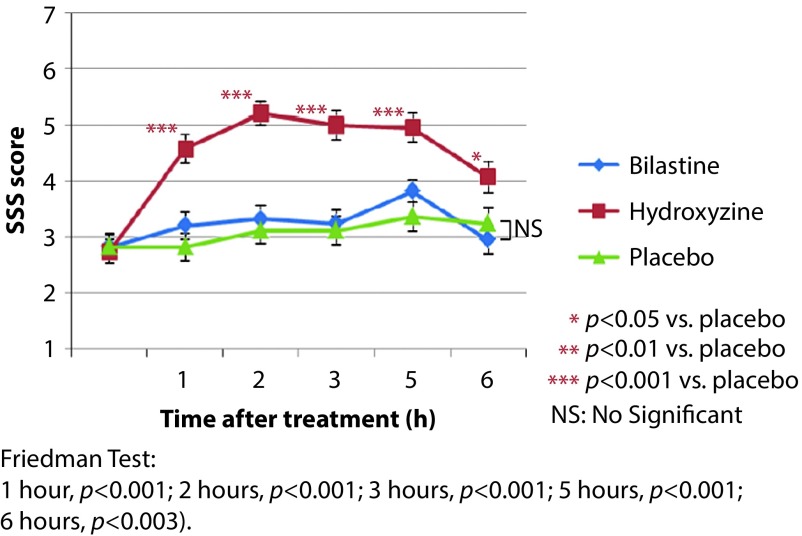
With respect to bilastine, a recent study (BISCAT), conducted under the supervision of Pierre Valk (TNO, Soesterberg, the Netherlands), evaluated the effects of a single dose of 20 mg on flying ability in healthy male volunteers under conditions of simulated cabin pressure. This was a phase IV, single-centre, randomised, double-blind, placebo-controlled crossover study using single doses of bilastine 20 mg, hydroxyzine 50 mg (as a positive control) and placebo. Between treatment phases, individuals were subjected to a washout period of at least 1 week [24]. Under simulated cabin altitude, subjects were evaluated using the following standard assessment tools: Vigilance and Tracking Task (VigTrack), measuring vigilance and tracking performance (analogous to in-flight tasks undertaken by fighter pilots), and the Multi-Attribute Task (MAT) battery, measuring ability to perform multiple tasks simultaneously (similar to in-flight activities that aircraft crew members have to perform), and the Stanford Sleepiness Scale, measuring subjective sleepiness [24]. For all three assessment tools, the effect of bilastine was similar to that of placebo over the entire 6-hour study period. In contrast, hydroxyzine significantly impaired the ability of subjects to perform each of the evaluative tasks and resulted in a significantly greater increase in sleepiness scores (Figure 1) [24].
Figure 1.
BISCAT (Bilastine in Simulated Cabin Altitude Test) study: Stanford Sleepiness Scale (SSS) scores.
As is the case for all second-generation antihistamines, it is prudent to advise patients for whom psychomotor performance is critical to undertake a trial of treatment to establish their individual response to bilastine prior to performing such tasks. It is also worth informing them that rarely some individuals may experience drowsiness which may have a detrimental effect on their psychomotor skills.
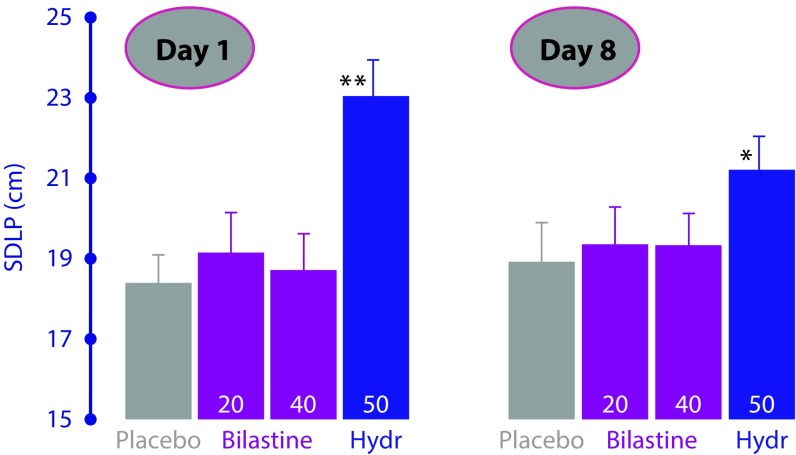
The same considerations apply to the use of bilastine in individuals performing other tasks requiring psychomotor skills such as driving a car. The ‘real-life’ effect of bilastine on driving performance was assessed in an on-the-road driving test in a double-blind, four-way crossover study in 22 healthy volunteers who randomly received the recommended dose of bilastine (20 mg) or double the recommended dose (40 mg) once-daily, hydroxyzine 50 mg (active control) or placebo for 8 consecutive days [25]. The primary efficacy variable, SDLP (standard deviation of lateral position) in the road-tracking test, was assessed on the first and eighth day of each treatment period. There were no significant differences between bilastine 20 mg or double the recommended dosage (40 mg) and placebo in terms of SDLP values on days 1 and 8 (Figure 2). In contrast, despite a reduction in the degree of driving impairment by the eighth day of treatment, SDLP was significantly increased with hydroxyzine compared with placebo on both evaluation days, demonstrating a lack of tolerance over time to its sedative effects. Importantly, there were no effects on driving performance after single and repeated doses of bilastine, thus suggesting its safe use in traffic at doses up to 40 mg [26].
Figure 2.
Effects on driving performance, assessed by mean standard deviation of lateral position (SDLP) on days 1 and 8 of daily bilastine 20 mg or 40 mg, hydroxyzine 50 mg (active control) or placebo in 22 healthy volunteers.
*p<0.01; **p<0.001 vs placebo. Data taken from Conen et al. [25].
In a positron emission tomography (PET) study in healthy volunteers, the brain histamine-1 receptor occupancy of bilastine (after a 20 mg dose) was significantly lower than that of hydroxyzine (25 mg) in all five regions of the cerebral cortex [27]. Bilastine was not associated with subjective sedation or objective impairment of psychomotor performance. This demonstrates the low potential for bilastine to penetrate the blood-brain barrier. Thus, bilastine has satisfied relevant pharmacological, clinical (subjective and objective) and PET criteria to be considered as a reliable non-sedating antihistamine [27].
Patients with renal disorders
Can bilastine be used in patients who have had a kidney transplant?
Cyclosporine, which is used in immunosuppressive regimens in kidney-transplant patients, can cause deterioration in renal function, and it has been shown to interact with drugs metabolised via the CYP P450 system [28]. This is not a concern for bilastine which is not metabolised and does not show any interaction with CYP 450. Cyclosporine is also a potent P-gp inhibitor, and for this reason, it might increase a drug’s plasma concentration when drug elimination is P-gp mediated.
Overall, the use of bilastine in patients with allergic rhinoconjunctivitis or urticaria undergoing organ or tissue transplantation and treated with an immunosuppressant such as cyclosporine would only be recommended in individuals with normal or mildly impaired renal function and who are being closely monitored. The bilastine summary of product characteristics (SmPC) advises that the drug should not be used in patients treated with cyclosporine with moderate to severe renal impairment [29].
Can bilastine be used in patients on renal dialysis?
The extent to which a drug is affected by dialysis is determined primarily by its physicochemical characteristics such as molecular size and water solubility, and its pharmacokinetic properties such as absorption, plasma clearance, protein binding and volume of distribution. In addition to these properties of the drug, technical aspects of the dialysis procedure may also determine the extent to which a drug is removed by dialysis [30]. Unfortunately, few in vivo studies have been published, and the pharmacokinetic behaviour of only a small number of drugs has been investigated in dialysis patients. Therefore, many guidelines for drug dosing during continuous renal replacement therapy (CRRT) are extrapolated from experiences with chronic haemodialysis or from theoretical considerations [30]. The Dialysis of Drugs guidelines includes an accompanying table which is a reference regarding the effect of dialysis on drug clearance. The table includes antihistamines such as cetirizine, desloratadine and fexofenadine, but as bilastine is a new drug, there are no specific studies or recommendations. We consider its physicochemical characteristics to be similar to those of fexofenadine, and therefore the dialysability of bilastine might be similar to that of fexofenadine, although there are no data on dialysed patients being administered bilastine. The Dialysis of Drugs guidelines 2013 recommendations [30] for fexofenadine are conventional haemodialysis: NO/NS, NO indicates that dialysis does not have a clinically important effect on plasma clearance and supplemental dosing is not usually required / NS indicates that the type of membrane was not specified; high permeability haemodialysis: ND indicates that no data exist on drug dialysability with type of dialysis; peritoneal dialysis: U indicates significant drug removal is unlikely based on physicochemical characteristics of the drug such as protein binding, molecular size, or volume of distribution.
Can bilastine be used in patients with urinary retention/dysuria?
Some antihistamines, mainly first-generation products, may cause urinary retention in patients with benign prostatic hypertrophy (BPH). This is due to an interaction with muscarinic receptors and as a result of poor receptor selectivity. Bilastine has not been studied in a subgroup of subjects with a diagnosis of BPH with urinary retention and/or dysuria. Nevertheless, based on its receptor-selectivity profile, which was clearly established in preclinical studies, and the results from which are included in the SmPC section 5.1 (pharmacological properties) ‘without affinity for muscarinic receptors’, it seems reasonable to think that bilastine will not increase the risk of urinary retention/dysuria [29].
Different patient groups
Can bilastine be used by patients with acute urticaria?
Bilastine is approved for the ‘symptomatic treatment of urticaria’ including acute urticaria, and current guidelines for the treatment of acute and chronic urticaria recommend non-sedating H1-antihistamines such as bilastine [31].
Is bilastine useful in patients with ‘pruritus’?
Pruritus is a frequent dermatologic problem that increases in incidence with age. In some patients, the condition may be so severe that it affects sleep and quality of life. The most common cause of itching is skin disease, but it may also be an important dermatologic clue to the presence of underlying systemic causes such as renal disease, cholestasis, myeloproliferative disorders and hyperthyroidism, neuropathic causes, and psychogenic causes [32]. A single mechanism cannot explain all causes of pruritus, and whilst histamine has classically been associated with the disorder, with the exception of allergic conditions, histamine must be considered only one of several chemical mediators of itch [33].
Management of pruritus should be directed at the underlying cause. Antihistamines are beneficial in the treatment of allergic causes of pruritus such as urticaria and have classically been used in other types of itching, especially the older first-generation products. However, other than providing night-time sedation to assist sleep, first-generation antihistamines are not uniformly effective in treating all causes of pruritus, and they may be associated with adverse effects related to sedation with daytime drowsiness and anticholinergic properties, particularly in elderly patients [32]. Pruritus or itch is the most common dermatological complaint amongst patients aged over 65 years. It generally results from dry skin, peripheral vasoconstriction, endocrine disorders, vitamin A deficiency and/or drug therapy [34]. Treatment is based upon general measures including emollient creams, and drug therapy with calcineurin inhibitors, capsaicin inhibitors, serotonin-reuptake inhibitors, gabapentin, or antihistamines. Recently, in Japan, bilastine has also been approved for pruritus associated with several skin diseases such as dermatitis/eczema and cutaneous pruritus. This approval was based on clinical trials results showing that bilastine produced an improvement from baseline in symptoms and QoL, with a good safety profile [35].
Can bilastine be used in obese patients?
Throughout the clinical development, there was no impact of weight on the pharmacological properties of bilastine. For example, during Phase I studies, no association between pharmacokinetic parameters and body weight were observed [7,29]. Consequently, there is no reason to believe that the efficacy and/or safety of bilastine will vary in patients with different levels of obesity.
Can bilastine be used by coeliac patients?
The tablets manufactured by Faes Farma (Spain) do not contain gluten and are therefore suitable for coeliac patients [29]. However, different companies in other countries manufacture bilastine tablets, and we cannot comment about these products in terms of whether they contain gluten or not.
Can bilastine be used by patients with ocular hypertension or glaucoma?
Bilastine has not been specifically studied in patients with glaucoma or ocular hypertension, but based on its pharmacological profile and receptor selectivity there are no specific contraindications to its use in such patients [29]. Specifically, bilastine 10 μM did not exhibit significant affinity (<10% displacement) in recombinant human muscarinic receptor binding studies [36]. Likewise, at concentrations up to 100 μM, bilastine did not antagonise acetylcholine-induced concentrations in isolated guinea pig ileum preparations [36]. In C6 cell cultures (rat glioma) which stably expressed M1–M5 muscarinic receptors, bilastine 100 μM did not inhibit acetylcholine-mediated intracellular calcium increase induced by stimulation of all muscarinic receptors [37]. Thus, on the basis of the available evidence, bilastine should not affect ocular pressure, but carefully monitoring such patients is clinically advisable.
Can bilastine be used by patients with atopic dermatitis?
Second-generation non-sedating antihistamines generally have only a weak effect in patients with atopic dermatitis [38]. Patients with concomitant urticaria, asthma, rhinitis, or allergic conjunctivitis may exhibit greater benefit because of the associated pathology.
What do we know about the use of bilastine in geriatric patients?
The safety and efficacy of bilastine have been demonstrated in a limited number of subjects >65 years of age (n=34) enrolled in controlled clinical studies. A small open-label study provided safety data for 150 patients aged ≥65 years with urticaria and/or allergic rhinoconjunctivitis treated with bilastine 20 mg once daily for 3 months [39]. The incidence of adverse events for bilastine in elderly patients is similar to findings in younger patients and demonstrates a favourable safety profile with a low incidence of treatment-emergent adverse events (TEAEs). These data concord with results from previous studies and are within the incidence range reported in the approved SmPC for bilastine [29]. The pharmacokinetic parameters of bilastine are comparable in healthy subjects >65 years of age and younger subjects, and no dosage adjustments are considered necessary in this patient population.
Can antihistamines such as bilastine be legitimately used by professional sportspersons?
Yes. None of the antihistamines, including bilastine, have ever been on the prohibited list of drugs on the official website of the World Anti-doping Agency (WADA) as a nonapproved drug or as a masking agent [40].
General queries
Can bilastine be taken with fruit juice?
Fruit juices, especially grapefruit juice, have been shown to reduce the bioavailability of some drugs by inhibiting OAT (organic anion transporter) P1A2-mediated uptake transport [41]. The flavanone naringin was the main causal component for the interaction, suggesting that other flavonoids in fruits and vegetables might also produce this effect. The duration of the inhibition lasted between 2 and 4 hours, indicating the interaction was avoidable, with an appropriate interval of time between juice and drug consumption [41]. The plasma bilastine Cmax, AUC(0-t), and AUC(0-inf) values were approximately 33%, 24% and 24% lower, respectively, for subjects receiving bilastine 20 mg concomitantly with grapefruit, compared to bilastine alone [42]. The in vitro inhibitory effects of bilastine on 12 human transporters were investigated and no clinically relevant changes were recorded [43]. Furthermore, the transport of bilastine by multidrug resistance protein 1 (MDR1), breast-cancer resistance protein (BCRP), OAT1, OAT3, and organic cation transporter2 (OCT2) was also investigated in vitro, and only MDR1 active transport of bilastine was considered potentially relevant. Finally, bilastine did not appear to be a substrate for BRCP, OCT2, OAT1 or OAT3, and it was concluded that clinically relevant drug-drug interactions resulting from inhibition of these drug transporters by bilastine would be unlikely. Thus, based on in vitro data, the only clinically relevant interaction between bilastine and membrane-transporter systems is with MDR1, and this would be avoided if bilastine were taken 2 hours before or 1 hour after fruit juices or food [43].
How long do we have to wait to perform a skin-prick test on a patient previously treated with bilastine?
Based on the pharmacokinetic properties of bilastine, it is recommended that a 5-day washout period is allowed between the last dose of bilastine and performing a skin-prick allergy test.
How quickly does bilastine start to act?
Based upon findings from a Vienna Challenge Chamber Study, the onset of action of bilastine was approximately 1 hour after drug administration. This was based upon the first statistically significant (p<0.05) reduction in total nasal symptom score after drug application [44]. In a recent study evaluating suppression of wheal-and-flare response induced by intradermal histamine in healthy volunteers, the greatest inhibition in wheal area was produced by bilastine, and it was significantly superior to desloratadine and rupatadine from 1 to 12 hours (p<0.001). Bilastine was also significantly superior to desloratadine and rupatadine for flare inhibition throughout the study, with an onset of action at 30 minutes and significantly reduced itching scores, as compared to placebo, 1 hour after administration [45].
What is the maximum duration of therapy for bilastine?
Generally speaking, bilastine should be discontinued once symptoms have resolved, and it should be recommenced if symptoms reappear. However, in perennial allergic rhinitis, continued treatment is advisable during the period of allergen exposure. In clinical studies to date, the longest period of treatment has been 12 months in patients with perennial allergic rhinitis [46]. In this open-phase study, following a controlled comparison of bilastine with cetirizine 10 mg and placebo, bilastine was well tolerated. On the basis of these findings, it seems reasonable to think that bilastine should be administered for as long as symptoms persist.
Can bilastine be administered by nasogastric tube?
Whilst no data are available for the enteral administration of bilastine, there is no reason to think that safety would be an issue, since doses up to 11 times the recommended dose have been used without negative consequences. If bioavailability is compromised for any reason, then the drug may be less effective when administered by the enteral route.
What do we know about photosensitivity reactions to bilastine?
Photosensitivity or photoallergic reactions are acquired, immunologically mediated reactions to a drug or chemical, initiated by the formation of photoproducts when that drug or chemical is exposed to UVA/UVB or visible light. Drug products that do not absorb between 290 and 700 nm of the electromagnetic spectrum will not be photoactivated, that is, they cannot be direct photochemical photosensitisers [47]. The ultraviolet/visible radiation-absorption spectrum for bilastine is 253 nm, which is below the minimum of the range for which photosafety testing is recommended. Therefore, photosafety testing for bilastine has not been performed, as photoallergy is highly improbable.
Can bilastine be used prophylactically before allergen exposure may be expected?
The approved SmPC states that bilastine is indicated for the treatment of symptoms associated with allergic rhinoconjunctivitis and urticaria, and it is not approved for prophylactic management of these diseases [29].
Can the dosage of bilastine be increased in patients with chronic urticaria?
The approved dosage of bilastine in the SmPC is 20 mg once daily [29]. However, consensus reports have suggested that doses of non-sedating H1-antihistamines may be increased two- to four-fold the normal therapeutic dose in patients with urticaria [31]. The Phase III clinical study performed to evaluate the efficacy of bilastine in the symptomatic treatment of urticaria exclusively used the approved 20 mg dosage [48]. Bilastine 20 mg, 40 mg or 80 mg once daily for 7 days was effective in reducing critical temperature thresholds in 19 of 20 patients with cold contact urticaria (CCU) [49]. The increased efficacy of bilastine with four-fold up-dosing was without sedation and supports urticaria treatment guidelines. Additionally, during the clinical development programme for bilastine, supratherapeutic doses have been studied. In Phase I studies, doses up to about ten-fold higher (in single-dose and multiple-dose studies) were administered to assess pharmacokinetics, tolerability and safety [7]. No clinically significant trends in ECG, vital signs or physical examination findings were observed. In another study, five-fold higher doses (100 mg), as well as 20 mg in combination with ketoconazole, were evaluated in a ‘Thorough QT/QTc’ study performed to assess the cardiac safety of the bilastine [50,51]. Doses of bilastine up to four times the therapeutic dose (80 mg) were found to be well tolerated in CNS safety studies. The results of these studies support the fact that bilastine at therapeutic and supratherapeutic doses, and even when combined with ketoconazole, is safe from a cardiac point of view. Furthermore, bilastine 20 mg–40 mg did not cause psychomotor impairment or affect driving ability [25,26]. In addition, throughout the clinical development programme, the adverse events profile of bilastine has not differed from that of placebo [29]. Should the physician, after considering the risk-benefit ratio, decide to follow the proposed ‘Up-Dosing Guidelines’ [49], bilastine should be considered as a possible candidate because of its excellent safety profile.
Does bilastine have a negative impact on the environment?
The world is trying to become more and more eco-friendly, and questions about environmental impact are being asked more frequently. Indeed, the EMA now requires an Environmental Risk Assessment (ERA) for all novel medicines for human use. In line with this requirement, the predicted environmental concentration of bilastine in surface water was determined, as well as its effect on aquatic systems. No environmental concerns were observed in this study, and bilastine can be considered eco-friendly [52].
Conclusions
The pharmacological and clinical profile of bilastine has been extensively researched, and it has proven to be a safe and effective non-sedating H1-antihistamine in patients with allergic disorders such as rhinoconjunctivitis and urticaria [7,8]. Whilst large numbers of patients have been treated with bilastine during the clinical trials’ programme, it is important to note that these studies included well-defined groups of patients treated under well-controlled conditions. In everyday life, patients do not always reflect the strict inclusion criteria used in the clinical trials’ programme, since they may have coexisting conditions and/or be taking concomitant medications. This is particularly likely for a drug such as bilastine which is approved for use in common allergic diseases involving large numbers of patients. The most frequently encountered queries fielded by the Medical Information Specialists included the potential for bilastine to be used in patients currently being treated with drugs such as anticoagulants, antiretrovirals, antituberculosis regimens, corticosteroids, digoxin, oral contraceptives, and proton pump inhibitors. Most of these medicines were not allowed in patients included in the clinical trials’ programme, and so advice needs to be based upon the pharmacological profiles of the drugs involved and expert opinion. Similar considerations apply to specific patient groups such as subjects with renal impairment and women who are breastfeeding or who are trying to become pregnant. Some questions related to topics such as indication, duration of treatment and prophylactic usage, which are well covered in the SmPC, suggests that this resource is not being used well in everyday practice. Overall, this analysis of questions received by Medical Information Specialists at Faes Farma highlights gaps in our knowledge regarding the optimal use of bilastine. Expert opinion based on an understanding of the science can help in the decision-making process, but more research is needed to provide evidence-based answers in some circumstances.
Acknowledgements
We thank Steve Clissold, PhD, Content Ed Net, for editorial assistance in the preparation of this manuscript; funding for editorial assistance was provided by Faes Farma S.A.
Abbreviations
- AME
Aviation Medical Examiner
- AR
allergic rhinitis
- ATCSs
air traffic control specialists
- BCRP
breast-cancer resistance protein
- BPH
benign prostatic hypertrophy
- CCU
cold contact urticaria
- CRRT
continuous renal replacement therapy
- EMA
European Medicines Agency
- ERA
Environmental Risk Assessment
- FAA
Federal Aviation Administration
- GPs
general practitioners
- MAT
multi-attribute task
- MDR1
multidrug resistance protein 1
- OAT
organic anion transporter
- OCT2
organic cation transporter2
- P-gp
p-glycoprotein
- PET
positron emission tomography
- SDLP
standard deviation of lateral position
- SmPC
summary of product characteristics
- TEAEs
treatment-emergent adverse events
- WADA
World Anti-doping Agency
Footnotes
Disclosure and potential conflicts of interest: All authors are employees of Faes Farma SA, 48940-Leioa, Bizkaia, Spain. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: http://www.drugsincontext.com/wp-content/uploads/2017/02/dic.212500-COI.pdf.
Contributions: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors contributed equally to the study design, the collection, analysis and interpretation of data and the writing of the manuscript.
Funding declaration: This research was supported by FAES FARMA SA.
Correct attribution: Copyright © 2017 Leceta A, Sologuren A, Valiente R, Campo C, Labeaga L. https://doi.org/10.7573/dic.212500. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Provenance: Submitted, two set of peer review comments, Editor-in-Chief evaluation and acceptance
Peer review evaluation: 23 December 2016
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 14 Weller Street, London, SE1 1QU, UK
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009
For all manuscript and submissions enquiries, contact Dr Gordon Mallarkey, Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact Peter Clarke peter.clarke@bioexcelpublishing.com
References
- 1.Fierro L, Savulich D, Fraser C. Historical perspective of technologies used in medical communications, past, present, and future. Drug Inf J. 2009;43:705–12. http://dx.doi.org/10.1177/009286150904300608. [Google Scholar]
- 2.Barbary K, Holmes J, Hermes-DeSantis E. Evaluation of the evolution of the pharmaceutical industry based medical information dissemination to healthcare professionals. [cited December 2015]. Available from: www.pharmafellows.rutgers.edu.
- 3.Stockley IH. In: Stockley’s drug interactions. 10th ed. Baxter K, Preston C, editors. London: Pharmaceutical Press; 2013. [Google Scholar]
- 4.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370:185–91. doi: 10.1016/S0140-6736(07)61092-7. http://dx.doi.org/10.1016/S0140-6736(07)61092-7. [DOI] [PubMed] [Google Scholar]
- 5.Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M, Bates DW. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–16. doi: 10.1001/jama.289.9.1107. http://dx.doi.org/10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 6.Lucero ML, Gonzalo A, Mumford R, Betanzos M, Alejandro A. An overview of bilastine metabolism during preclinical investigations. Drug Chem Toxicol. 2012;35(Suppl 1):18–24. doi: 10.3109/01480545.2012.682651. http://dx.doi.org/10.3109/01480545.2012.682651. [DOI] [PubMed] [Google Scholar]
- 7.Church MK. Safety and efficacy of bilastine: a new H(1)-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin Drug Saf. 2011;10:779–93. doi: 10.1517/14740338.2011.604029. http://dx.doi.org/10.1517/14740338.2011.604029. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Ansótegui I, Canonica GW, Zuberbier T, Baena-Cagnani CE, Bachert C, Cruz AA, González SN, Kuna P, Morais-Almeida M, Mullol J, Ryan DP, Sánchez-Borges M, Valiente R, Church MK. Establishing the place in therapy of bilastine in the treatment of allergic rhinitis according to ARIA: evidence review. Curr Med Res Opin. 2012;28:131–39. doi: 10.1185/03007995.2011.648263. http://dx.doi.org/10.1185/03007995.2011.648263. [DOI] [PubMed] [Google Scholar]
- 9.Keleş N. Treatment of allergic rhinitis during pregnancy. Am J Rhinol. 2004;18:23–28. [PubMed] [Google Scholar]
- 10.Vlastarakos PV, Manolopoulos L, Ferekidis E, Antsaklis A, Nikolopoulos TP. Treating common problems of the nose and throat in pregnancy: what is safe? Eur Arch Otorhinolaryngol. 2008;265:499–508. doi: 10.1007/s00405-008-0601-4. http://dx.doi.org/10.1007/s00405-008-0601-4. [DOI] [PubMed] [Google Scholar]
- 11.Mazzotta P, Loebstein R, Koren G. Treating allergic rhinitis in pregnancy. Safety considerations. Drug Saf. 1999;20:361–75. doi: 10.2165/00002018-199920040-00005. http://dx.doi.org/10.2165/00002018-199920040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Etwel F, Hutson JR, Madadi P, Gareri J, Koren G. Fetal and perinatal exposure to drugs and chemicals: novel biomarkers of risk. Annu Rev Pharmacol Toxicol. 2014;54:295–315. doi: 10.1146/annurev-pharmtox-011613-135930. http://dx.doi.org/10.1146/annurev-pharmtox-011613-135930. [DOI] [PubMed] [Google Scholar]
- 13.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–85. doi: 10.1016/j.contraception.2011.07.013. http://dx.doi.org/10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg L, Olsen J, S⊘rensen HT. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. Eur J Clin Pharmacol. 1999;55:139–44. doi: 10.1007/s002280050608. http://dx.doi.org/10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 15.Choi JS, Han JY, Kim MY, Velázquez-Armenta EY, Nava-Ocampo AA. Pregnancy outcomes in women using inhaled fluticasone during pregnancy: a case series. Allergol Immunopathol (Madr) 2007;35:239–42. doi: 10.1157/13112989. http://dx.doi.org/10.1157/13112989. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) Reviewer guidance: evaluating the risks of drug exposure in human pregnancies. [cited December 2015]. Available from: www.fda.gov.
- 17.EMA Committee for Medicinal Products for Human Use. Guideline on Risk Assessment of medicinal products on human reproduction and lactation from data to labelling. [cited December 2015]. Available from: www.ema.europa.eu.
- 18.Hu Y, Sieck DE, Hsu WH. Why are second-generation H1-antihistamines minimally sedating? Eur J Pharmacol. 2015;765:100–6. doi: 10.1016/j.ejphar.2015.08.016. http://dx.doi.org/10.1016/j.ejphar.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Federal Aviation Administration. Introduction to Aviation Physiology. [last accessed December 2015]. Available from: www.faa.gov/pilots/training/airman_education/media/IntroAviationPhys.pdf.
- 20.Pilot Medical Solutions. Air Traffic Control Specialists (ATCS) [cited October 2016]. Available from: https://www.leftseat.com/atc/atcsmeds.htm.
- 21.Sen A, Akin A, Craft KJ, Canfield DV, Chaturvedi AK. Federal Aviation Administration, Office of Aerospace Medicine; 2007: Report No. DOT/FAA/AM-07/12. First-generation H1 antihistamines found in pilot fatalities of Civil Aviation Accidents, 1990–2005. [PubMed] [Google Scholar]
- 22.Canfield DV, Dubowski KM, Chaturvedi AK, Whinnery JE. Federal Aviation Administration, Office of Aerospace Medicine; 2012: Report No. DOT/FAA/AM-11/13. Drugs and alcohol in Civil Aviation Accident pilot fatalities from 2004–2008. [Google Scholar]
- 23.Valk PJ, Van Roon DB, Simons RM, Rikken G. Desloratadine shows no effect on performance during 6 h at 8,000 ft simulated cabin altitude. Aviat Space Environ Med. 2004;75:433–8. [PubMed] [Google Scholar]
- 24.Valk PJL, Simons R, Jetten AM, Valiente R, Labeaga L. Cognitive performance effects of bilastine 20 mg during 6 hours at 8000 ft cabin altitude. Aerosp Med Hum Perform. 2016;87:622–7. doi: 10.3357/AMHP.4522.2016. http://dx.doi.org/10.3357/AMHP.4522.2016. [DOI] [PubMed] [Google Scholar]
- 25.Conen S, Theunissen EL, Van Oers AC, Valiente R, Ramaekers JG. Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. J Psychopharmacol. 2011;25:1517–23. doi: 10.1177/0269881110382467. http://dx.doi.org/10.1177/0269881110382467. [DOI] [PubMed] [Google Scholar]
- 26.Jáuregui I, Ramaekers JG, Yanai K, Farré M, Redondo E, Valiente R, Labeaga L. Bilastine: a new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Expert Opin Drug Saf. 2016;15:89–98. doi: 10.1517/14740338.2016.1112786. http://dx.doi.org/10.1517/14740338.2016.1112786. [DOI] [PubMed] [Google Scholar]
- 27.Farré M, Pérez-Mañá C, Papaseit E, Menoyo E, Pérez M, Martin S, Bullich S, Rojas S, Herance JR, Trampal C, Labeaga L, Valiente R. Bilastine vs. Hydroxyzine: Occupation of Brain Histamine H1 Receptors Evaluated by Positron Emission Tomography in Healthy Volunteers. Br J Clin Pharmacol. 2014;78:970–80. doi: 10.1111/bcp.12421. http://dx.doi.org/10.1111/bcp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandimmun Neoral (cyclosporine) SmPC. [cited November 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sandimmun_30/WC500158912.pdf.
- 29.Bilaxten SmPC. Approved Summary of Product Characteristics Date of revision of the text November 2010. [last accessed November 2016]. Bilastine 20 mg tablets. Marketing authorisation holder FAES FARMA. [Google Scholar]
- 30.Dialysis of Drugs NPA 2013. [cited November 2016]. Available from: www.homedialyzorsunited.org.
- 31.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF, Gimenez-Arnau A, Godse K, Gonçalo M, Grattan C, Hébert J, Hide M, Kaplan A, Kapp A, Abdul Latiff AH, Mathelier-Fusade P, Metz M, Nast A, Saini SS, Sánchez-Borges M, Schmid-Grendelmeier P, Simons FE, Staubach P, Sussman G, Toubi E, Vena GA, Wedi B, Zhu XJ, Maurer M. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–87. doi: 10.1111/all.12313. http://dx.doi.org/10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 32.Yosipovitch G, Bernhard JD. Chronic pruritus. NEJM. 2013;368:1625–34. doi: 10.1056/NEJMcp1208814. http://dx.doi.org/10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 33.Green D, Dong X. The cell biology of acute itch. J Cell Biol. 2016;213:155–61. doi: 10.1083/jcb.201603042. http://dx.doi.org/10.1083/jcb.201603042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward JR, Bernhard JD. Willan’s itch and other causes of pruritus in the elderly. Int J Dermatol. 2005;44:267–73. doi: 10.1111/j.1365-4632.2004.02553.x. http://dx.doi.org/10.1111/j.1365-4632.2004.02553.x. [DOI] [PubMed] [Google Scholar]
- 35.Bilanoa SmPC. Bilastine 20 mg tablets. Marketing authorization holder TAIHO PHARMACEUTICAL Co. Ltd. (Japan) Approved Summary of Product Characteristics, September 2016. [last accessed December 2016]. Available in https://ss.pmda.go.jp/en_all/search.x?q=bilanoa&x=0&y=0&ie=UTF-8&page=1.
- 36.Corcóstegui R, Labeaga L, Innerárity A, Berisa A, Orjales A. Preclinical pharmacology of bilastine, a new selective histamine H1 receptor antagonist: receptor selectivity and in vitro antihistaminic activity. Drugs R D. 2005;6:371–84. doi: 10.2165/00126839-200506060-00005. http://dx.doi.org/10.2165/00126839-200506060-00005. [DOI] [PubMed] [Google Scholar]
- 37.Wolff SC, Brubaker K, Navratil T, Fulcher EH, Lankford JR, Boyer JL. Evaluation of muscarinic receptor antagonism by antihistamines. XXVI Congress of the European Academy of Allergology and Clinical Immunology. Göteborg (Sweden) June 9–13, 2007. Abstract 365. Allergy. 2007;62(Suppl 83):138. doi: 10.1111/j.1398-9995.2007.01405.x. http://dx.doi.org/10.1111/j.1398-9995.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 38.Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, Gieler U, Lipozencic J, Luger T, Oranje AP, Schäfer T, Schwennesen T, Seidenari S, Simon D, Ständer S, Stingl G, Szalai S, Szepietowski JC, Taïeb A, Werfel T, Wollenberg A, Darsow U. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26(8):1045–60. doi: 10.1111/j.1468-3083.2012.04635.x. http://dx.doi.org/10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 39.Sologuren A, Viñas R, Cordón E, Elisabeth S, Forés MM, Senán MR. [Postmarketing study to assess the safety profile of bilastine 20 mg in elderly patients with rhinoconjunctivitis and/or urticaria]. Simposio Internacional Sociedad española de alergología e inmunología clínica (SEAIC), Sevilla (Spain), October 22–24, 2015. J Investig Allergol Clin Immunol. 2015;25(Suppl 1):65. [Abstract] [Google Scholar]
- 40.World Anti-doping Agency (WADA) The prohibited list. [cited December 2015]. Available from: www.wada-ama.org.
- 41.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70:645–55. doi: 10.1111/j.1365-2125.2010.03722.x. http://dx.doi.org/10.1111/j.1365-2125.2010.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crean C, Valiente R, Sologuren A, McLaverty D. Effect of grapefruit juice on the pharmacokinetics of bilastine. 36th Annual Meeting of the American College of Clinical Pharmacology; September 9–11, 2007; San Francisco, USA. J Clin Pharmacol. 2007;47(9):1198. Abstract 71. [Google Scholar]
- 43.Lucero ML, Gonzalo A, Ganza A, Leal N, Soengas I, Ioja E, Gedey S, Jahic M, Bednarczyk D. Interactions of bilastine, a new oral H1 antihistamine, with human transporter systems. Drug Chem Toxicol. 2012;35(Suppl 1):8–17. doi: 10.3109/01480545.2012.682653. http://dx.doi.org/10.3109/01480545.2012.682653. [DOI] [PubMed] [Google Scholar]
- 44.Horak F, Zieglmayer P, Zieglmayer R, Lemell P. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm Res. 2010;59:391–8. doi: 10.1007/s00011-009-0117-4. http://dx.doi.org/10.1007/s00011-009-0117-4. [DOI] [PubMed] [Google Scholar]
- 45.Antonijoan R, Coimbra J, García-Gea C, Puntes M, Gich I, Campo C, Valiente R, Labeaga L. Comparative efficacy of bilastine, desloratadine and rupatadine in the suppression of wheal and flare response induced by intradermal histamine in healthy volunteers. Curr Med Res Opin. 2017;33:129–36. doi: 10.1080/03007995.2016.1240665. http://dx.doi.org/10.1080/03007995.2016.1240665. [DOI] [PubMed] [Google Scholar]
- 46.Sastre J, Mullol J, Valero A, Valiente R on behalf of Bilastine Study Group. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo in the treatment of perennial allergic rhinitis. Curr Med Res Opin. 2012;28:121–30. doi: 10.1185/03007995.2011.640667. http://dx.doi.org/10.1185/03007995.2011.640667. [DOI] [PubMed] [Google Scholar]
- 47.Pharmacology and Toxicology. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); May, 2003. Guidance for Industry, Photosafety Testing. [Google Scholar]
- 48.Zuberbier T, Oanta A, Bogacka E, Medina I, Wesel F, Uhl P, Antépara I, Jáuregui I, Valiente R Bilastine International Working Group. Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo-controlled study. Allergy. 2010;65:516–28. doi: 10.1111/j.1398-9995.2009.02217.x. http://dx.doi.org/10.1111/j.1398-9995.2009.02217.x. [DOI] [PubMed] [Google Scholar]
- 49.Krause K, Spohr A, Zuberbier T, Church MK, Maurer M. Up-dosing with bilastine results in improved effectiveness in cold contact urticaria. Allergy. 2013;68:921–8. doi: 10.1111/all.12171. http://dx.doi.org/10.1111/all.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyl B, Kabbaj M, Azzam S, Sologuren A, Valiente R, Reinbolt E, Roupe K, Blanco N, Wheeler W. Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. J Clin Pharmacol. 2012;52:893–903. doi: 10.1177/0091270011407191. http://dx.doi.org/10.1177/0091270011407191. [DOI] [PubMed] [Google Scholar]
- 51.ICH Harmonized Tripartite Guideline E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. 2005. [last accessed December 2015]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/Step4/E14_Guideline.pdf.
- 52.Lucero ML, Peither A, Ledo F. Bilastine: an environmental risk assessment. Drug Chem Toxicol. 2015;38:460–8. doi: 10.3109/01480545.2014.992438. http://dx.doi.org/10.3109/01480545.2014.992438. [DOI] [PubMed] [Google Scholar]