Abstract
Objective:
Investigate and confirm the association between sympathoadrenal activation, endotheliopathy and poor outcome in trauma patients.
Background:
The association between sympathoadrenal activation, endotheliopathy, and poor outcome in trauma has only been demonstrated in smaller patient cohorts and animal models but needs confirmation in a large independent patient cohort.
Methods:
Prospective observational study of 424 trauma patients admitted to a level 1 Trauma Center. Admission plasma levels of catecholamines (adrenaline, noradrenaline) and biomarkers reflecting endothelial damage (syndecan-1, thrombomodulin, and sE-selectin) were measured and demography, injury type and severity, physiology, treatment, and mortality up till 28 days were recorded.
Results:
Patients had a median ISS of 17 with 72% suffering from blunt injury. Adrenaline and noradrenaline correlated with syndecan-1 (r = 0.38, P < 0.001 and r = 0.23, P < 0.001, respectively) but adrenaline was the only independent predictor of syndecan-1 by multiple linear regression adjusted for age, injury severity score, Glascow Coma Scale, systolic blood pressure, base excess, platelet count, hemoglobin, prehospital plasma, and prehospital fluids (100 pg/mL higher adrenaline predicted 2.75 ng/mL higher syndecan-1, P < 0.001). By Cox analyses adjusted for age, sex, injury severity score, Glascow Coma Scale, base excess, platelet count and hemoglobin, adrenaline, and syndecan-1 were the only independent predictors of both <24-hours, 7-day and 28-day mortality (all P < 0.05). Furthermore, noradrenaline was an independent predictor of <24-hours mortality and thrombomodulin was an independent predictor of 7-day and 28-day mortality (all P < 0.05).
Conclusions:
We confirmed that sympathoadrenal activation was strongly and independently associated with endothelial glycocalyx and cell damage (ie, endotheliopathy) and furthermore that sympathoadrenal activation and endotheliopathy were independent predictors of mortality in trauma patients.
Keywords: endotheliopathy, mortality, syndecan-1, thrombomodulin, trauma
Trauma is a major cause of death and disability worldwide and despite the introduction of damage control resuscitation1 mortality remains high.2 Approximately one quarter of the trauma patients present with laboratory defined coagulopathy upon admission, and this is associated with a three to fourfold increase in mortality.3 In the literature, laboratory coagulopathy also designated trauma induced coagulopathy (TIC) is described as an over activation of the protein C pathway accompanied by hyperfibrinolysis4 or as representing the hemorrhagic phenotype of disseminated intravascular coagulation.5 Common for both these explanations is that they are based on measuring concentrations of pro- and anticoagulant coagulation factors, platelet count, and fibrin degradation products.
The introduction of the cell based model of hemostasis in the late 1990 s taught us that to adequately assess hemostatic competence whole blood that includes blood cellular elements should be used6 and consequently, assays reflecting this were required. When studying trauma patients at admission with whole blood thrombelastography (TEG), we found that with increasing injury severity, the hemostatic profile became progressively more hypocoagulable and hyperfibrinolytic, which seemed counterintuitive given the substantial risk of exsanguination these patients faced.7 To fully comprehend these findings, we speculated that the endothelium should also be included as a pivotal part of the vascular compartment and we hypothesized that the hemostatic changes observed in whole blood represented an evolutionary adapted response to a potential life-threatening condition.8 The vascular endothelium comprises a single layer of cells that lines every vessel in the body, covers a total surface area of 5000 m2 and has a total weight of 1 kg.9 On the luminal part of the endothelium lays the endothelial glycocalyx, a 0.2 to 1 μm thick negatively charged antiadhesive and anticoagulant carbohydrate-rich surface layer that protects the endothelium and maintains vascular barrier function.10–12 The endothelium is instrumental for balancing hemostasis and for maintaining the homeostasis between the circulating blood and the extravascular space/surrounding tissues.13,14 Furthermore, given the immediate nature of changes in hemostatic competence in severely injured trauma patients we further speculated that activation of the sympathoadrenal activation, with release of large amounts of catecholamines was an important driver of this condition.8
In independent, but small, studies of trauma patients, we found that (i) high circulating syndecan-1, a marker of endothelial glycocalyx degradation,15 was associated with inflammation, hypocoagulability, and increased mortality;16 (ii) the trauma-induced catecholamine surge was closely associated with biomarkers of tissue and endothelial damage, glycocalyx degradation, hypocoagulability including hyperfibrinolysis and independently predicted mortality;17 and (iii) the syndecan-1 levels decreased after resuscitation with plasma.18 We also found that an important determinant of the hypocoagulability observed by whole blood TEG, in these patients, was because of endogenous heparinization induced by the constituents released from the glycocalyx in response to the trauma and shock.19 Collectively, these data indicated that endothelial damage and/or dysfunction significantly contributed to outcome and that sympathoadrenal activation was an important driver of early shock induced coagulopathy.20
Introduction of early administration of plasma and platelets along with red blood cells (RBCs) to trauma patients with severe hemorrhage, not only reduced early deaths caused by exsanguination21 but also led to a reduction in development of multiple organ failure.22 Interestingly, Kozar et al23 found in a rat model of hemorrhagic shock that resuscitation with plasma, as opposed to normal saline, had an endothelial- and glycocalyx rejuvenating effect related to increased mRNA levels of Syndecan-1 in the endothelial cells. More recently, adiponectin was identified as an important component in FFP to attenuate endothelial damage and improve lung vascular barrier function in mice after shock and resuscitation and collectively, these data suggest a pivotal role of the endothelium for outcome in trauma patients with hemorrhagic shock.24,25
The aim of the current study was therefore to validate and confirm our preliminary findings presented above in a larger, and independent, cohort of trauma patients and to more comprehensively characterize the endothelium by analyzing also soluble thrombomodulin (sTM), an endothelial cell damage marker, and soluble E-selectin (sE-selectin), an endothelial cell activation marker, which both have been significantly associated with poor outcome in patients with other types of acute critical illness.26–28
METHODS
Setting and Patients
This prospective observational study was conducted under an approved Institutional Review Board (IRB) (Universal Study, HSC-GEN-12–0059), which included all adult trauma patients (≥16 yrs) at the highest level of activation at Memorial Hermann Hospital Texas Medical Center (MHH-TMC). The criteria for highest activation include: Glasgow Coma Score ≤10, heart rate >120 beats per minute, systolic blood pressure ≤90, respiratory rate <10 or >29 per minute, intubation, penetrating injury to torso, groin, head or neck, amputation proximal to ankle or wrist, paraplegia, quadriplegia, uncontrolled external hemorrhage, fracture to pelvis or two or more long bone fractures, and receiving blood en route. The study took place over 18 months from March 2012 to September 2013. This was an opportunistic prospective study that ran parallel to three other clinical studies at MHH-TMC; patients admitted to the other studies were not included in this analysis. Samples were collected when research staff was not directly involved in the other studies and available to process the samples. The IRB approval was obtained for delayed consent, obtained from the patient or their legally authorized representative within 72 hours of admission, or as soon as possible. For patients who were discharged or died within 24 hours of admission, a waiver of consent was obtained. If consent could not be obtained, the patient was excluded from the study and their blood samples destroyed. The study excluded pregnant women and prisoners.
In the current study, plasma biomarkers from a total of 561 patients were investigated. Of these, only patients suffering from blunt or penetrating injury were included with the requirement that data on ISS and adrenaline, noradrenaline, syndecan-1, and thrombomodulin were available. This excluded burn injury patients (n = 64) and an additional 73 patients [ie, nontrauma (n = 2), missing ISS (n = 42), and missing biomarkers (n = 29)]. The excluded nonburn injury patients (n = 73) did not differ significantly from the 424 included patients with regard to age median 43 years (interquartile range, IQR 27–55), sex (73% males), and <24-hours, 7-day, and 28-day mortality (4%, 14%, and 15%, respectively).
Clinical Data and Blood Samples
Information on baseline demography, clinical, physiologic, and laboratory tests were retrospectively collected from patient records and the institutional trauma registry. The data included admission vital signs, blood count test results, pH, base excess (BE), conventional plasma based coagulation tests [prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR)], prehospital fluids, 24-hour blood transfusions (packed RBCs, plasma, and apheresis platelets), 24-hour crystalloid infusions, complications, injury severity score (ISS), and patient outcomes (mortality up to 28 days).
Blood samples were collected in citrated tubes from each patient immediately upon hospital admission. Patients from whom blood samples could not be obtained were excluded from analysis. Upon hospital admission, 20 mL of blood was obtained. Blood was transferred into vacutainer tubes containing 3.2% citrate and inverted to ensure proper anticoagulation. After spinning, plasma was aliquoted and frozen for later analysis. The enzyme linked immunosorbent assay (ELISA) analyses were conducted by trained laboratory technicians, according to the manufactures recommendations at the Hemostasis Research Laboratory, Section for Transfusion Medicine, Capital Region Blood Bank, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
ELISA
Soluble biomarkers of sympathoadrenal activation (adrenaline, noradrenaline), endothelial cell activation (sE-selectin),29,30 endothelial glycocalyx (syndecan-1),15 and cell (thrombomodulin)29–31 damage were measured by commercially available immunoassays in EDTA plasma according to the manufactures recommendations. Adrenaline and noradrenaline (2-CAT ELISA, Labor Diagnostica Nord GmbH & Co. KG, Nordhorn, Germany) have lower limit of detection (LLD) 10 pg/mL (adrenaline, normal reference <100 pg/mL) and 50 pg/mL (noradrenaline, normal reference <600 pg/mL), respectively. Syndecan-1 (Diaclone Nordic Biosite, Copenhagen, Denmark) has LLD 4.94 ng/mL, soluble thrombomodulin (sTM, Nordic Biosite, Copenhagen, Denmark) has LLD 0.31 ng/mL, sE-selectin (IBL International GMBH, Hamburg, Germany) has LLD 0.3 ng/mL, and sVE-cadherin (R&D Systems Europe, Ltd., Abingdon, UK) has LLD 0.113 ng/mL.
Statistics
Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and SPSS 22 (IBM Corporation, New York, NY).
Simple correlations were investigated by Pearson correlations with results displayed as the product-moment correlation coefficient Pearson's r (or r2) and P values. To investigate the independent association between sympathoadrenal activation and endothelial glycocalyx and cell damage (syndecan-1, thrombomodulin, and sE-selectin), univariate and multivariate adjusted linear regression analysis were performed, including variables that were either found to correlate with or were expected to influence endothelial damage: age, ISS, GCS, SBP, BE, platelet count, hemoglobin, prehospital plasma, and administered prehospital fluids (Table 2). Results are presented as regression coefficients (β) with 95% confidence intervals (CI) and P values.
TABLE 2.
Univariate and Multivariate Linear Regression Analysis of Variables Associated With Circulating Levels of Biomarkers Reflecting Endothelial Glycocalyx and Cell Damage in 424 Trauma Patients Trauma Patients Admitted to a Level I Trauma Centre in the United States
| Predictors of Syndecan-1 | Predictors of Thrombomodulin | ||||||||
| Univariate | Adjusted (R = 0.40) | Univariate | Adjusted (R = 0.29) | ||||||
| β (95%CI) | P | β (95%CI) | P | β (95 CI) | P | β (95%CI) | P | ||
| Age | 10 yrs | 0.45 (−4.25–5.16) | 0.851 | 0.34 (−4.01–4.68) | 0.879 | 0.36 (0.20–0.52) | 0.000 | 0.32 (0.15–0.5) | 0.000 |
| ISS (ED) | 1 point | 1.73 (1.06–2.41) | 0.000 | 0.48 (−0.30–1.25) | 0.229 | 0.03 (0.00–0.05) | 0.019 | 0.00 (−0.03–0.03) | 0.890 |
| GCS (ED) | 1 point | −1.92 (−3.49–0.36) | 0.016 | 0.17 (−1.48–1.83) | 0.837 | −0.05 (−0.10–0.01) | 0.088 | −0.01 (−0.07–0.06) | 0.879 |
| SBP (ED) | 10 mmHg | −6.23 (−8.93–3.53) | 0.000 | −2.69 (−5.51–0.14) | 0.062 | −0.03 (−0.13–0.06) | 0.507 | −0.01 (−0.12–0.1) | 0.857 |
| BE (ED) | 1 mEg/L | −4.24 (−5.9–2.58) | 0.000 | −0.91 (−2.73–0.91) | 0.325 | −0.07 (−0.13–0.01) | 0.029 | −0.03 (−0.11–0.04) | 0.375 |
| Platelet count (ED) | 10*109/L | −1.31 (−2.36–0.26) | 0.014 | −0.62 (−1.62–0.37) | 0.220 | −0.04 (−0.07–0.00) | 0.068 | −0.03 (−0.07–0.01) | 0.113 |
| Hemoglobin (ED) | 1 g/dL | −5.15 (−9.41–0.89) | 0.018 | −2.17 (−6.31–1.98) | 0.305 | −0.19 (−0.34–0.04) | 0.014 | −0.17 (−0.33–0.00) | 0.048 |
| Plasma (prehospital) | 1 unit | 42.48 (19.22–65.75) | 0.000 | 6.02 (−16.78–28.81) | 0.604 | 0.72 (−0.15–1.59) | 0.104 | 0.36 (−0.55–1.27) | 0.432 |
| Crystalloids (prehospital) | 100 mL | 1.23 (−0.47–2.93) | 0.155 | −0.64 (−2.55–1.27) | 0.508 | −0.01 (−0.07–0.05) | 0.758 | −0.01 (−0.09–0.06) | 0.758 |
| Adrenaline | 100 pg/mL | 3.68 (2.79–4.57) | 0.000 | 2.75 (1.54–3.96) | 0.000 | 0.02 (−0.01–0.06) | 0.213 | 0.02 (−0.02–0.07) | 0.328 |
Regression coefficients (β) with 95% CI and P (and R, for the multivariate models) are displayed. Predicted change in plasma syndecan-1 (ng/mL) or soluble thrombomodulin (ng/mL) levels associated with one unit increase in: Age (10 yrs older), ISS 1 point higher, GCS score, SBP 10 mmHg higher, BE 1 mEq/L higher, 1 point higher, platelet count (10 platelets*109/L higher), hemoglobin 1 g/dL higher, plasma (prehospital, per 1 unit transfused), crystalloids volume (prehospital, per 100 mL administered) and plasma adrenaline level (100 pg/mL higher).
BE indicates base excess; CI, confidence intervals; ED, emergency department; GCS, Glascow Coma Scale; ISS, injury severity score; SBP, systolic blood pressure.
The predictive value of sympathoadrenal activation (adrenaline, noradrenaline) and endothelial damage (syndecan-1, thrombomodulin) for <24-hour, 7-day, and 28-day mortality was investigated by univariate and multivariate adjusted Cox proportional-hazards models, the latter after adjusting for age, sex, ISS, GCS, BE, platelet count, and hemoglobin. Data are presented as relative hazard ratio (HR) with 95% CI, Walds χ2 and P values. To reveal the influence of missing values on the results, multiple imputation analysis (five imputed dataset and a merge of these) were conducted and the linear regression analysis and Cox proportional-hazards models were repeated on imputed data. This did not change the results for any of the analyses and results from the raw data are displayed in the manuscript. Data are presented as medians with IQR or as n (proportions). P values <0.05 were considered significant.
RESULTS
Patients
A total of 424 patients were included in the current study with a median age of 40 years, 77% were male, median ISS was 17, and 72% suffered from blunt injury (Table 1). Further details on demography, injury severity and type, admission physiology, biomarker levels, transfusions, and outcome are displayed in Table 1.
TABLE 1.
Demography, Injury Type and Severity, Admission Physiology, Biomarker Levels, Transfusions, and Outcome in 424 Prospectively Enrolled Trauma Patients Admitted to a Level I Trauma Centre in the United States
| Unit | Median (IQR) | |
| Demography | ||
| N | 424 | |
| Age | yrs | 40 (27–55) |
| Sex | male [n (%)] | 326 (76.9%) |
| Race | W / BL /H /Others | 231 (54%) / 67 (16%) / 103 (24%) / 23 (6%) |
| Injury type and severity | ||
| MOI | Blunt injury | 306 (72%) |
| ISS | score | 17 (9–26) |
| Severe TBI (AIS head ≥3) | n (%) | 180 (42%) |
| GCS | score | 12 (3–15) |
| Transfer | n (%) | 168 (40%) |
| Mode of Transport | HEL/AMB/Others | 238 (56%) / 168 (40%) / 18 (4%) |
| Admission physiology and biochemistry | ||
| SBP (ED) | mmHg | 124 (106–142) |
| BE (ED) | mEq/L | −3 (-6–1) |
| pH (ED) | 7.32 (7.25–7.37) | |
| Platelet count (ED) | 109/L | 220 (180–269) |
| Hemoglobin (ED) | g/dL | 13.1 (11.9–14.4) |
| PT | sec | 14.2 (13.4–15.3) |
| INR | ratio | 1.1 (1–1.2) |
| aPTT | sec | 29.2 (26.3–32.5) |
| Biomarkers | ||
| Adrenaline | pg/mL | 162 (53–364) |
| Noradrenaline | pg/mL | 848 (332–1501) |
| Syndecan-1 | ng/mL | 25 (13–60) |
| Thrombomodulin | ng/mL | 5.2 (3.84–7.19) |
| sE-selectin | ng/mL | 39 (23–58) |
| Transfusions | ||
| Transfused | n (%) | 138 (61.1%) |
| RBC (prehospital) | n (%) | 38 (10.4%) |
| Plasma (prehospital) | n (%) | 29 (7.9%) |
| Platelets (prehospital) | n (%) | 0 (0%) |
| Crystalloids (prehospital) | n (%) | 156 (39.6%) |
| Crystalloids volume (prehospital) | mL | 0 (0–200) |
| Outcome | ||
| Hospital LOS | days | 5 (1–15) |
| ICU LOS | days | 1 (0–5) |
| Ventilator | days | 1 (0–3) |
| Mortality (<24 hrs) | n (%) | 19 (4.9%) |
| Mortality (7days) | n (%) | 62 (16.1%) |
| Mortality (28 days) | n (%) | 73 (17.2%) |
Data are presented as medians (IQR) or n (%).
AIS head >3.
AIS indicates Abbreviated Injury Score; AMB, ambulance; aPTT, activated partial thromboplastin time; BE, base excess; BL, Blacks; ED, emergency department; GCS, Glascow Coma Scale; H, Hispanics; HEL, helicopter; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range; ISS, injury severity score; LOS, length of stay; MOI, mechanism of injury; PH, prehospital; PT, prothrombin time; RBC, red blood cells; SBP, systolic blood pressure; TBI, severe traumatic brain injury; W, Whites.
Sympathoadrenal Activation and Endothelial Damage
To investigate the association between sympathoadrenal activation and endothelial damage, we investigated simple correlations between catecholamine levels and biomarkers reflecting endothelial damage followed by linear regression analysis.
By simple correlations, plasma adrenaline and noradrenaline correlated with plasma syndecan-1 (r = 0.38, P < 0.001 and r = 0.23, P < 0.001, respectively) whereas neither adrenaline nor noradrenaline correlated with thrombomodulin (r = 0.06, P = 0.213 and r = 0.07, P = 0.149, respectively) or sE-selectin (r = –0.09, P = 0.094 and r = –0.01, P = 0.886, respectively). When stratifying patients according to injury type, the correlation between plasma adrenaline and syndecan-1 levels persisted within in both blunt (r = 0.43, P < 0.001) and penetrating (r = 0.24, P = 0.013) injury patients.
By linear regression analysis, univariate predictors of higher syndecan-1 were higher ISS and lower GCS, SBP, BE, platelet count and hemoglobin, and prehospital plasma and higher adrenaline but in the adjusted model, higher plasma adrenaline was the only independent predictor of higher syndecan-1 levels (Table 2). Univariate predictors of higher thrombomodulin were higher age and ISS and lower BE and hemoglobin (whereas adrenaline was not) but only higher age and lower hemoglobin were independent predictors of higher thrombomodulin (Table 2). We did not perform linear regression analysis for sE-selectin as this biomarker neither correlated with catecholamines nor were predictive for mortality (see below).
As depicted in Figure 1, the combined highest level plasma adrenaline, injury severity, shock, inhospital transfusion, and 28-day mortality were associated with excessively increased syndecan-1 levels (Figure 1A–D).
FIGURE 1.
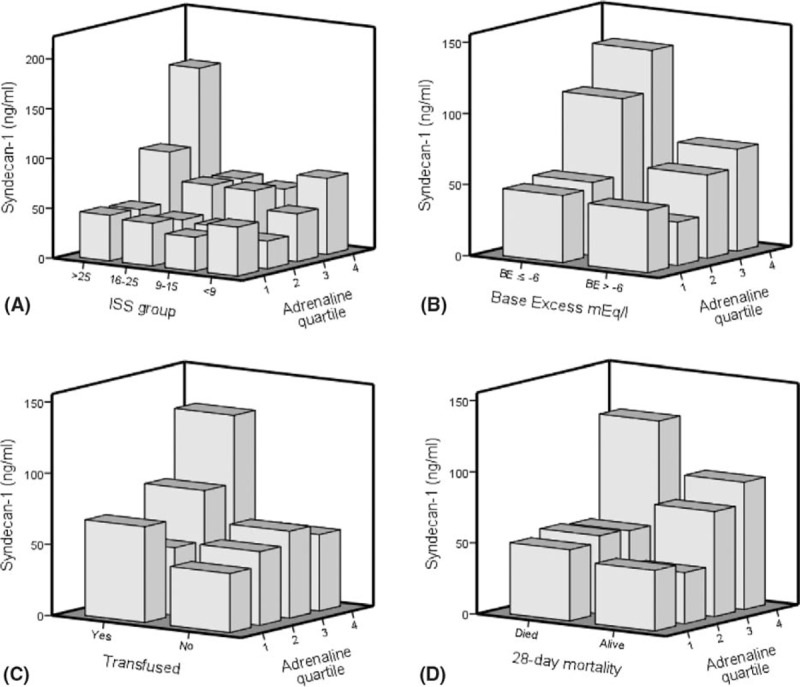
3-D plots displaying associations between plasma adrenaline, injury severity, base excess, transfusions, mortality, and plasma levels of endothelial glycocalyx damage in 424 trauma patients admitted to a Level I Trauma Centre in the United States. Injury severity score (ISS) were categorized into four groups (ISS <9, ISS 9–15, ISS 16–25, and ISS >25) and plasma adrenaline were categorized into quartiles (Q1 28 pg/mL (IQR 16–28), Q2 93 pg/mL (IQR 77–124), Q3 263 pg/mL (IQR 217–316), and Q4 884 pg/mL (IQR 659–1901). Transfusions refers to inhospital transfusions (yes, no). The 3-D plots display: (A) ISS, adrenaline quartile, and syndecan-1 (ng/mL); (B) Base excess, adrenaline quartile, and syndecan-1 (ng/mL); (C) Transfusions, adrenaline quartile, and syndecan-1 (ng/mL); and (D) 28-day mortality, adrenaline quartiles, and syndecan-1 (ng/mL).
Outcome
The predictive value of sympathoadrenal activation and endothelial damage for mortality was investigated by univariate and adjusted Cox proportional-hazards models.
In the Cox proportional-hazards analyses, higher age, higher ISS, and lower GCS, BE and hemoglobin, and higher adrenaline, noradrenaline (P = 0.007, P = 0.137, and P = 0.032, respectively) syndecan-1 and thrombomodulin levels were univariate predictors of <24-hour, 7-day, and 28-day mortality. The only exceptions were that GCS did not predict <24-hours mortality; noradrenaline did not predict 7-day mortality and hemoglobin did not predict 7-day and 28-day mortality (Table 3). Plasma sE-selectin did not predict mortality (data not shown).
TABLE 3.
Univariate and Multivariate Cox Proportional Hazards Models of Variables Associated With 24-Hours, 7-Day, and 28-Day Mortality in 424 Trauma Patients Trauma Patients Admitted to a Level I Trauma Centre in the United States
| Covariable | Biomarker: Adrenaline | Biomarker: Syndecan-1 | Biomarker: Thrombomodulin | ||||||||||
| Univariate | Adjusted | Adjusted | Adjusted | ||||||||||
| HR (95%CI) | Wald | P | HR (95%CI) | Wald | P | HR (95%CI) | Wald | P | HR (95%CI) | Wald | P | ||
| <24-hour mortality | |||||||||||||
| Biomarker | Univariate | 1.06 (1.03–1.09) | 19.0 | <0.001 | 1.06 (1.03–1.09) | 18.0 | <0.001 | 1.09 (0.98–1.21) | 2.5 | 0.116 | |||
| Biomarker | Adjusted | 1.06 (1.02–1.09) | 10.5 | 0.001 | 1.04 (1.00–1.08) | 3.9 | 0.047 | 1.03 (0.9–1.17) | 0.2 | 0.674 | |||
| Age | 10 Yrs | 1.26 (1.02–1.57) | 4.5 | 0.034 | 1.30 (1.02–1.66) | 4.5 | 0.035 | 1.2 (0.95–1.51) | 2.3 | 0.132 | 1.20 (0.95–1.51) | 2.3 | 0.131 |
| Sex | Male | 0.52 (0.20–1.31) | 1.9 | 0.163 | 0.69 (0.23–2.10) | 0.4 | 0.519 | 0.7 (0.25–1.97) | 0.5 | 0.495 | 0.78 (0.28–2.19) | 0.2 | 0.636 |
| ISS | 1 Point | 1.03 (1.00–1.06) | 4.1 | 0.042 | 1.00 (0.96–1.04) | 0.0 | 0.833 | 1.00 (0.96–1.04) | 0.0 | 0.994 | 1.01 (0.97–1.05) | 0.2 | 0.690 |
| GCS (ED) | 1 Point | 0.92 (0.85–1.01) | 3.3 | 0.070 | 0.98 (0.88–1.08) | 0.2 | 0.627 | 0.98 (0.88–1.08) | 0.2 | 0.673 | 0.98 (0.89–1.09) | 0.1 | 0.726 |
| BE (ED) | 1 mEq/L | 0.90 (0.84–0.95) | 12.7 | <0.001 | 0.93 (0.86–1.01) | 2.9 | 0.088 | 0.94 (0.88–1.00) | 3.3 | 0.069 | 0.92 (0.86–0.98) | 6.4 | 0.012 |
| Platelet count (ED) | 10*109/L | 0.94 (0.88–1.00) | 3.6 | 0.058 | 0.97 (0.9–1.04) | 0.9 | 0.348 | 0.97 (0.91–1.04) | 0.6 | 0.439 | 0.96 (0.90–1.03) | 1.1 | 0.295 |
| Hemoglobin (ED) | 1 g/dL | 0.83 (0.69–1.00) | 4.0 | 0.046 | 0.96 (0.76–1.23) | 0.1 | 0.767 | 0.96 (0.76–1.22) | 0.1 | 0.758 | 0.95 (0.75–1.22) | 0.1 | 0.710 |
| 7-day mortality | |||||||||||||
| Biomarker | Univariate | 1.06 (1.04–1.08) | 34.3 | <0.001 | 1.04 (1.02–1.06) | 12.9 | <0.001 | 1.11 (1.05–1.17) | 13.9 | <0.001 | |||
| Biomarker | Adjusted | 1.06 (1.03–1.08) | 25.1 | <0.001 | 1.03 (1.00–1.05) | 4.9 | 0.027 | 1.07 (1.00–1.14) | 4.3 | 0.037 | |||
| Age | 10 Yrs | 1.48 (1.30–1.67) | 37.5 | <0.001 | 1.46 (1.28–1.67) | 30.8 | <0.001 | 1.44 (1.26–1.64) | 28.2 | <0.001 | 1.4 (1.23–1.61) | 24.3 | <0.001 |
| Sex | Male | 0.61 (0.36–1.04) | 3.3 | 0.070 | 0.68 (0.38–1.23) | 1.6 | 0.207 | 0.83 (0.47–1.47) | 0.4 | 0.529 | 0.84 (0.47–1.48) | 0.4 | 0.539 |
| ISS | 1 Point | 1.03 (1.02–1.05) | 15.7 | <0.001 | 1.00 (0.98–1.02) | 0.2 | 0.692 | 1.01 (0.99–1.03) | 2.0 | 0.158 | 1.02 (1.00–1.04) | 3.8 | 0.051 |
| GCS (ED) | 1 Point | 0.94 (0.90–0.98) | 7.5 | 0.006 | 0.96 (0.91–1.01) | 2.7 | 0.099 | 0.97 (0.92–1.02) | 1.2 | 0.272 | 0.97 (0.92–1.03) | 1.0 | 0.307 |
| BE (ED) | 1 mEq/L | 0.95 (0.91–1.00) | 4.3 | 0.038 | 1.00 (0.94–1.05) | 0.0 | 0.865 | 0.97 (0.93–1.02) | 1.3 | 0.250 | 0.97 (0.93–1.02) | 1.4 | 0.241 |
| Platelet count (ED) | 10*109/L | 0.97 (0.94–1.01) | 2.8 | 0.094 | 0.97 (0.94–1.01) | 2.4 | 0.124 | 0.98 (0.95–1.02) | 1.0 | 0.312 | 0.98 (0.95–1.02) | 0.9 | 0.339 |
| Hemoglobin (ED) | 1 g/dL | 0.91 (0.82–1.02) | 2.5 | 0.117 | 0.98 (0.86–1.11) | 0.1 | 0.724 | 1.00 (0.88–1.14) | 0.0 | 0.976 | 1.00 (0.88–1.13) | 0.0 | 0.996 |
| 28-day mortality | |||||||||||||
| Biomarker | Univariate | 1.05 (1.03–1.07) | 36.5 | <0.001 | 1.04 (1.02–1.06) | 14.3 | <0.001 | 1.10 (1.04–1.15) | 12.3 | <0.001 | |||
| Biomarker | Adjusted | 1.06 (1.04–1.08) | 27.5 | <0.001 | 1.03 (1.00–1.05) | 5.2 | 0.022 | 1.06 (1.00–1.13) | 3.9 | 0.049 | |||
| Age | 10 Yrs | 1.41 (1.26–1.59) | 34.3 | <0.001 | 1.41 (1.24–1.60) | 28.9 | <0.001 | 1.39 (1.23–1.58) | 27.4 | <0.001 | 1.36 (1.20–1.54) | 22.7 | <0.001 |
| Sex | Male | 0.63 (0.38–1.03) | 3.4 | 0.066 | 0.66 (0.38–1.15) | 2.2 | 0.139 | 0.83 (0.49–1.43) | 0.4 | 0.507 | 0.82 (0.48–1.4) | 0.5 | 0.460 |
| ISS | 1 Point | 1.03 (1.02–1.05) | 15.4 | <0.001 | 1.00 (0.98–1.02) | 0.0 | 0.841 | 1.01 (0.99–1.03) | 1.9 | 0.171 | 1.02 (1.00–1.04) | 3.4 | 0.065 |
| GCS (ED) | 1 Point | 0.94 (0.90–0.98) | 8.5 | 0.004 | 0.96 (0.91–1.00) | 3.3 | 0.070 | 0.97 (0.92–1.02) | 1.6 | 0.203 | 0.97 (0.93–1.02) | 1.3 | 0.250 |
| BE (ED) | 1 mEq/L | 0.96 (0.92–1.01) | 2.8 | 0.097 | 1.01 (0.96–1.07) | 0.3 | 0.583 | 0.98 (0.94–1.03) | 0.6 | 0.442 | 0.98 (0.94–1.03) | 0.5 | 0.463 |
| Platelet count (ED) | 10*109/L | 0.97 (0.94–1) | 2.9 | 0.090 | 0.97 (0.94–1.01) | 2.7 | 0.098 | 0.98 (0.95–1.01) | 1.2 | 0.270 | 0.98 (0.95–1.01) | 1.2 | 0.284 |
| Hemoglobin (ED) | 1 g/dL | 0.92 (0.83–1.02) | 2.3 | 0.132 | 1.00 (0.89–1.13) | 0.0 | 0.953 | 1.01 (0.90–1.13) | 0.0 | 0.862 | 1.01 (0.90–1.14) | 0.0 | 0.830 |
Relative hazards (HR) with 95% CI, Wald and P values are displayed. The HR (95% CI) are associated with one unit increase in: Age (10 yrs older), Sex (male sex), ISS 1 point higher, GCS 1 point higher, BE 1 mEq/L higher, platelet count (10 platelets*109/L higher), hemoglobin (1 g/dL higher) and either adrenaline (100 pg/mL higher), syndecan-1 (10 ng/mL), and thrombomodulin (1 ng/mL higher).
BE indicates base excess; CI, confidence intervals; ED, emergency department; GCS, Glascow Coma Scale; ISS, injury severity score; SBP, systolic blood pressure.
In the adjusted models, only adrenaline and syndecan-1 were independent biomarker predictors of early <24-hour mortality together with age and BE (Table 3). Adrenaline, syndecan-1, and thrombomodulin levels were independent predictors of 7-day and 28-day mortality together with age (Table 3). In the adjusted models, noradrenaline predicted <24-hours mortality (P = 0.048) but not 7-day or 28-day mortality (data not shown).
DISCUSSION
The main findings of the current study were that plasma catecholamine levels at admission correlated with circulating levels of syndecan-1, an endothelial glycocalyx constituent, and that adrenaline levels independently predicted higher syndecan-1 levels—that is, a surrogate marker of glycocalyx degradation (endotheliopathy of trauma). Furthermore, admission adrenaline and syndecan-1 were independent predictors of both early and late mortality.
The finding here of an independent association between higher syndecan-1 levels and mortality of trauma patients underscores the importance of the state of the endothelium and its related constituents for outcome in these critically ill patients, and is in alignment with our previous findings in smaller cohorts of trauma patients.16,17 The results are also in agreement with findings in other patient groups facing acute critical illness. In a large cohort of patients with varying degrees of sepsis, we found a dose-dependent increase in syndecan-1 levels with increased sepsis severity and mortality.26 Moreover, syndecan-1 was here independently associated with organ failure, such as hepatic and renal failure.26 In patients with acute myocardial infarction, being an acute critical illness though with minute tissue damage but where cardiogenic shock have systemic manifestations, we also found that higher syndecan-1 levels were independently associated with increased all-cause mortality at 30 days.27 From a pathophysiological and mechanistic point of view, it could be speculated that the increased shedding of glycocalyx results in an increased activation and damage of the endothelial cells resulting in a prothrombotic phenotype that leads to microvascular thrombosis and ensuing organ failure.8 Here, this is supported by an independent association of the endothelial cellular damage marker sTM and increased 7-day and 28-day mortality. Johansen et al26 reported in patients with sepsis that the level of sTM independently predicted organ failure of the liver and the kidneys and multiorgan failure and mortality; and in resuscitated out of hospital cardiac arrest patients, sTM was also a strong and independent predictor of 30-day mortality.28 Moreover, loss of endothelial integrity causes capillary leakage with fluid extravasation, hypotension and shock, and in the current study we found that lower systolic blood pressure and BE were strongly associated with circulating syndecan-1, supporting this notion.
Traumatic injury immediately activates the sympathoadrenal system with release of large amounts of catecholamines that exert widespread dose-dependent effects on metabolism and the vascular system.32,33 We proposed that patients with excessive trauma activation, the sympathoadrenal system becomes maladaptive and contribute to organ damage.8 Thus, in high concentrations, catecholamines directly damage the endothelium resulting in local edema, endothelial cell swelling, necrosis, and progressive deendothelialization.34 In the current study, we found that plasma catecholamines correlated strongly with plasma syndecan-1 and higher adrenaline was the only independent predictor of higher syndecan-1 levels. Moreover, the combined highest level plasma adrenaline, injury severity, and transfusions (and mortality) was associated with excessively increased syndecan-1, confirming our previous findings that sympathoadrenal activation is of critical importance for development of traumatic endotheliopathy. Furthermore, adrenaline and syndecan-1 were independent predictors of early <24-hours mortality and late 7-day and 28-day mortality, whereas sTM only independently predicted 7-day and 28-day mortality. The finding here, of a strong correlation between levels of catecholamines upon admission and outcome, is in alignment with findings from other patient groups suffering from acute critical illness. In patients resuscitated from out-of-hospital cardiac arrest, we reported that high plasma adrenaline was associated with increased 7-day, 30-day, and 180-day mortality.28 Similarly, in patients with acute myocardial infarction, stratified into quartiles based on plasma adrenaline levels, one quartile higher plasma adrenaline was independently associated with both increased 30-day and long-term mortality.27 In patients with varying degree of infection, including sepsis, we also found that catecholamines together with syndecan-1 and sTM independently predicted increased 28-day mortality.28
According to our proposed hypothesis, sympathoadrenal activation is a pivotal driver of the traumatic endotheliopathy, so modulation of this response by limiting the catecholamine surge, is expected to be beneficial for outcome in these patients. Interestingly, Bukur et al35 recently reported that in 663 critically injured patients (ISS≥25) admitted to the intensive care unit, those 98 patients receiving beta-blockers upon admission, had significantly lower inhospital mortality (11% vs 19%, P = 0.006). Stepwise logistic regression identified beta-blocker use as an independent protective factor for mortality these patients. Similarly, Cotton et al36 reported that in patients with severe traumatic brain injury, beta-blocker exposure before the trauma was associated with a significant reduction in mortality, which was even more impressive, considering that the group on beta-blocker therapy was older, more severely injured, and had lower predicted survival than the comparators.36 Together, these findings support that downstream effects of excessive sympathoadrenal activation may also be harmful in shocked patients 8,37 making it tempting to speculate if low-dose beta-blockers may be beneficial in these patients.
The current study has important limitations inherent to its design. Being an observational study precludes from cause and effect conclusions but merely points towards significant associations that requires further investigation. Moreover, as a single-center study, this limits the generalizability of the results, although our findings here support previous reports from independent trauma cohorts.16,18,19 Furthermore, the selection of biomarkers analyzed in this study does not preclude that other markers may also be of importance for the development and progression of traumatic endotheliopathy. Unique for the current study ∼10% of the patients received transfusions prehospital and the median prehospital crystalloid use was 0. This may have influenced the levels of endothelial biomarkers at admission given that administration of crystalloids has been shown to increase the circulating levels of these markers and contribute to endothelial damage.
In conclusion, the finding of the current study supports the critical role of endotheliopathy for outcome in severely traumatized patients and that sympathoadrenal activation is an important driver of this condition. Future randomized controlled trials investigating the effect of sympathoadrenal modulation and endothelial protection and repair are highly warranted.
Acknowledgments
The authors would like to thank laboratory technicians Karen Dyeremose, Marie Helena Stjernkvist, and Mehwish Jubeen Hussain for their skilled technical assistance.
Footnotes
Disclosure: The authors declare no conflicts of interest.
REFERENCES
- 1.Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peden M., McGee K., Krug E. Injury: A leading cause of the global burden of disease, 2000. Peden, M., McGee, K., and Krug, E. Available at: http://whqlibdoc.who.int/publications/2002-9241562323.pdf 2002. Geneva, Switzerland, World Health Organization; Accessed February 12, 2017. [Google Scholar]
- 3.Cardenas JC, Wade CE, Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol 2014; 21:404–409. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma 2008; 65:748–754. [DOI] [PubMed] [Google Scholar]
- 5.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost 2001; 27:585–592. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman M, Monroe DM, Roberts HR. Cellular interactions in hemostasis. Haemostasis 1996; 26:12–16. [DOI] [PubMed] [Google Scholar]
- 7.Johansson PI, Stissing T, Bochsen L, et al. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med 2009; 17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson PI, Ostrowski SR. Acute coagulopathy of trauma: Balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses 2010; 75:564–567. [DOI] [PubMed] [Google Scholar]
- 9.Aird WC. Endothelium as an organ system. Crit Care Med 2004; 32:S271–S279. [DOI] [PubMed] [Google Scholar]
- 10.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch 2000; 440:653–666. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwdorp M, Meuwese MC, Vink H, et al. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol 2005; 16:507–511. [DOI] [PubMed] [Google Scholar]
- 12.Rehm M, Zahler S, Lotsch M, et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 2004; 100:1211–1223. [DOI] [PubMed] [Google Scholar]
- 13.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998; 91:3527–3561. [PubMed] [Google Scholar]
- 14.Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 2006; 26:41–48. [DOI] [PubMed] [Google Scholar]
- 15.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116:1896–1906. [DOI] [PubMed] [Google Scholar]
- 16.Johansson PI, Stensballe J, Rasmussen LS, et al. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein c depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg 2011; 254:194–200. [DOI] [PubMed] [Google Scholar]
- 17.Johansson PI, Stensballe J, Rasmussen LS, et al. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma Acute Care Surg 2012; 72:428–436. [DOI] [PubMed] [Google Scholar]
- 18.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE 2011; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg 2012; 73:60–66. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb JB. A Novel and Potentially Unifying Mechanism for Shock Induced Early Coagulopathy. Ann Surg 2011; 254:201–202. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 2009; 66:41–48. [DOI] [PubMed] [Google Scholar]
- 23.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg 2011; 112:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma 2010; 69 Suppl:S55–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng X, Cao Y, Huby MP, et al. Adiponectin in fresh frozen plasma contributes to restoration of vascular barrier function after hemorrhagic shock. Shock 2016; 45:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansen ME, Johansson PI, Ostrowski SR, et al. Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost 2015; 41:16–25. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowski SR, Pedersen SH, Jensen JS, et al. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care 2013; 17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson PI, Bro-Jeppesen J, Kjaergaard J, et al. Sympathoadrenal activation and endothelial damage are inter correlated and predict increased mortality in patients resuscitated after out-of-hospital cardiac arrest: A post hoc sub-study of patients from the TTM-trial. PLoS ONE 2015; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blann A, Seigneur M. Soluble markers of endothelial cell function. Clin Hemorheol Microcirc 1997; 17:3–11. [PubMed] [Google Scholar]
- 30.Blann AD. Endothelial cell activation, injury, damage, and dysfunction: separate entities or mutual terms? Blood Coagul Fibrinolysis 2000; 11:623–630. [DOI] [PubMed] [Google Scholar]
- 31.Ishii H, Uchiyama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost 1991; 65:618–623. [PubMed] [Google Scholar]
- 32.Jaattela A, Alho A, Avikainen V, et al. Plasma catecholamines in severely injured patients: a prospective study on 45 patients with multiple injuries. Br J Surg 1975; 62:177–181. [DOI] [PubMed] [Google Scholar]
- 33.Davies CL, Newman RJ, Molyneux SG, et al. The relationship between plasma catecholamines and severity of injury in man. J Trauma 1984; 24:99–105. [DOI] [PubMed] [Google Scholar]
- 34.Makhmudov RM, Mamedov Y, Dolgov VV, et al. Catecholamine-mediated injury to endothelium in rabbit perfused aorta: a quantitative analysis by scanning electron microscopy. Cor Vasa 1985; 27:456–463. [PubMed] [Google Scholar]
- 35.Bukur M, Lustenberger T, Cotton B, et al. Beta-blocker exposure in the absence of significant head injuries is associated with reduced mortality in critically ill patients. Am J Surg 2012; 204:697–703. [DOI] [PubMed] [Google Scholar]
- 36.Cotton BA, Snodgrass KB, Fleming SB, et al. Beta-blocker exposure is associated with improved survival after severe traumatic brain injury. J Trauma 2007; 62:26–33. [DOI] [PubMed] [Google Scholar]
- 37.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 2009; 24:293–316. [DOI] [PubMed] [Google Scholar]


