Abstract
Heart failure with preserved ejection fraction (HFpEF) is the most common form of heart failure (HF) in older adults, particularly women, and is increasing in prevalence as the population ages. With morbidity and mortality on par with HF with reduced ejection fraction, it remains a most challenging clinical syndrome for the practicing clinician and basic research scientist. Originally considered to be predominantly caused by diastolic dysfunction, more recent insights indicate that HFpEF in older persons is typified by a broad range of cardiac and non-cardiac abnormalities and reduced reserve capacity in multiple organ systems. The globally reduced reserve capacity is driven by: 1) inherent age-related changes; 2) multiple, concomitant co-morbidities; 3) HFpEF itself, which is likely a systemic disorder. These insights help explain why: 1) comorbidities are among the strongest predictors of outcomes; 2) approximately 50% of clinical events in HFpEF patients are non-cardiovascular; 3) clinical drug trials in HFpEF have been negative on their primary outcomes. Embracing HFpEF as a true geriatric syndrome, with complex, multi-factorial pathophysiology and clinical heterogeneity could provide new mechanistic insights and opportunities for progress in management.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is nearly exclusively found in older persons, particularly older women, in whom 90% of new HF cases are HFpEF [1]. The prevalence of HFpEF is rising, with morbidity, mortality, and healthcare costs now equal to HF with reduced ejection fraction (HFrEF) [2–5]. Outcomes following hospitalization for decompensated HFpEF are poor, with about 1/3 of patients rehospitalized or dead within 90 days of discharge [6]. Its pathophysiology is poorly understood, and no medication trials have had positive effect on their primary endpoints. Consequently, there are no class A guideline recommendations for improving clinical outcomes in patients with HFpEF [7]. This syndrome has proven challenging partly due to its association with many common co-morbidities and marked heterogeneity in presentation. The co-morbidities including diabetes mellitus (DM), atherosclerosis, renal dysfunction, chronic obstructive lung disease(COPD), anemia, sarcopenia, obesity, etc., significantly influence cardiovascular structure and function and global organ system reserve as well as long-term prognosis. In this review, we address translational and clinical research into HFpEF, providing an overview of HFpEF for both the clinical and basic research scientist, including epidemiology and pathophysiology. We discuss potential mechanisms involving the heart and other organs, including left ventricular systolic, diastolic and chronotropic reserve, stiffening of the ventricles and vasculature, low nitric oxide bioavailability and protein kinase G (PKG) activity, altered myocardial energetics, neurohormonal activation and autonomic imbalance, arterial vasodilatory dysfunction, and abnormal skeletal muscle mass, quality, composition, and function. A review of current and potential novel treatments is discussed in relation to evolving key concepts.
Definition of HFpEF
The controversy surrounding HFpEF has, in part, been attributable to varying definitions employed to define the syndrome. This syndrome was historically considered to be caused exclusively by left ventricular (LV) diastolic dysfunction. In 2000, the Framingham study suggested specific criteria for definite, probable, and possible diastolic HF [8]. All 3 categories required definitive evidence of HF and a normal LVEF. Objective evidence of diastolic dysfunction (ie, abnormal LV relaxation, filling, or distensibility indexes measured during cardiac catheterization) was recommended for the diagnosis of definite but not for the diagnosis of probable or possible diastolic HF [8].This classification was criticized for a lack of sensitivity due to the requirement of determination of EF within 72 h of presentation and invasive demonstration of LV diastolic dysfunction—a situation which can be challenging to achieve clinically. Further, Gandhi and colleagues reported stable EF upon immediate echo assessment of acutely decompensated HFpEF patients, refuting the suggestion that transient systolic dysfunction or ischemia mediates symptoms [9]. In 2001, data from Zile et al., supported the idea that almost all patients with HFpEF have Doppler evidence of diastolic dysfunction making these parameters unnecessary because of lack of specificity and sensitivity for identifying HFpEF and they concluded that objective evidence of abnormal LV relaxation, filling, or distensibility is not necessary to make the diagnosis of diastolic HF [10]. The need for invasive demonstration of LV diastolic abnormalities was also questioned, because these were shown to be uniformly present in patients with clinical HF and a normal EF [11].
Importantly, most measures used to assess diastolic function (echocardiographic or radionuclide techniques or invasive measurements) do not assess the key passive component of diastole, have significant variability, and have considerable overlap with normal aging, hypertension, and other comorbidities common in HFpEF and thus lack specificity for HFpEF. Furthermore, using direct invasive measurements, Kawaguchi et al show that during exercise, patients with HFpEF were able to increase preload volume with little if any effect on the ventricular end-diastolic pressure-volume relation, despite a substantial prolongation of time constant of relaxation [12]. Such a finding is supported by data from multiple sources indicating that even in well-characterized, symptomatic HFpEF, many patients do not have echo-Doppler indexes of diastolic dysfunction that differ greatly from that expected based on age and comorbidities [13;14]. These findings suggested that abnormalities of intrinsic diastolic function may not always be present during or completely explain the occurrence of HFpEF [15].
In acknowledgement of these considerations, as well as data supporting a broader paradigm for HFpEF pathophysiology and outcomes, diagnostic criteria for HFpEF have evolved and the most recent U.S. guideline has not included the requirement for ‘diastolic dysfunction’ or any specific cardiac parameter, such as LV hypertrophy or left atrial (LA) dilation, significantly elevated B-type natriuretic peptide (BNP). Instead, the 2013 American College of Cardiology/American Heart Association (ACC/AHA) HF management guideline takes a practical, phenomenological approach to HFpEF. It states that the diagnosis of HFpEF is based on: 1) typical symptoms and signs of HF; 2) normal or near normal LVEF; 3) no other obvious factors to account for the apparent HF symptoms, including significant valvular abnormalities [16]. In contrast, the older proposed European Society of Cardiology (ESC) recommendations suggested also requiring the presence of LV diastolic dysfunction and / or increased BNP for the diagnosis of HFpEF, along with symptoms and signs of HF and normal or mildly abnormal LV function [17]. However in support of the more recent 2013 ACC/AHA guideline, studies of patients with all the clinical hallmarks of HF and an EF>50% showed that many patients have modest diastolic dysfunction under resting conditions [18;19]. Furthermore, similar changes can be seen in elderly patients with hypertensive heart disease with no clinical HF, and diastolic dysfunction in HFpEF patients may not be greater than age-matched sedentary controls and has not prevent a fruitful target for intervention [10;15;20–22]. Overall, these newer guidelines may facilitate progress in understanding the pathogenesis and optimal therapy of the large population of elderly patients in the community who have HF symptoms and preserved EF in that they do not assume a specific mechanism of their disorder, and thereby allow for a phenomenological, iterative approach based on actual observations in humans rather than theory. However, objective evidence of cardiac dysfunction including diastolic dysfunction, elevated natriuretic peptides, and structural evidence of cardiovascular abnormalities including LV hypertrophy and / or increased LA size can be helpful to support a diagnosis of typical HFpEF, but need not be mandatory unless one is seeking a specific subgroup with fortified risk, such as for a clinical event trial. Even in that event, the results of such a trial would need to be interpreted in regard to generalizability to the overall HFpEF population.
A strategy that has been proposed but not well explored is utilizing formal cardiopulmonary exercise testing to support the diagnosis of HFpEF, with the potential advantage of objectively confirming that the patient has an exercise limitation compared to that expected for age and gender, and providing potential insight into the mechanisms of exercise limitation [23].
Key Knowledge Gaps:
Do these existing guidelines have a similar sensitivity in patients who have increased filling pressures only during exercise but not at rest?
What will be the effect of phenotypic diversity of HFpEF on these guidelines?
What is the impact of important co-morbidities on diagnostic thresholds?
What does formal cardiopulmonary exercise testing add for diagnosis and management of HFpEF patients?
Epidemiology of HFpEF
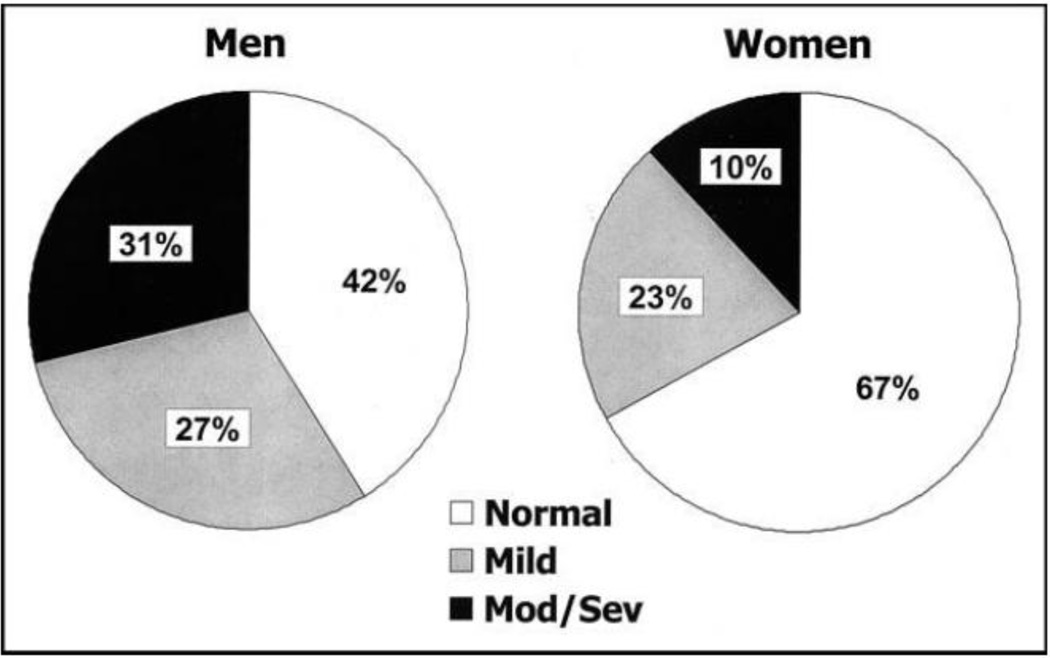
HFpEF is the predominant form of HF in older adults, increasing in prevalence as the population ages. The annual incidence of HF in both men and women doubles with every decade after age 65, and the prevalence increases from less than 0.5% in the age group of 20–39 years to more than 10% in those 80 years and older [24]. In westernized countries, HFpEF patients are older, predominantly female (Figure 1) [25], and with a high prevalence of hypertension, DM, and atrial fibrillation (AF) [4–6;16;25–27]. In some studies, mortality in HFpEF is similar to HFrEF, with less than 50% five-year survival in community HFpEF cohorts [6;28]. The pooled death rate in HFpEF was 121[95% confidence interval:117, 126] deaths per 1000 patient-years in a meta-analysis of 31 studies [29]. Outcomes following hospitalization for decompensated HFpEF are poor with about 1/3 of patients rehospitalized or dead within 90 days of discharge [6].Non-cardiovascular hospital readmissions and mortality are more frequent in HFpEF than in HFrEF and the number of co-morbidities correlate with increased all-cause hospitalization and mortality [30].
Figure 1.
Systolic function by gender among participants with congestive heart failure
Mild = mildly reduced systolic function; Mod-Sev = moderately to severely reduced systolic function.
Pathophysiological considerations in HFpEF
Cardiac aging
Recent studies have defined aging as an important factor in the HFpEF epidemic [31;32]. Aging may contribute independently to deterioration of diastolic function [33–38;38–40]. Specific alterations in structural and function in aging, such as ventriculararterial stiffening, vascular dysfunction, impaired [Ca2+]i regulation, decreased β-adrenergic reserve, and physical deconditioning [35–40;40–43], have been identified as important contributing causes for HFpEF. As observed by Borlaug et al, LV stiffness increases with normal aging, despite excellent control of blood pressure and reductions in LV mass [44]. Many of these processes are linked to greater adiposity, and weight gain is associated with increases in LV diastolic stiffness, even after adjustment for changes in arterial afterload [45]. Aging leads to β-adrenoceptor (AR) desensitization. Although aging may has no effects on resting heart rate, contractility, or cardiac output (CO), it blunts the capacity to enhance heart rate, systolic function, and CO in response to β-AR stimulation and exercise. Aging is also associated with impaired endothelium-dependent vasodilatation [46;47]. As described by Borlaug, et al, in HFpEF, these combined limitations are exaggerated above and beyond what is seen in normal aging [48]. In this section, we briefly review age-related contributors to HFpEF, an area of paramount importance.
Age related determinants of diastolic dysfunction
LV diastolic stiffness
Cardiac aging-induced oxidative stress and mitochondrial damage are responsible for triggering the increased cardiomyocyte death including necrosis, apoptosis and autophagy. This process induces initially a compensatory remodeling characterized by the alterations of extracellular matrix composition involving the synthesis of myofibroblasts, the degradation of collagen through transforming growth factor β signaling. Aging also produces shift in the balance between the matrix metalloproteinases (MMPs) and the tissue inhibitors of matrix metalloproteinases (TIMPs), ultimately leading to increased matrix accumulation [49–51;51–54]. These alterations lead to an increase in remaining myocyte size and in myocardial thickness [37;55], with consequent reactive fibrosis that increases cardiac stiffness and reduces the cardiac compliance [56].
An emerging theory for the pathogenesis of HFpEF proposes that a systemic proinflammatory state produced by aging, causes coronary microvascular endothelial inflammation. The inflamed coronary microvascular endothelial cells, as evidenced by the upregulated expression of endothelial adhesion molecules [57–59] produce reactive oxygen species (ROS), which in turn may produce nitric oxide (NO) synthase uncoupling and decrease the bioavailability of NO [60]. In this construct, low NO bioavailability and ROS lead to decreased cyclic guanosine monophosphate and PKG activity, and perhaps localized microdomains of potentially ischemic tissue. Thus, this inflammation ultimately results in increased interstitial fibrosis and cardiomyocytes stiffness that contributes to high diastolic LV stiffness and HF development [57,13].
Cardiac aging is also associated with mitochondrial dysfunction, as evidenced by increased mitochondrial protein oxidation, and decreased level of function of the components of the mitochondrial apparatus [61–63;63;64]. Efficiency of macroautophagy or mitophagy, the process that eliminates dysfunctional mitochondria, progressively declines in cardiomyocytes during aging [65;66]. Furthermore, locally elevated levels of angiotensin II are observed in aging hearts, this in turn binds to the angiotensin receptor-1, which has been shown to stimulate the NOX4 isoform of NADPH oxidase on the mitochondrial membrane [67;68]. ROS generated by NADPH oxidase may set off a ROS-mediated- ROS generation propagating a vicious cycle of oxidative stress damaging mitochondrial components further exacerbating the above cycle and ultimately leads to fibrosis [69;70].
Active diastolic relaxation
Aging plays a fundamental role in modifying the active diastolic relaxation properties of the myocytes and causing the delayed ventricular relaxation due to impaired Ca2+ cycling/handling [38;39;71;72]. Recently, in multiple separate studies it has been shown that the increased oxidative stress observed in senescent myocardium leads to oxidative damage of the sarcoplasmic reticulum Ca2+ ATPase (SERCA) pump, thus decreasing its Ca2+-sequestering activity and prolonging diastolic relaxation. [73–75]
Chronotropic incompetence
Aging is associated with increased sympathetic nervous system activity characterized by elevated plasma norepinephrine and epinephrine circulating levels, due to increased spillover from tissues (including the heart) and reduced plasma clearance of catecholamine, but a diminished positive inotropic response to β-AR stimulation [76–78]. Both animal and human studies of aging indicate a decline in heart rate, cardiac contractility, CO and EF in response to β-AR stimulation and exercise [79–81]. With aging, cardiac β1-ARs are down-regulated, and β1- and β2-ARs are desensitized from GS, reducing the increment in myocardial contraction and response to adrenergic-induced stressors. Loss of positive inotropic response to catecholamine in cardiac β-AR is proposed to cause progression and clinical abnormalities in cardiac aging, [38;71;82–86] although the mechanism responsible is unclear. In aging, the blunted responses to β-AR adrenergic stimulation also contribute to the impaired force-frequency relationship. The abnormal LV arterial coupling and diastolic dysfunction present at rest may be exacerbated during exercise.
Other factors in cardiac aging
One of these may be deposition of amyloid proteins such as wide-type transthyretin (wtTTR). The wtTTR deposition has a clear association with sex and aging and is almost exclusively seen in men aged 65 and older [87–89]. As aging is associated with increased oxidative stress, oxidative modification of wtTTR may account for the association of the senile systemic amyloidosis with age. Senile amyloidosis due to deposition of wtTTR in the myocardial interstitium and intramural coronary vessels is associated with LV wall thickening, diastolic dysfunction, and HFpEF. Additional data showed that, in a U.S. community based sample, approximately 30% of subjects aged 75 and older with HFpEF had cardiac deposits of wtTTR [90]. Recent autopsy data of HF hearts with an EF>40% at time of diagnosis found overall increased prevalence of wtTTR deposition in the LV and/or intramural coronary vessels compared to control subjects and was associated with more fibrosis [91].
MicroRNAs (miRNAs) are endogenous small noncoding RNAs, 20–23- nucleotides in length, which have emerged as important post-translational regulators of numerous cardiovascular processes, from myocardial infarction to cardiac aging [92;93]. More recently, exciting studies have revealed that miRNA-34a has been implicated in cardiac aging and might have an important role in cardiac aging via effects on apoptosis, DNA damage, and telomere shortening [94]. It was demonstrated that, in HFrEF, microRNA-21 (miR-21) could inhibit the apoptosis of cardiac fibroblasts, leading to cardiac hypertrophy and myocardial fibrosis, but the role of miR-21 in HFpEF remains unknown. Recent study suggested that miR-21 promoted the development of HFpEF by up-regulating the expression of anti-apoptotic gene Bcl-2 and thereby suppressing the apoptosis of cardiac fibrosis [95].
LV structure, remodeling and diastolic dysfunction in HFpEF
Significant LV hypertrophy was previously thought to be a uniform characteristic of HFpEF. However, some HFpEF patients have concentric remodeling without hypertrophy, or even normal LV geometry [96–98]. Furthermore, Maurer and colleagues found no significantly increased LV mass in older HFpEF patients compared to controls with hypertension but not HF [99]. In addition, Solomon et al found that only 8% of patients with well-defined, symptomatic HFpEF had significant LV hypertrophy [100]. Thus, contrary to prior assumptions, LV hypertrophy may not be a unique or fundamental feature of HFpEF.
At the structural level, both increased myocyte diameter and myofibrillar density have been observed with increased collagen content in HFpEF [13;101]. However, the magnitude of increase in fibrosis in HFpEF patients appears modest at most [102], its role in HFpEF pathophysiology is uncertain, and agents known to reduce fibrosis have not improved outcomes in HFpEF patients [103–105].
HFpEF patients who have functional abnormalities in diastole can have prolonged rate of LV pressure decay during isovolumic relaxation, [11;12;106;107] impaired mitral annular longitudinal motion, impaired LV ‘untwisting’ that occurs during early diastole and increased passive diastolic stiffness [11;107–112]. These abnormalities have been observed to be more pronounced during the stress of exercise, such that the LV fills at the expense of LA hypertension [107–110;112]. Borlaug et al. showed an upward and leftward shift of the end-diastolic pressure-volume relationship in HFpEF, attributing increased filling pressures to intrinsic ventricular stiffness and reduced diastolic filling time at higher heart rates [107].
Causes of myocardial stiffening can be divided into factors altering the extracellular space and those intrinsic to the myocyte. In HFpEF patients, excessive collagen type I deposition may result from an imbalance between exaggerated synthesis and depressed degradation and decreased matrix degradation because of down regulation of MMPs and upregulation of TIMPs [113,114]. Increases cardiac myocyte stiffness in HFpEF appears mediated in part by hypophosphorylation of titin, related to cyclic guanosine monophosphate (GMP) deficiency from increased nitroso- oxidative stress [115]. Increases in fibrosis, alteration in matrix homeostasis and increased oxidative burden are exacerbated by the proinflammatory environment seen in normal aging and in HFpEF patients (see above). In addition as explained in the cardiac aging section, cardiac amyloid deposition may be another plausible biologic mechanism in the genesis of HFpEF [116].
Key Knowledge Gaps:
Why have agents known to alter central hemodynamics or reduce fibrosis or block prohypertrophic and profibrotic effects not improved outcomes in HFpEF patients?
Do newer novel therapies targeting the central hemodynamics in different ways have any role to improve exercise capacity, quality of life and clinical outcome?
Chronotropic incompetence, cardiovascular reserve and systolic dysfunction
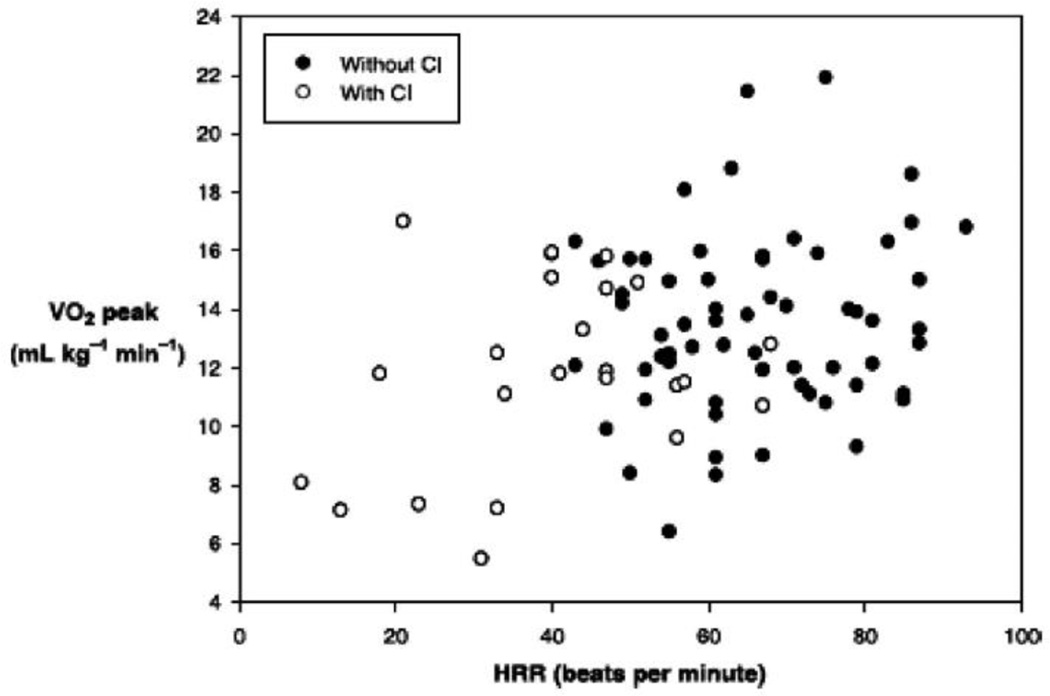
The CO increases through integrated enhancements in venous return, contractility, heart rate, and peripheral vasodilation [117]. Abnormalities in each of these components have been identified in HFpEF. Chronotropic incompetence (CI), the inability of heart rate to increase adequately during physical exertion, has been observed in greater than 25% of patients with HFpEF contributing to exercise intolerance [118–120]. Across reports from a variety of sources, lower heart rate at peak exercise (CI) has been the most consistently reported cardiac abnormality during exercise in HFpEF. In some studies, CI is the only mechanism accounting for reduced CO during exercise in HFpEF and the primary or sole cardiac contributor to exercise intolerance [121]. Of note, this same phenomenon occurs in patients with HFrEF in whom it contributes importantly to reduced CO(Figure 2) [122]. Further, HFpEF patients also have abnormal heart rate recovery after exercise, due to autonomic dysfunction, an independent risk factor for cardiac death [119;123].
Figure 2.
Relationship of heart rate reserve (HRR) to peak exercise oxygen consumption (V̇O2 peak) in older patients with heart failure with reduced ejection fraction and those with heart failure with preserved ejection fraction, with (○) and without (●) chronotropic incompetence (CI). There is a significant correlation between HRR and V̇o2 peak in those with (R=0.39 P=0.04) and without CI (R=0.41 P=0.01).
Some but not all reports indicate that HFpEF patients can have ventricular stiffening with exertion, blunted increases in LV end diastolic volume, despite elevations in filling pressure[124;125], and blunted increases in EF, contractility, and longitudinal systolic shortening velocities during exercise [48;108;109;118;126;127]. Although EF is preserved at rest, enhancement in EF with stress may be significantly limited in HFpEF[118;128], due to increased end systolic volume [118;121;129]. Regional measures of function, observed by tissue Doppler indices, are impaired [130]. HFpEF patients were found to have reduced longitudinal and circumferential strain by speckle tracking compared to age and gender matched hypertensive patients with diastolic dysfunction but without clinical HF [131].
Ventricular-arterial stiffening and vascular dysfunction
Ventricular and vascular stiffening increase with age, are further exacerbated by co-morbidities such as hypertension, obesity, DM, chronic kidney disease and are abnormally elevated in patients with HFpEF [3;132]. Combined ventricular-vascular stiffening means that small changes in LV filling volumes can induce wide swings in arterial blood pressure and thus increase cardiac work with little increase in stroke volume [9;12]. Acute after load elevation in the setting of ventricular-vascular stiffening increases cardiac metabolic demand, and the energy cost for CO [12]. This may also contribute to the relatively larger end systolic volume with exercise and reduced exercise EF. The inability to vasodilate, to accommodate increased boluses of blood without increases in pressure, together with previously described limitations in systolic reserve, leads to dynamic limitations in ventricular-vascular coupling with exercise in patients with HFpEF [109;118]. This was demonstrated by Tartiere-Kesri et al., who showed a steep increase in proximal afterload after moderate exercise that is underestimated at rest and is associated with unfavorable ventricular- arterial coupling and exercise intolerance [133]. Conduit artery (aorta and large artery) stiffening occurs as part of the normal aging process which can be accentuated by many of the diseases associated with HFpEF. Both aortic distensibility [134] and carotid artery distensibility [135] are severely reduced in elderly HFpEF patients and correlate with their degree of exercise intolerance and objectively measured peak exercise O2 uptake.
Reduced exercise limb blood flow has also been noted in HFpEF patients using magnetic resonance imaging (MRI) [136]. Haykowsky et al using high resolution brachial artery ultrasound to assess flow-mediated dilation and healthy age matched controls, found no reduction in endothelial function in HFpEF patients who were free of clinically significant coronary, cerebrovascular, and peripheral arterial disease [137]. This finding of no difference in flow-mediated arterial dilation in HFpEF compared to age-matched, healthy controls was replicated in a separate study using a different technique (MRI) and location (femoral artery) [138]. These studies overall suggest that large vessel endothelial dysfunction may not be an inherent feature of HFpEF. However, flow-mediated vasodilation in large conduit arteries (e.g. femoral) may differ from that observed in the microvasculature (as described in later studies).
Microvascular endothelial dysfunction as measured by peripheral artery tonometry was impaired in HFpEF compared with controls and correlated with reduced exercise capacity and greater symptoms [118]. Similarly in another study, microvascular endothelial dysfunction was found to be an independent predictor of poorer prognosis, mainly hospital readmission, in patients with HFpEF [139]. Recently in an autopsy-based study, Mohammed and colleagues at the Mayo Clinic showed considerably reduced cardiac microvascular density in HFpEF patients which was independent of coronary artery disease and hypertension and in adjusted analyses appeared to account for the increased fibrosis [13]. Their findings suggest that co morbidities other than hypertension may perpetuate microvascular rarefaction [13]. The findings by Mohammed et al parallel the finding by Kitzman et al who reported a 50% reduction in capillary density in thigh skeletal muscle in HFpEF compared to controls which correlated with their reduced exercise capacity [140]. Advanced age and common HFpEF comorbidities such as obesity, systemic hypertension and DM have been shown to be associated with microvascular dysfunction in the heart as well as other organs, including skeletal muscle [141;142]. This supports an over-arching hypothesis for HFpEF pathogenesis as originally proposed by Paulus: a systemic pro-inflammatory state that results in systemic arterial and microvascular dysfunction [57]. Nevertheless, this appealing hypothesis is supported by growing evidence, including a recent report that HFpEF patients have increased levels of tumor necrosis factor-α (TNF-α) and its type–2 receptor, and the latter was elevated even more than in HFrEF [143].
Left Atrial Dysfunction
Prolonged increases in LV volume and/or LV end diastolic pressure can induce LA enlargement. The extent of LA enlargement can provide an integrated assessment of both the severity and duration of dysfunction [144;145]. An enlarged LA is a marker of important valvular (mitral regurgitation or stenosis), atrial (including AF and infiltrative processes such as amyloid), or ventricular pathology [146]. Both LV systolic and diastolic dysfunction impact LA size. Thus, LA volume is not helpful in discriminating between the types of cardiac dysfunction that cause the elevations in pressure or volume. Therefore, while HFpEF patients often have LA enlargement [144;146–148], increased LA size is not specific for HFpEF.
In HFpEF, there is also evidence of impaired atrial compliance and reduction of atrial pump function. This is not only observed in the resting condition but also, importantly, during exercise, indicating a significant impairment of atrial contractile reserve [149;150]. Tissue Doppler strain and speckle tracking strain showed reduced LA strain in HFpEF but remained unchanged in those with LV hypertrophy although both conditions share similar abnormalities including increased LV mass and LA volume [151]. Furthermore, atrial dyssynchrony is common in HFpEF. These factors likely contribute to the development of new onset or progression of atrial arrhythmias, a frequent complication of HFpEF [152]. LA function is associated with pulmonary vascular disease and right heart failure in HFpEF, supporting efforts to improve LA function in this cohort [153].The importance of LA function in HFpEF is underscored by observations that AF can be poorly tolerated in patients with HFpEF, especially if there is a rapid ventricular response and reduced LV filling time [154]. In a community based study; AF occurred in two thirds of HFpEF patients at some point in the natural history and associated with increased risk of death [155]. Recently in a RELAX ancillary study, AF identified an HFpEF cohort with more advanced disease and associated with significantly reduced exercise capacity [156].
RV dysfunction, pulmonary vascular disease and other hemodynamic factors
Pulmonary hypertension is common in patients with HFpEF, particularly during exercise and predicts increased mortality in HFpEF [157;158]. Increases in LA pressure preferentially increase pulsatile right ventricle (RV) loading and the increase in pulmonary artery pressures in HFpEF is not simply the result of passive LA hypertension [158]. Moreover, pulmonary pressure increases with aging and is correlated with ventricular-vascular stiffening, both common risk factors for HFpEF [159]. Furthermore, a large percentage of patients have co-existing COPD that can worsen pulmonary hypertension [160]. The pulmonary pressure decreases more precipitously with acute vasodilator therapy in HFpEF than in HFrEF, suggesting increased RV end-systolic elastance [161]. The RV in HFpEF demonstrates increased diastolic stiffness [162]. In addition, the presence of AF was an additional independent predictor of RV dysfunction in HFpEF [162]. In a community based study, RV dysfunction assessed by echocardiography was common in HFpEF patients, and associated with poorer outcomes [163]. Right-sided chamber and LA enlargement in HFpEF can lead to a marked increases in total cardiac volume, thus creating the substrate for increased pericardial restraint and diastolic ventricular interaction [158;164].
Patients with‘ pre-clinical’ HFpEF often have impaired natriuretic function [165], and even seemingly well compensated HFpEF patients often have elevated plasma volume [166]. Some view volume overload and congestion as key contributors to HF development and progression [167]. Overall, patients with HFpEF have several reserve impairments combining to cause symptomatic HF especially after a hemodynamic challenge, but the dominant contributors appear to differ from patient to patient [118,168].
Peripheral factors: perfusion and skeletal muscle function
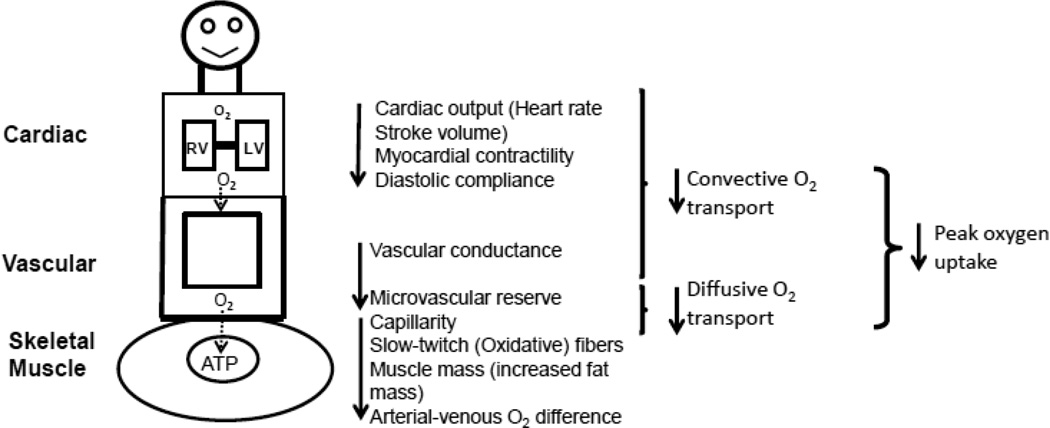
The primary symptom in chronic HFpEF patients is severe exercise intolerance, which can be measured objectively as decreased peak exercise O2 uptake (peak V̇O2) and is associated with severely reduced quality of life [48;121;125;126;169–172]. V̇O2 is equal to the product of CO and arterial-venous oxygen content difference (AVO2Diff). Therefore, the reduced peak V̇O2 in patients with HFpEF can be caused at least partly by decreased oxygen delivery to or impaired oxygen utilization by the exercising skeletal muscles (Figure 3) [173].
Figure 3.
Determinants of exercise intolerance in patients with HFpEF. ATP, adenosine triphosphate; LV, left ventricle; O2, oxygen; RV, right ventricle.
Some cross-sectional studies suggest that decreased peak V̇O2 in elderly HFpEF patients is due primarily to reduced peak CO secondary to blunted chronotropic, inotropic, and vasodilator reserve [48;126].Other studies suggest reduced peak exercise V̇O2in HFpEF is due to reductions in both peak CO and A-VO2 Diff [121;125;172] or primarily due to reduced peak A-VO2 Diff secondary to impaired skeletal muscle oxidative metabolism [169]. Haykowsky and colleagues found that compared with age matched healthy controls [121], while reduced CO was present (due primarily to reduced peak heart rate), peak A-VO2Diff was also significantly reduced, and the change in AVO2Diff from rest to peak exercise was the strongest independent predictor of the reduced peak VO2 in elderly HFpEF patients [121]. Furthermore, in a separate study, these investigators found that improved peak A-VO2 Diff accounted for the nearly all of the improvement in peak VO2 following exercise training [174]. A recent meta-analysis of the 6 randomized controlled trials to date of the effect of exercise training in patients with HFpEF indicated that exercise training increased peak VO2 and quality of life without any significant change in resting diastolic or systolic function [175]. Thus, the beneficial effects of exercise training in HFpEF patients appear to be mediated predominantly through effects in the periphery without altering endothelial function or arterial stiffness, suggesting that abnormalities in the quality or quantity of skeletal muscle function and / or perfusion may not only contribute to the reduced peak VO2 in older HFpEF patients but improvements in these factors may be largely responsible for improved exercise performance with exercise training [174].
Skeletal Muscle Mass, Oxygen Utilization and Exercise Intolerance
Lower extremity skeletal muscle is an important predictor of physical functional performance [176]. The loss of skeletal muscle and age-related alterations in skeletal muscle are major factors in the age-associated decline in peakV̇O2 [177–179]. Loss of skeletal muscle in HFpEF patients is concerning because due both aging and HF effects add to further reduce muscle mass; muscle mass is a major determinant of exercise capacity and strength; and skeletal muscle loss in aging and HF patients is strongly associated with increased disability, hospitalizations, and death [180–184]. This can be further exacerbated by sedentary behavior as symptoms worsen [185].
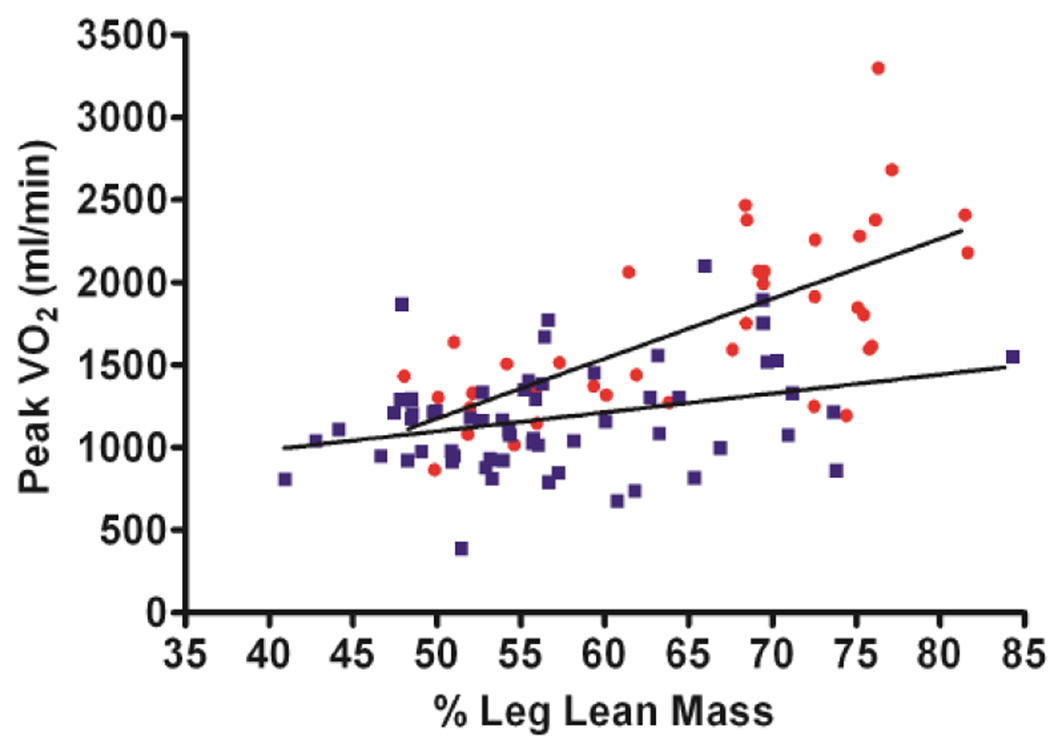
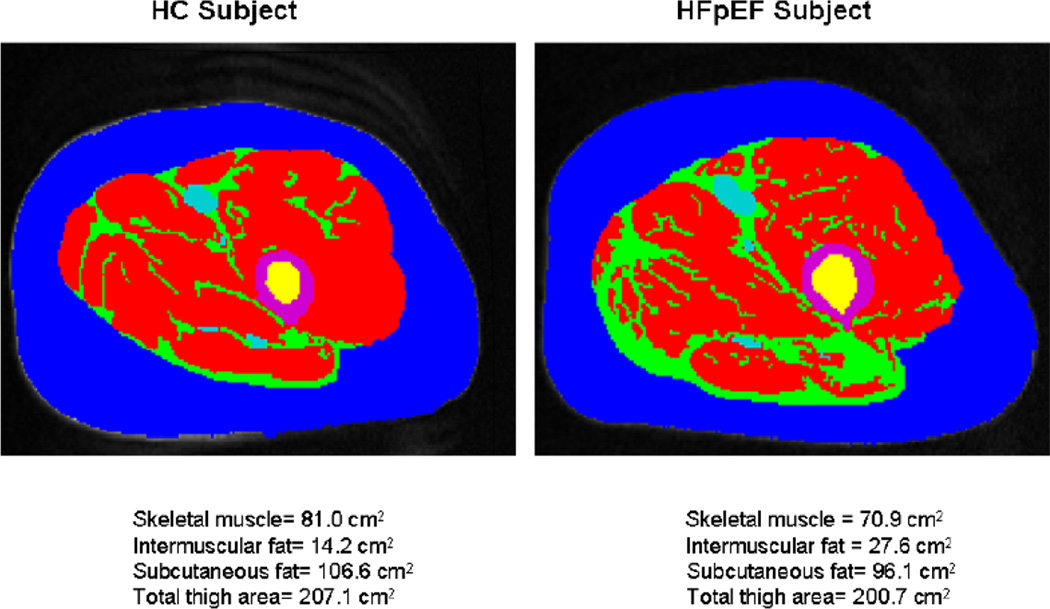
Using dual energy x-ray absorptiometry, Haykowsky and colleagues found percent body fat and percent leg fat were significantly increased, whereas percent body lean and leg lean mass were significantly reduced, in older HFpEF patients versus healthy controls [186]. Moreover, the slope of the relation of peak V̇O2 with percent leg lean mass was markedly reduced in the HFpEF versus healthy control group (Figure 4) [186]. These data suggest that poor “quality” of skeletal muscle may contribute to the reduced peak V̇O2 found in older HFpEF patients. These investigators extended these results by directly characterizing thigh muscle composition using phase-contrast MRI, which showed abnormal fat infiltration into the thigh skeletal muscle and that this was associated with reduced peak exercise V̇O2 in HFpEF(Figure 5) [187]. Together, these findings support the concept that altered skeletal muscle composition (remodeling) contributes to exercise intolerance in older patients with HFpEF.
Figure 4.
Relationship between peak VO2 (ml/min) and percent leg lean mass in heart failure with preserved ejection fraction (HFpEF) and healthy controls (HC) HFpEF (filled squares) and HC (filled circles)
Figure 5.
Magnetic resonance imaging axial image of the mid-thigh in a patient with heart failure with preserved ejection fraction (HFpEF) and healthy controls (HC)
Red = Skeletal muscle; green = Intermuscular fat (IMF); blue = Subcutaneous fat; purple = femoral cortex; yellow = femoral medulla. IMF (green) is substantially increased in the patient with HFpEF compared with the HC despite similar subcutaneous fat.
There are multiple mechanisms whereby adipose infiltration into skeletal muscle could impair exercise capacity in HFpEF. Heinonen et al, [188] using positron emission tomography, found that adipose tissue blood flow adjacent to the active muscles increased sevenfold during continuous isometric knee-extension exercise in non-obese younger healthy sedentary women. Thus, increased thigh intermuscular fat in older patients with HFpEF may “steal” blood that would normally be delivered to the active muscles during exercise thereby reducing perfusive oxygen delivery to the thigh muscle. Furthermore, increased intermuscular fat area is associated with reduced mitochondrial mass, biogenesis, and oxidative metabolism [189]. Indeed, Bhella et al [169], using phosphate-31 magnetic resonance spectroscopy during and after performing static leg lifts, showed impaired skeletal muscle oxidative metabolism in patients with HFpEF. Thus, a number of potential intermuscular fat-mediated structural and biochemical alterations may decrease oxygen transport to and/or utilization by the active muscles.
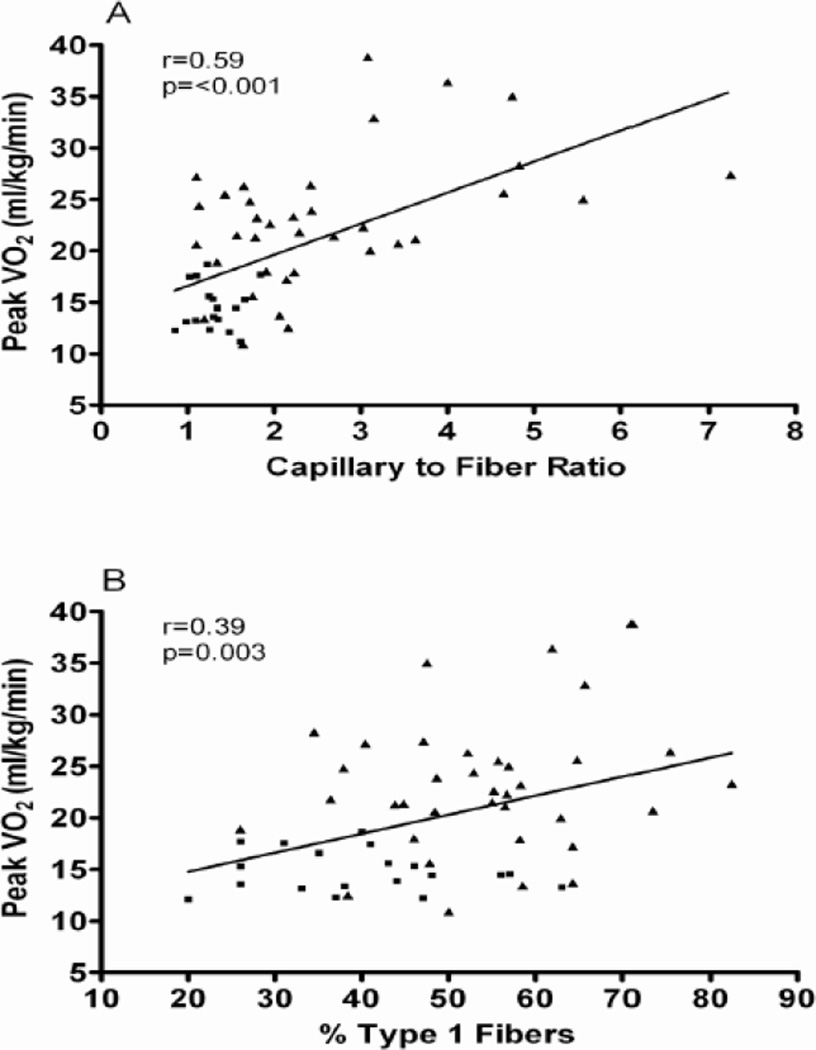
Kitzman and Haykowsky also showed that compared with healthy control subjects, older HFpEF patients had a shift in skeletal muscle fiber type distribution with a reduced percentage of slow twitch type I fibers and reduced type I-to-type-II fiber ratio and reduced capillary-to-fiber ratio [140]. Furthermore, both the capillary-to-fiber ratio and percentage of type I fibers were significant, independent predictors of peak V̇O2(Figure 6)[140]. While speculative, a reduction in the percentage of type I fibers could be associated with reduced oxidative capacity (such as shown by Bhella et al) and mitochondrial density and thereby contribute to the reduced peak V̇O2 in HFpEF. In aging and in HF, muscle blood flow (perfusive and diffusive O2 delivery) assumes an important role in limiting V̇O2 kinetics [190]. Therefore, the reduced capillary-to-fiber ratio in HFpEF patients would be expected to result in a decreased diffusive capacity for O2 transport to active skeletal muscle during exercise and limiting exercise capacity [191].
Figure 6.
Relationship of capillary-to-fiber ratio (A) and percentage of type I muscle fibers (B) with peak O2 uptake (VO2) in older patients with heart failure with preserved ejection fraction (■) and age-matched healthy control subjects (▲).
In these studies, age-matched, sedentary HC subjects were recruited and screened and excluded if they had any chronic medical illness or regularly undertook vigorous exercise. As such, we cannot exclude a contribution of the comorbidities that are common in HFpEF, such as hypertension and DM [192]. However, the pattern of altered skeletal muscle fiber type and capillary-to-fiber ratio that observed in elderly HFpEF patients is strikingly similar to that reported by others in HFrEF patients [193–196], and the fiber type alteration is dissimilar to that seen with aging alone [197]. Indeed, this finding of nearly 50% lower capillary density in thigh muscles nicely parallels with a recent report showing microvascular rarefaction in cardiac muscle [13]. If a systemic process is responsible, perhaps mediated by circulating factors, then adverse effects on striated muscle in both cardiac and skeletal muscle compartments would be expected.
Potential causes for the skeletal muscle abnormalities might include neuroendocrine activation, sympathetic overdrive, oxidative stress, inflammation, abnormal Ca2+ cycling and excitation-contraction coupling, and deconditioning (though skeletal muscle dysfunction has been shown to occur in HF with reduced ejection fraction in the absence of deconditioning) [179]. In addition to this, it is known that aging results in alterations in skeletal muscle, including a reduction in the relative number of type II fibers [197] and in capillary density [198], and that these are associated with a decline in physical performance. Taken together, these findings may help explain why older HFpEF patients have such severely reduced exercise capacity, and why this has not improved with multiple medications tested in trials [104;199].
Key Knowledge Gaps:
Do pre-existing skeletal muscle characteristics determine responses to HF or is the converse true - skeletal muscle alterations are a consequence of the disease process? Or are both of these striated muscle types – cardiac and skeletal – affected by a common systemic factor that triggers HFpEF?
Would approaches to "phenotype" the predominant mechanism(s) of HFpEF (central versus peripheral) in the individual patient would help improve understanding of pathophysiology and optimize treatment approaches in HFpEF?
Do we need to study the skeletal muscle morphology and function of much older, sedentary, non-HF controls in future studies?
Co- morbidities in HFpEF and a systemic proinflammatory state
Non-cardiac co-morbidities are highly prevalent in HFpEF [30]. The most important are obesity, hypertension, DM, COPD, anemia, chronic kidney disease, AF, and peripheral artery disease. Paulus and Tschope proposed that co morbidities and especially obesity induce a systemic inflammatory state, which induces oxidative stress in the coronary microvascular endothelium. This reduces myocardial nitric oxide (NO) bioavailability and leads to reduced PKG activity in cardiomyocytes, which therefore become stiff and hypertrophied [57]. Aging is associated with a systemic proinflammatory state, increasing cytokines levels (interleukin-6, tumor necrosis factor-α, soluble ST2, and pentraxin 3) [200–203] that may lead to functional declines in multiple organs even in absence of a specific disease [204]. The recognition of the importance of co-morbidities in HFpEF has led some to question whether HFpEF simply represents a collection of co-morbidities in elderly breathless patients, rather than a distinct disease entity [205]. However, mortality in patients in HFpEF trials is much higher despite a 'lower' co-morbidity burden compared with non-HFpEF trial patients [206]. Similarly, adjustment for co-morbidities does not fully account for the more severe morbidities observed in HFpEF compared with gender/age-matched healthy controls and hypertensive patients without HF [7]. These findings suggest that HFpEF is a distinct entity aside from its associated co-morbidities.
Frailty
Due to the aging and increasingly complex nature of our patients, frailty has become a high-priority theme in cardiovascular medicine. The Cardiovascular Health Study cohort showed that frailty, as evidenced by slow gait speed and muscular weakness, strongly predicts hospital admission in older adults newly diagnosed with HF [207]. Patients with chronic HF who were frail had a higher risk of mortality at 1 year (17% vs. 5%), HF hospitalizations (21% vs. 13%), and impaired quality of life [208]. This relationship between frailty and HF may potentially be explained by the common inflammatory, metabolic, and autonomic abnormalities seen in both clinical entities. Rather than being simply a result of deconditioning, recent data suggest that frailty and muscular abnormalities may directly contribute to the HFpEF syndrome, a finding similar to HFrEF, where skeletal muscle abnormalities can be independent of physical activity and deconditioning [209;210].
Key Knowledge Gaps:
How much do aging, non-cardiac comorbidities, contribute to the pathogenesis of HFpEF?
What novel interventions would directly address the adverse impact of multiple co-morbidities and frailty in older patients with HFpEF?
Treatment of HFpEF
Pharmacological interventions
Targeting the Renin Angiotensin Aldosterone System (RAAS) pathways has long been considered a logical intervention for HFpEF, based on animal models as well as human hypertensives without HF and its link to interstitial fibrosis and fluid imbalance [211–214]. Of the three large randomized trials of Angiotensin-converting enzyme inhibitor (ACE-I) / angiotensin II type I receptor blocker (ARB) performed to date in HFpEF, only the CHARM-Preserved study found nominal benefit for candesartan in reducing HF hospitalizations [HR 0.86 (95 % CI 0.74–1.0), p =.051] over three years of follow-up. However, most importantly, none of the trials showed benefit for their primary endpoints [215]. The PEP-CHF study evaluated perindopril in elderly HF patients (≥70 years old) with LVEF > 40%, showed no overall difference in mortality and or need for HF hospitalizations [216;216. I-PRESERVE was a very large, multi-center, randomized, controlled, blinded, international trial of HFpEF. It enrolled 4,128 patients and randomly assigned them to the ARB, irbesartan or placebo. Mortality or rates of hospitalizations for cardiovascular causes were not improved by treatment with an ARB [105].
Kitzman et al studied a 12-month, randomized controlled trial of the ACEI enalapril in elderly patients with established HFpEF, and showed no improvement in exercise capacity or quality of life [199]. The Aldo-DHF trial of 12 months treatment of spironolactone improved some measures of diastolic function, though maximal exercise capacity, clinical symptoms, and quality of life were not changed [104]. The RAAM-PEF trial of 6 months treatment of eplerenone vs. placebo, showed reductions in circulating markers of collagen turnover and modest improvements in diastolic function [217]. In addition to this, in the OPTIMIZE-HF registry, aldosterone antagonists had no effect on all-cause mortality or hospitalization [218]. The large TOPCAT trial of spironolactone aldosterone inhibitor failed to show statistically significant benefit for the clinical composite primary end -point (cardiovascular mortality, aborted cardiac arrest, or hospitalization for the management of HF). A modest decline in hospitalizations was observed [103]. However, the marked regional differences in patient populations and responses to spironolactone in TOPCAT somewhat confounds the interpretation of these overall findings. A post hoc regional analysis indicates significant regional differences among enrolled patients and that the cohort from the Americas most closely matched characteristics observed in other randomized trials and also appeared most responsive to spironolactone [219]. Although these post-hoc data cannot be definitive, in the absence of stronger data, they may be informative in patients with HFpEF who have similar risk profiles to those of enrolled from the Americas.
Slowing the heart rate should result in an increase in the diastolic filling period in an abnormally stiff LV with prolonged relaxation, potentially allowing greater filling at high heart rates. In addition, there is a high prevalence of CI in HFpEF, and limitations in chronotropic reserve might be a key factor contributing to reduced CO and exercise capacity [48;109]. However, the role of β-blockers remains uncertain and data to date have not been encouraging. Both carvedilol (the J-DHF study) and nebivolol (ELANDD study) had neutral effects on their primary outcomes in HFpEF patients.220;221 In the OPTIMIZE-HF registry, discharge use of beta-blockers appeared to exert no effect on 1 year mortality or hospitalization rates of HFpEF patients [222].
In the Digitalis Interaction Group trial (DIG), a subgroup of 988 patients with EF >45% was randomized to placebo or to digoxin. There were no significant reductions in the amount of hospitalizations or mortality secondary to HF, although trends towards decreased hospitalization and improved exercise tolerance were noted [223].
Finally, Holland et al. collected data from 53,878 patients enrolled in 30 published reports, including 18 randomized controlled trials (n = 11,253) and 12 observational studies (n = 42,625). Their analysis suggested that pharmacotherapy of HFpEF may improve exercise tolerance but not mortality [224]. However, their conclusions regarding exercise capacity are based on data from only 183 total treated patients drawn from 6 trials. Further, if one excludes the largest trial, which enrolled elderly men after myocardial infarction with EF as low as 40%, then over a third of the remaining patients were drawn from a single, adequately powered study which was decidedly neutral. Thus, while this timely meta-analysis supports a positive trend, additional trials focused on the important outcome of exercise capacity in HFpEF are needed. Holland et al speculated that future trials might be more fruitful if they select patients with “endorsed” or “objective” criteria of HFpEF, and select more “homogenous” samples. While potentially attractive, this approach could also reduce generalizability of findings to the broader HfpEF population.
Key Knowledge Gaps:
Why did all studies, across a range of classes of agents shown to be beneficial for HFrEF and / or hypertension and ischemic heart disease, show no improvement in their primary outcomes in HFpEF?
Are there overarching, systemic processes in HFpEF that underlie the global impairments in combined cardiac reserve that might be targeted therapeutically to improve overall clinical outcomes?
Novel pharmacological agents
Phosphodiesterase 5 inhibitors (PDE5I) increase cGMP levels by blocking their catabolism, thus augmenting PKG activity in multiple organs relevant to HF. In the recent RELAX trial, sildenafil did not improve 6-minute walk distance or quality of life, and was associated with modest worsening of renal function and increases in neurohormone levels [225]. The DILATE-1 study showed that riociguat, a soluble guanylate cyclase stimulator, did not have any impact on the primary end -point of peak change in mean pulmonary artery pressure in patients with HFpEF and pulmonary hypertension [226].
By blocking the activity of several guanosine triphosphate binding proteins, and inhibiting some of the inflammatory processes described above, statins can suppress LV hypertrophy and decrease collagen synthesis in experimental models [227;228]. Even though observational data in HFpEF patients suggest a mortality benefit with use of HMG-Co-A reductase inhibitors, despite neutral outcomes in HFrEF patients, definitive trials have not been performed yet and might be difficult given existing wide-spread use of statins for multiple indications in many HFpEF patients [229;230] In a seven-day study, ivabradine, a selective sinus node If sodium channel inhibitor that reduces HR without affecting contractility or lusitropy increased peak VO2 and reduced exercise E/e’ ratio in 61 patients with HFpEF [231].
Neprilysin is a zinc-dependent metalloprotease that degrades biologically active natriuretic peptides and does not affect the biologically inactive NTproBNP [18]. The PARAMOUNT study randomized 301 HFpEF patients to valsartan vs. LCZ696, a combination ARB/neprilysin inhibitor. Compared to valsartan alone, the LCZ696 group had significantly lower NT-pro BNP levels and at 36 weeks, decreased LA size and showed a trend toward improved functional class [18]. The findings of this phase-2 study are promising and a large, multi-center trial, PARAGON, is underway comparing LCZ696 to valsartan in patients with HFpEF.
Anemia is highly prevalent in HFpEF and carries a poor prognosis; leading to the hypothesis that epoeitin-alfa would improve submaximal exercise capacity and ventricular remodeling. However, in a well-designed randomized trial, after 24 weeks of therapy there was no change in 6-minute walk distance or LV end diastolic volume [232].
A small open-label study found that administration of alagebrium chloride, the compound that breaks glucose cross-links, was associated with slightly reduced LV mass and improved diastolic filling, however, there were no changes in EF, BP, peak VO2 and aortic distensibility (the latter were the primary outcomes) [233]. The RALI-DHF study showed that ranolazine, which blocks inward sodium current and reduces intracellular calcium, improved some measures of diastolic function [234]. Reduction of filling pressures did occur with the ranolazine but appeared to also decrease CO [235].
Serelaxin, a recombinant form of human relaxin-2, administered to acute HF patients, caused in improvement of symptoms and prevention of organ damage with a reduction in 180-day mortality, compared with placebo [236;237]. Compared with HFrEF, serelaxin was well tolerated and effective in relieving dyspnea and had a similar effect on short- and long-term outcome, including survival improvement in HFpEF patients [238]. In HFpEF patients, treatment with a sitaxsentan sodium selective endothelin type A receptor antagonist appeared to increase exercise time on the treadmill. However, no expired gas testing was reported, there appeared to be no improvement in patients with class II NYHA symptoms, and there were no improvements in any of the secondary endpoints such as left ventricular mass or diastolic function [239]. This agent (as were other endothlelin type A antogonsits) was not beneficial in multiple outcomes trials of HFrEF; it had hepatotoxicity, and has been removed from development. A non-pharmacological approach using SERCA2 gene treatments by an adenovirus has been tested with some promising results in HFrEF [240]. In a dosing study, the lowest frequency of HF exacerbations were seen in those receiving the greatest dose [241], whether this approach could be beneficial in HFpEF is unknown. The strategy of replacement of miRNAs of interest or of blockade of potentially harmful miRNAs (anti-MIRs) is currently being tested in pre-clinical studies [94].
A number of small molecules and pharmacological agents have been shown to increase TTR’s native-state stability and kinetic barriers to misfolding and aggregation [242]. Two of these compounds with the most robust clinical data are diflunisal and tafamidis. Preliminary data from an open label trial also suggest efficacy in persons with familial TTR cardiomyopathy [243], although randomized trials in persons with wild-type disease have not been performed. Active clinical research programs are also testing strategies of ribonucleic acid (RNA) interference through the use of small interfering RNAs (siRNAs) and antisense nucleotides to silence the TTR gene. Using these new technologies, Phase I clinical studies are ongoing to test drug safety and tolerability in individuals with transthyretin amyloidosis. As with tafamidis, none of these studies specifically test drug effects in persons with cardiac amyloidosis from wild-type disease [244;245].
Thus, while there are some signs of promise, novel agents tested for HFpEF to date have fared only a little better than the standard agents adapted from treatment of hypertension, ischemic heart disease, and HFrEF. This further suggests the possible need for a revised paradigm regarding the pathogenesis and outcomes of HFpEF.
Why Did Trials of These Pharmacological Agents Not Show Benefit in HFpEF?
HFpEF is strongly influenced by aging, a systemic process affecting all organ systems. The overwhelming majority of the patients with HFpEF have multiple co-morbidities that further ensure phenotypic heterogeneity and multifactorial pathophysiology. These co-morbidities may play a much greater role in the development of symptoms and treatment response than previously recognized. If so, they may not be addressed by agents and strategies that are primarily or solely targeted at cardiac function. Furthermore, more recent data, discussed above, indicates that HFpEF may be a systemic disorder, and involves important contributions from peripheral abnormalities of vascular and skeletal muscle function that have not been addressed in trials to date [14].
Animal models of HFpEF, which can initially suggest promise, do not mimic the integrative co-existence of co-morbidities so common in HFpEF patients. Furthermore non-cardiovascular hospital readmissions and mortality are more frequent in HFpEF than in HFrEF [30;246]. The cardiovascular drugs might therefore may have a limited effect in a condition where the non-cardiovascular mode of death is more common than in HFrEF. It seems clear that HFpEF in older persons is typified by a much broader range of cardiac and non-cardiac abnormalities and reduced reserve capacity in multiple organ systems.
Non-pharmacological Strategies
Exercise Training
The primary chronic symptom in patients with HFpEF, even when well compensated, is severe exercise intolerance. It is an independent predictor of morbidity and mortality and is increasingly a leading outcome in pharmacologic trials of HFpEF. Due to this, interest has recently focused on exercise training as a potential treatment modality. Kitzman and colleagues performed the first randomized, single-blinded trial comparing the effects of 16 weeks of endurance exercise training versus attention control in 53 older (mean age = 70 years) patients with HFpEF. Three months of exercise training increased peak VO2, ventilatory anaerobic threshold, 6-minute walk distance, and physical quality-of-life scores [43]. These results were confirmed in a subsequent multicenter study of 64 HFpEF patients randomized to 3 months of combined exercise training and strength training [247]. In a second, separate, randomized, attention-controlled, single- blind trial of 4 months upper and lower extremity endurance exercise training, Kitzman et al found a significant increase in peak VO2 without altering carotid arterial stiffness or brachial artery flow mediated dilation [248]. Taken together, exercise training is an effective non-pharmacologic therapy in clinically stable patients with HFpEF to improve exercise tolerance. The improvement in peak VO2 appears to be primarily caused by favorable microvascular or skeletal muscle adaptations that increase diffusive oxygen transport or oxygen utilization by the working muscles [173;175].
Key Knowledge Gaps:
Are there pharmacologic strategies that can be combined with exercise training in HFpEF to facilitate an improvement in exercise capacity?
What is the optimal “dose” (frequency, duration, intensity) and modality of exercise training that will be most effective in HFpEF?
Does exercise training ameliorate skeletal muscle alterations in HFpEF?
Can exercise interventions be implemented safely in very elderly, frail patients with HFpEF?
Nutritional Strategies
In multiple ‘salt-sensitive’ experimental models of HFpEF, high sodium consumption exacerbates oxidative stress and adverse cardiovascular remodeling [249–251]. In a recent proof-of-concept study, consumption of sodium-restricted DASH diet for 21 days in 13 hypertensive HFpEF patients resulted in improvement in relaxation and stiffness based measures of diastolic function and ventricular–vascular coupling ratio and decrease in arterial elastance [220;252].
Device Therapy
Lack of effective therapy in HFpEF patients has driven a search for device based strategies. Strategies being examined include creation of an interatrial septal defect with LA shunt which can serve as a ‘relief’ valve to decompress LA pressure. A computer simulation interatrial shunt study reduced left-sided CO with a marked reduction in pulmonary capillary wedge pressure [253]. Recently, a pilot study showed an interatrial septal device was successfully implanted in a cohort of HFpEF patients and resulted in improved hemodynamics at rest, with encouraging early clinical response [254].
The baroreflex activation therapy (BAT) produced by stimulating the carotid sinuses using an implanted device (Rheos) is being studied for the treatment of hypertension, a key, frequent precursor to and co-morbidity in HFpEF. The potential benefits of BAT include regression of LV hypertrophy, normalization of the sympathovagal balance, inhibition of the RAAS, arterio- and venodilation, and preservation of renal function. The potential for BAT for HFpEF, the HOPE4HF trial (a randomized outcomes trial designed to evaluate the clinical safety and efficacy of BAT in the HFpEF population) [255] is underway. Additional devices record invasive hemodynamics from either the RV, pulmonary artery or LA and have been used to adjust medical therapy based on invasive hemodynamics in an attempt to avert acute or severe decompensation [256] and hemodynamically guided management of patients with HFpEF reduced the hospitalization compared with standard HF management strategies [257]. A micro-ventricular assist device implanted (off pump) via a mini-thoracotomy, in a right subclavicular subcutaneous pocket (like a pacemaker) with drainage of blood from the LA and output it in the subclavian artery appears to interrupt the progressive hemodynamic deterioration in HFrEF [258]. However its role in HFpEF is yet to be defined. Cardiac resynchronization therapy in a patient with HFpEF showed improvement in functional class and in exercise capacity [259]. Thus, use of a device-based approach in symptomatic HFpEF may provide novel means to improve hemodynamic and warrants further investigation.
Conclusion
Recent work indicates that HFpEF patients frequently have functional abnormalities in multiple cardiovascular and non-cardiovascular domains with reduced reserve capacity in multiple organ systems and is likely a system disorder. HFpEF is a true geriatric syndrome, with complex, multi-factorial pathophysiology and clinical heterogeneity. Progress in HFpEF may be made by incorporating these concepts of heterogeneity and multifactorial contributions, and searching for the common threads and links with other debilitating disorders common among the elderly.
Highlights.
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF.
Despite the strong public health importance, its pathogenesis is poorly understood.
HFpEF is a systemic disorder with complex, multi-factorial pathophysiology and clinical heterogeneity.
Progress in HFpEF may be made by incorporating these concepts of heterogeneity and multifactorial contributions.
Acknowledgments
Supported in part by NIH grant R01AG18915, P30AG021332.
Dr. Kitzman reports the following potential financial conflicts of interest: consulting for GSK, Relypsa, Regeneron, Abbvie; grant support from Novartis; stock ownership in Gilead Sciences and Relypsa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No other members of the writing group have conflicts of interest to declare.
References
- 1.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction: Prevalence, Therapies, and Outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, et al. Costs for Heart Failure With Normal vs Reduced Ejection Fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed SF, Borlaug BA, Roger VrL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, et al. Comorbidity and Ventricular and Vascular Structure and Function in Heart Failure With Preserved Ejection Fraction / Clinical Perspective. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi SK, Powers JE, Fowle KM, Rankin KM, Nomeir AM, Kitzman DW, et al. The Pathogenesis of Acute Pulmonary Edema Associated with Hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure. Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: the systemic nature of heart failure with preserved ejection fraction. Circulation. 2015;131:522–524. doi: 10.1161/CIRCULATIONAHA.114.014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the management of heart-failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 18.Solomon S, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 19.Kitzman D, Upadhya B. Heart failure with preserved ejection fraction: a heterogenous disorder with multifactorial pathophysiology. J Am Coll Cardiol. 2014;63:457–459. doi: 10.1016/j.jacc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 21.Melenovsky V, Borlaug B, Rosen B, Hay I, Ferruci L, Morell C, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Maurer MS, Hummel SL. Heart Failure With a Preserved Ejection Fraction: What Is in a Name? J Am Coll Cardiol. 2011;58:275–277. doi: 10.1016/j.jacc.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Guazzi M, Myers J, Peberdy M, Bensimhon D, Chase P, Arena R. Cardiopulmonary exercise testing variables reflect the degree of diastolic dysfunction in patients with heart failure-normal ejection fraction. J Cardiopulm Rehabil Prev. 2010;30:165–172. doi: 10.1097/HCR.0b013e3181d0c1ad. [DOI] [PubMed] [Google Scholar]
- 24.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics --2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, et al. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Yancy C, Lopatin M, Stevenson L, De Marco T, Fonarow G. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 29.Meta-analysis Global Group. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 30.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population With Heart Failure and Preserved Versus Reduced Ejection Fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottdiener J, Bartz T, DeFilippi C, Kop W, Kitzman D, Barasch E, et al. Echocardiographic and biomarker phenotype of heart failure with preserved ejection fraction (HFPEF) in older individuals in comparison to hypertension without heart failure (HTN), elderly with risk factors, and healthy aging. Importance of myocyte injury, fibrosis, LV hypertrophy, and diastolic load. J Am Coll Cardiol. 2012;59:E852. [Google Scholar]
- 32.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer R, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, et al. Beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. doi: 10.3389/fphys.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roger V. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ertl G, Ruschitzka F. The Year in Cardiology 2013: heart failure. Eur Heart J. 2014;35:470–473. doi: 10.1093/eurheartj/eht555. [DOI] [PubMed] [Google Scholar]
- 37.North B, Sinclair D. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakatta E. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 39.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 40.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16(12):1492–1526. doi: 10.1089/ars.2011.4179. Ref Type: Conference Proceeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braunwald E. Research advances in heart failure: a compendium. Circ Res. 2013;113:633–645. doi: 10.1161/CIRCRESAHA.113.302254. [DOI] [PubMed] [Google Scholar]
- 42.Spinale F, Zile M. Integrating the myocardial matrix into heart failure recognition and management. Circ Res. 2013;113:725–738. doi: 10.1161/CIRCRESAHA.113.300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borlaug B, Redfield M, Melenovsky V, Kane G, Karon B, Jacobsen S, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2:489–499. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 47.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 48.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonnema D, Webb C, Pennington W, Stroud R, Leonardi A, Clark L, et al. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of vetalloproteinases (TIMPs) J Card Fail. 2007;13:530–540. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobaczewski M, Chen W, Frangogiannis N. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bujak M, Frangogiannis N. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, et al. Myocardial fibrosis in transforming growth factor-beta (1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 54.Brooks W, Conrad C. Myocardial fibrosis in transforming growth factor beta(1) heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 55.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 56.Boyle A, Shih H, Hwang J, Ye J, Lee B, Zhang Y, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulus W, Tschope C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 58.Csiszar A, Ungvari Z, Koller A, Edwards J, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 59.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cucoranu I, Clempus R, Dikalova A, Phelan P, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 61.Dai D, Rabinovitch P. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink J, Rovio A, Bruder C, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 63.Vermulst M, Wanagat J, Kujoth G, Bielas J, Rabinovitch P, Prolla T, et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 64.Vermulst M, Bielas J, Kujoth G, Ladiges WC, Rabinovitch PS, Prolla TA, et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- 65.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 66.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox. Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai D, Johnson S, Villarin J, Chin M, Nieves-Cintron M, Chen T, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai D, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana L, et al. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mollnau H, Wendt M, Szocs K, Lasseque B, Schulz E, Oelze M, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 70.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao R, Tomhave E, Wang D, Ji X, Boluyt M, Cheng H, et al. Age-associated reductions in cardiac beta1- and beta2-adrenergic responses without changes in inhibitory G proteins or receptor kinases. J Clin Invest. 1998;101:1273–1282. doi: 10.1172/JCI1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakatta E. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 73.Babusikova E, Lehotsky J, Dobrota D, Racay P, Kaplan P. Age-associated changes in Ca(2+)-ATPase and oxidative damage in sarcoplasmic reticulum of rat heart. 2012;61(5):453–460. doi: 10.33549/physiolres.932320. Ref Type: Conference Proceeding. [DOI] [PubMed] [Google Scholar]
- 74.Lancel S, Qin F, Lennon S, Zhang J, Tong X, Mazzini M, et al. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin F, Siwik D, Lancel S, Zhang J, Kuster G, Luptak I, et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng A, Callister R, Johnson D, Seals D. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 77.Esler M, Turner A, Kaye D, Thompson J, Kingwell B, Morris M, et al. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 78.Corbi G, Conti V, Russomanno G, Longobardi G, Furgi G, Filippelli A, et al. Adrenergic signaling and oxidative stress: a role for sirtuins? Front Physiol. 2013;4:324. doi: 10.3389/fphys.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rinaldi B, Corbi G, Boccuti S, Filippelli W, Rengo G, Leosco D, et al. Exercise training affects age-induced changes in SOD and heat shock protein expression in rat heart. Exp Gerontol. 2006;41:764–770. doi: 10.1016/j.exger.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli A, et al. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxid Med Cell Longev. 2012;2012:6. doi: 10.1155/2012/728547. Article ID 728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci (Elite Ed) 2012;4:768–778. doi: 10.2741/417. [DOI] [PubMed] [Google Scholar]
- 82.Kaye D, Esler M. Sympathetic neuronal regulation of the heart in aging and heart failure. Cardiovasc Res. 2005;66:256–264. doi: 10.1016/j.cardiores.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 83.Esler M, Thompson J, Kaye D, Turner A, Jennings G, Cox H, et al. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- 84.Fraticelli A, Josephson R, Danziger R, Lakatta E, Spurgeon H. Morphological and contractile characteristics of rat cardiac myocytes from maturation to senescence. Am J Physiol Heart Circ Physiol. 1989;257:H259–H265. doi: 10.1152/ajpheart.1989.257.1.H259. [DOI] [PubMed] [Google Scholar]
- 85.Thomas S, Rich M. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3:381–387. doi: 10.1016/j.hfc.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, Ma X, Ye F, Zhang J, Zhou X, Wang Z, et al. Expressions of cardiac sympathetic norepinephrine transporter and beta1-adrenergic receptor decreased in aged rats. J Zhejiang Univ Sci B. 2009;10:203–210. doi: 10.1631/jzus.B0820213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dharmarajan K, Salomon S, Helmke S, Tereyua S, Rapezzi C, Falk R, et al. Genotype and phenotypic characteristics of persons with cardiac smyloidosis from the Multinational Transthyretin Amyloidosis Outcomes Survey (THAOS) Registry. J Card Fail. 2011;17:S69. [Google Scholar]
- 88.Bhuiyan T, Helmke S, Patel AR, Ruberg FL, Packman J, Cheung K, et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS) Circ Heart Fail. 2011;4:121–128. doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 90.Mirzoyev SA, Edwards WD, Mohammed SF, Donovan JL, Roger VL, Grogan DR, et al. Cardiac amyloid deposition is common in elderly patients with heart failure and preserved ejection fraction. Circulation. 2010;122 [Google Scholar]
- 91.Mohammed S, Mirzoyev S, Edwards W, Dogan A, Grogan D, Dunlay S, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zamore P, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 93.Sayed D, Hong C, Chen I, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 94.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 95.Dong S, Ma W, Hao B, Hu F, Yan L, Yan X, et al. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol. 2014;7:565–574. [PMC free article] [PubMed] [Google Scholar]
- 96.Lam CSP, Roger VrL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, et al. Cardiac Structure and Ventricular-Vascular Function in Persons With Heart Failure and Preserved Ejection Fraction From Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and Ventricular Systolic Stiffening in Hypertensive Heart Disease: Insights Into the Pathogenesis of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and Significance of Alterations in Cardiac Structure and Function in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 99.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular Structure and Function in Hypertensive Participants With Heart Failure and a Normal Ejection Fraction: The Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 100.Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, et al. Effect of Intensive Versus Standard Blood Pressure Lowering on Diastolic Function in Patients With Uncontrolled Hypertension and Diastolic Dysfunction. Hypertension. 2010;55:241–248. doi: 10.1161/HYPERTENSIONAHA.109.138529. [DOI] [PubMed] [Google Scholar]
- 101.van Heerebeek L, Borbely A, Niessen HWM, Bronzwaer JGF, van der Velden J, Stienen GJM, et al. Myocardial Structure and Function Differ in Systolic and Diastolic Heart Failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 102.Su M, Lin L, Tseng Y, Chang C, Wu C, Lin J, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–997. doi: 10.1016/j.jcmg.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 103.Pitt B, Pfeffer M, Assmann S, Boineau R, Anand I, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 104.Edelmann F Aldo-DHF investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 105.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 106.Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, et al. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J. 2009;30:3027–3036. doi: 10.1093/eurheartj/ehp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borlaug BA, Jaber WA, Ommen SR, Lam CSP, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, et al. The Pathophysiology of Heart Failure With Normal Ejection Fraction: Exercise Echocardiography Reveals Complex Abnormalities of Both Systolic and Diastolic Ventricular Function Involving Torsion, Untwist, and Longitudinal Motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 109.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, et al. Heart Failure With Preserved Ejection Fraction Is Characterized by Dynamic Impairment of Active Relaxation and Contraction of the Left Ventricle on Exercise and Associated With Myocardial Energy Deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 110.Tan YT, Wenzelburger F, Lee E, Heatlie G, Frenneaux M, Sanderson JE. Abnormal left ventricular function occurs on exercise in well-treated hypertensive subjects with normal resting echocardiography. Heart. 2010;96:948–955. doi: 10.1136/hrt.2009.185181. [DOI] [PubMed] [Google Scholar]
- 111.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. Cardiol Board Review. 1991;8:72–85. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 112.Ohara T, Niebel C, Stewart K, Charonko J, Pu M, Vlachos P, et al. Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: evaluation by color M-mode echocardiography. JACC Cardiovasc Imaging. 2012;5:861–870. doi: 10.1016/j.jcmg.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Weber K, Brilla C, Janicki J. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 114.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix Metalloproteinases/Tissue Inhibitors of Metalloproteinases: Relationship Between Changes in Proteolytic Determinants of Matrix Composition and Structural, Functional, and Clinical Manifestations of Hypertensive Heart Disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 115.van Heerebeek L, Hamdani N, Falcao-P I, Leite-Moreira A, Begieneman M, Bronzwaer J, et al. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 116.Mohammed S, Mirzoyev S, Edwards W, Dogan A, Grogan D, Dunlay S, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RD, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 118.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Phan T, Shivu G, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 120.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 121.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brubaker PH, Kitzman DW. Chronotropic Incompetence: Causes, Consequences, and Management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 124.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan M. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 126.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 127.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, et al. Left Ventricular Abnormal Response During Dynamic Exercise in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction at Rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 128.Ennezat P, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, et al. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 129.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with "isolated" diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 131.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired Systolic Function by Strain Imaging in Heart Failure with Preserved Ejection Fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melenovsky V, Borlaug B, Rosen B, Hay I, Ferruci L, Morell C, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 133.Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F, Cohen Solal A. Increased Proximal Arterial Stiffness and Cardiac Response With Moderate Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 134.Hundley W, Kitzman D, Morgan T, Hamilton C, Darty S, Stewart K, et al. Cardiac cycle-dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 135.Kitzman DW, Herrington DM, Brubaker P, Moore J, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puntawangkoon C, Kitzman D, Kritchevsky S, Hamilton C, Nicklas B, Leng X, et al. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. Journal of Cardiovascular Magnetic Resonance. 2009;11:48. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow mediated arterial; dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 139.Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y, et al. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2013;168:36–40. doi: 10.1016/j.ijcard.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 140.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 142.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Putko BN, Wang Z, Lo J, Anderson T, Becher H, Dyck JR, et al. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS ONE. 2014;9:e99495. doi: 10.1371/journal.pone.0099495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Douglas PS. The left atrium: a biomarker of chronicdiastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–1207. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 145.Rossi A, Vassanelli C. Left atrium: no longer neglected. Ital Heart J. 2005;6:881–885. [PubMed] [Google Scholar]
- 146.Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–1064. doi: 10.1016/j.ahj.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 147.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients >=65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 148.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 149.Melenovsky V, Borlaug B, Rosen B, Hay I, Ferruci L, Morell C, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 150.Tan Y, Wenzelburger F, Lee E, Nightingale P, Heatlie G, Leyva F, et al. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. 2010;96:1017–1023. doi: 10.1136/hrt.2009.189118. [DOI] [PubMed] [Google Scholar]
- 151.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovascu Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 152.Eicher JC, Laurent G, Mathe A, Barthez O, Bertaux G, Philip JL, et al. Atrial dyssynchrony syndrome: an overlooked phenomenon and a potential cause of 'diastolic' heart failure. Eur J Heart Fail. 2012;14:248–258. doi: 10.1093/eurjhf/hfr169. [DOI] [PubMed] [Google Scholar]
- 153.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015 doi: 10.1161/CIRCHEARTFAILURE.114.001667. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 154.Fung J, Sanderson J, Yip G, Zhang Q, Yu C. Impact of atrial fibrillation in heart failure with normal ejection fraction: a clinical and cchocardiographic study. J Card Fail. 2007;13:649–655. doi: 10.1016/j.cardfail.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 155.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123–130. doi: 10.1161/CIRCHEARTFAILURE.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lam CSP, Roger VrL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction: A Community-Based Study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guazzi M, Borlaug B. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 159.Lam C, Borlaug B, Kane G, Enders F, Rodeheffer R, Redfield M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. European Journal of Heart Failure. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of Vasodilation in Heart Failure With Preserved or Reduced Ejection Fraction: Implications of Distinct Pathophysiologies on Response to Therapy. J Am Coll Cardiol. 2012;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 162.Melenovsky V, Hwang S, Lin G, Redfield M, Borlaug B. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schwarz K, Singh S, Dawson D, Frenneaux M. Right ventricular function in left ventricular disease: pathophysiology and implications. Heart Lung Circ. 2013;22:507–511. doi: 10.1016/j.hlc.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 165.McKie P, Schirger J, Costello-Boerrigter L, Benike S, Harstad L, Bailey K, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58:2095–2103. doi: 10.1016/j.jacc.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Noumi B, Teruya S, Salomon S, Helmke S, Maurer M. Blood volume measurements in patients with heart failure and a preserved ejection fraction: implications for diagnosing anemia. Congest Heart Fail. 2011;17:14–18. doi: 10.1111/j.1751-7133.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Colombo P, Ganda A, Lin J, Onat D, Harxhi A, Iyasere J, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–190. doi: 10.1007/s10741-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shah S. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near-maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol. 2000;88:2138–2142. doi: 10.1152/jappl.2000.88.6.2138. [DOI] [PubMed] [Google Scholar]
- 171.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 172.Maeder MT, Thompson BR, Brunner-La Rocca H-P, Kaye DM. Hemodynamic Basis of Exercise Limitation in Patients With Heart Failure and Normal Ejection Fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 173.Haykowsky M, Brubaker P, Kitzman D. Role of Physical Training in Heart Failure with Preserved Ejection Fraction. Current Heart Failure Reports. 2012;9:101–106. doi: 10.1007/s11897-012-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, et al. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Experimental Gerontology. 2012;47:38–44. doi: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Franssen F, Wouters E, Schols A. The contribution of starvation, deconditioning and ageing to the observed alterations in peripheral skeletal muscle in chronic organ diseases. Clinical Nutrition. 2002;21:1–14. doi: 10.1054/clnu.2001.0485. [DOI] [PubMed] [Google Scholar]
- 178.Coats A, Clark A, Piepoli M, Volterrani M, Poole-Wilson P. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Middlekauff HR. Making the Case for Skeletal Myopathy as the Major Limitation of Exercise Capacity in Heart Failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Beavers K, Beavers D, Houston D, Harris T, Hue T, Koster A, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.von Haehling S, Horwich TB, Fonarow GC, Anker SD. Tipping the Scale: Heart Failure, Body Mass Index, and Prognosis. Circulation. 2007;116:588–590. doi: 10.1161/CIRCULATIONAHA.107.716662. [DOI] [PubMed] [Google Scholar]
- 182.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 183.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 184.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current donsensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 186.Haykowsky M, Brubaker P, Morgan T, Kritchevsky S, Eggebeen J, Kitzman D. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, et al. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. Jvf Applied Physiology. 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 189.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rossiter HB. Exercise: Kinetic Considerations for Gas Exchange, Comprehensive Physiology. John Wiley & Sons, Inc.; 2010. [DOI] [PubMed] [Google Scholar]
- 191.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stuart CA, McCurry MP, Marino A, South MA, Howell MEA, Layne AS, et al. Slow-Twitch Fiber Proportion in Skeletal Muscle Correlates With Insulin Responsiveness. J Clin Endocrinol Metab. 2013;98:2027–2036. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 194.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84:1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 195.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb F, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29 doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Toth M, Matthews DE, Ades PA, Tischler MD, Van Buren P, Previs M, et al. Skeletal muscle myofibrillar protein metabolism in heart failure: relationship to immune activation and functional capacity. Am J Physiol Endocrinol Metab. 2005;288:E685–E692. doi: 10.1152/ajpendo.00444.2004. [DOI] [PubMed] [Google Scholar]
- 197.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 198.Coggan AR, Spina RJ, King DS. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 199.Kitzman DW, Hundley WG, Brubaker P, Stewart K, Little WC. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, et al. Pentraxin 3 Is a New Inflammatory Marker Correlated With Left Ventricular Diastolic Dysfunction and Heart Failure With Normal Ejection Fraction. J Am Coll Cardiol. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 201.Kalogeropoulos A, Georgiopoulou V, Psaty B, Rodondi N, Smith A, Harrison D, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Collier P, Watson C, Voon V, Phelan D, Jan A, Mak G, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 203.Shah K, Kop W, Christenson R, Diercks D, Henderson S, Hanson K, et al. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem. 2011;57:874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 204.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 205.Packer M. Can Brain Natriuretic Peptide Be Used to Guide the Management of Patients With Heart Failure and a Preserved Ejection Fraction?: The Wrong Way to Identify New Treatments for a Nonexistent Disease. Circ Heart Fail. 2011;4:538–540. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 206.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJV. What Have We Learned About Patients With Heart Failure and Preserved Ejection Fraction From DIG-PEF, CHARM-Preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 207.Chaudhry SI, McAvay G, Chen S, Whitson H, Newman AB, Krumholz HM, et al. Risk Factors for Hospital Admission Among Older Persons With Newly Diagnosed Heart FailureFindings From the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61:635–642. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lupon J, Gonzalez B, Santaeugenia S, Altimir S, Urrutia A, Mas D, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61:835–842. [PubMed] [Google Scholar]
- 209.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 210.Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart Failure in Rats Causes Changes in Skeletal Muscle Morphology and Gene Expression That Are Not Explained by Reduced Activity. Circulation Research. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- 211.Groban L, Pailes NA, Bennett C, Carter CS, Chappell MC, Kitzman DW, et al. Growth Hormone Replacement Attenuates Diastolic Dysfunction and Cardiac Angiotensin II Expression in Senescent Rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 212.Groban L, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, et al. Progressive diastolic dysfunction in the female mRen(2).Lewis Rat: Influence of salt and ovarian hormones. Gerontol B Physiol Sci Sco Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- 213.Lasocki S, Iglarz M, Seince PF, Vuillaumier-Barrot S, Vicaut E, Henrion D, et al. Involvement of renin-angiotensin system in pressure-flow relationship: role of angiotensin-converting enzyme gene polymorphism. Anesthesiology. 2002;96:271–275. doi: 10.1097/00000542-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 214.Little WC, Wesley-Farrington DJ, Hoyle J, Brucks S, Robertson S, Kitzman DW, et al. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc.Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 215.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 216.Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 217.Deswal A, Richardson P, Bozkurt B, Mann D. Results of the Randomized Aldosterone Antagonism in Heart Failure With Preserved Ejection Fraction Trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 218.Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, et al. Aldosterone antagonists and outcomes in real-world older patients with heart failure and preserved ejection fraction. JACC: Heart Failure. 2013;1:40–47. doi: 10.1016/j.jchf.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 220.Hummel S, Seymour E, Brook R, Kolias T, Sheth S, Rosenblum H, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Conraads V, Metra M, Kamp O, De Keulenaer G, Pieske B, Zamorano J, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 222.Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical Effectiveness of Beta-Blockers in Heart Failure: Findings From the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of Digoxin on Morbidity and Mortality in Diastolic Heart Failure: The Ancillary Digitalis Investigation Group Trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of Treatment on Exercise Tolerance, Cardiac Function, and Mortality in Heart Failure With Preserved Ejection Fraction: A Meta-Analysis. J Am Coll Cardiol. 2011;57:1676–1686. doi: 10.1016/j.jacc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 225.Redfield M, Chen H, Borlaug B, Semigran M, Lee K, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (dilate-1): A randomized, double-blind, placebo-controlled, single-dose study. CHEST Journal. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 228.Martin J, Denver R, Bailey M, Krum H. In vitro inhibitory effects of atorvastatin on cardiac fibroblasts: implications for ventricular remodelling. Clin Exp Pharmacol Physiol. 2005;32:697–701. doi: 10.1111/j.1440-1681.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 229.Fukuta H, Sane DC, Brucks S, Little WC. Statin Therapy May Be Associated With Lower Mortality in Patients With Diastolic Heart Failure: A Preliminary Report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 230.Fukuta H, Little W. Observational studies of statins in heart failure with preserved systolic function. Heart Fail Clin. 2008;4:209–216. doi: 10.1016/j.hfc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 231.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 232.Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating Anemia in Older Adults with Heart Failure with a Preserved Ejection Fraction (HFPEF) with Epoetin Alfa: Single Blind Randomized Clinical Trial of Safety and Efficacy. Circ Heart Fail. 2013;6:254–263. doi: 10.1161/CIRCHEARTFAILURE.112.969717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX, deGroof RC. The Effect of Alagebrium Chloride (ALT-711), a Novel Glucose Cross-Link Breaker, in the Treatment of Elderly Patients with Diastolic Heart Failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 234.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier L. Ranolazine for the Treatment of Heart Failure With Preserved Ejection Fraction: Background, Aims, and Design of the RALI-DHF Study. Clin Cardiol. 2011;34:426–432. doi: 10.1002/clc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 236.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2012;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 237.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the relaxin in acute heart failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 238.Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, et al. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014;35:1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2:123–130. doi: 10.1016/j.jchf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 240.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly D, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 242.Bartolini M, Andrisano V. Strategies for the inhibition of protein aggregation in human diseases. ChemBioChem. 2010;11:1018–1035. doi: 10.1002/cbic.200900666. [DOI] [PubMed] [Google Scholar]
- 243.Falk R, Maurer M, Fedson S, Judge D, Zeldenrust S, Quyyumi A, et al. Tafamidis stabilizes transthyretin and improves clinical outcomes in transthyretin amyloid cardiomyopathy. J Card Fail. 2011;17:S56. [Google Scholar]
- 244.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 245.Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–618. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- 246.Kitzman DW, Upadhya B, Reeves G. Hospitalizations and prognosis in elderly patients with heart failure and preserved ejection fraction: time to treat the whole patient. JACC Heart Fail. 2015 doi: 10.1016/j.jchf.2015.01.009. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Edelmann F, Gelbrich G, Dungen H, Frohling S, Wachter R, Stahrenberg R, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 248.Kitzman D, Brubaker P, Herrington D, Morgan T, Stewart K, Hundley W, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rickard A, Morgan J, Tesch G, Funder J, Fuller P, Young M. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood Pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 250.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 251.Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, et al. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–294. doi: 10.1161/HYPERTENSIONAHA.108.111815. [DOI] [PubMed] [Google Scholar]
- 252.Hummel S, Seymour E, Brook R, Sheth S, Ghosh E, Zhu S, et al. Low-sodium DASH diet Improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013 doi: 10.1161/CIRCHEARTFAILURE.113.000481. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20:212–221. doi: 10.1016/j.cardfail.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 254.Sondergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796–801. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 255.Georgakopoulos D, Little WC, Abraham WT, Weaver FA, Zile MR. Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–178. doi: 10.1016/j.cardfail.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 256.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 1919;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 257.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 258.Meyns B, Klotz S, Simon A, Droogne W, Rega F, Griffith B, et al. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J Am Coll Cardiol. 2009;54:79–86. doi: 10.1016/j.jacc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 259.Penicka M, Kocka V, Herman D, Trakalova H, Herold M. Cardiac resynchronization therapy for the causal treatment of heart failure with preserved ejection fraction: insight from a pressure-volume loop analysis. Eur J Heart Fail. 2010;12:634–636. doi: 10.1093/eurjhf/hfq068. [DOI] [PubMed] [Google Scholar]