Abstract
Objective:
Laparoscopic sleeve gastrectomy (LSG) is performed almost as often in Europe as laparoscopic Roux-Y-Gastric Bypass (LRYGB). We present the 3-year interim results of the 5-year prospective, randomized trial comparing the 2 procedures (Swiss Multicentre Bypass Or Sleeve Study; SM-BOSS).
Methods:
Initially, 217 patients (LSG, n = 107; LRYGB, n = 110) were randomized to receive either LSG or LRYGB at 4 bariatric centers in Switzerland. Mean body mass index of all patients was 44 ± 11 kg/m2, mean age was 43 ± 5.3 years, and 72% of patients were female. Minimal follow-up was 3 years with a rate of 97%. Both groups were compared for weight loss, comorbidities, quality of life, and complications.
Results:
Excessive body mass index loss was similar between LSG and LRYGB at each time point (1 year: 72.3 ± 21.9% vs. 76.6 ± 20.9%, P = 0.139; 2 years: 74.7 ± 29.8% vs. 77.7 ± 30%, P = 0.513; 3 years: 70.9 ± 23.8% vs. 73.8 ± 23.3%, P = 0.316). At this interim 3-year time point, comorbidities were significantly reduced and comparable after both procedures except for gastro-esophageal reflux disease and dyslipidemia, which were more successfully treated by LRYGB. Quality of life increased significantly in both groups after 1, 2, and 3 years postsurgery. There was no statistically significant difference in number of complications treated by reoperation (LSG, n = 9; LRYGB, n = 16, P = 0.15) or number of complications treated conservatively.
Conclusions:
In this trial, LSG and LRYGB are equally efficient regarding weight loss, quality of life, and complications up to 3 years postsurgery. Improvement of comorbidities is similar except for gastro-esophageal reflux disease and dyslipidemia that appear to be more successfully treated by LRYGB.
Keywords: bariatric surgery, gastric bypass, morbid obesity, RCT, slevve gastrectomy
The last 30 years have brought a dramatic increase in obesity worldwide.1 Currently, bariatric surgery is the only efficient treatment option leading to sustainable weight loss and reduction in comorbidities in morbidly obese patients.2 Therefore, there has been a continuously rapid growth in the number of bariatric interventions.3 There are a multitude of different surgical procedures and each intervention has its own profile of advantages and disadvantages. Up to now, there is no clear consensus on which procedure should be applied in each respective case. Currently, laparoscopic gastric bypass (LRYGB) is regarded as the gold standard treatment, but this procedure is challenged by other procedures such as sleeve gastrectomy or single anastomosis gastric bypass, always expecting to find even more efficient but equally or even safer procedures.3 Laparoscopic gastric banding temporarily gained popularity due to its easy application and the reversibility of this procedure. In the meantime, an increasing number of trials have shown the considerable drawbacks of this procedure (low grade of efficiency combined with poor quality of life and a high reoperation rate), and this intervention has more or less been abandoned in Europe.3–5
In LRYGB, the stomach is divided into a small upper pouch and a larger gastric remnant, and the small intestine is rearranged in a way that, in the final state, from the small gastric pouch the ingested food enters the jejunum (alimentary limb) directly and is not exposed to digestive liquids until it reaches the entero-enterostomy (“Y intersection”) at about 150 cm, where the biliopancreatic limb (excluded gastric remnant plus duodenum, and approximately 50 cm of proximal jejunum) is reconnected and the remaining small intestine forms the common channel. This rearrangement of the small intestine bears the risk of a typical complication “the internal hernia,” where the small bowel becomes trapped and obstructed; a condition that usually requires reoperation and can potentially have fatal consequences if not recognized and treated adequately. Internal hernia can occur many years after the original operation and is found in 1% to 11% of LRYGB patients.6
In laparoscopic sleeve gastrectomy (LSG) a large portion of the stomach along the greater curvature is removed resulting in a “sleeve”-like tube; however, no rearrangement of the small bowel is performed. Hence, this particular complication is not found after LSG (a clear argument in its favor); however, efficiency in remission of comorbidities is still a matter of debate.
Other potential advantages of LSG are that the procedure seems technically easier than LRYGB, shows lower early morbidity and lower prevalence of dumping symptoms. However, the LSG procedure is irreversible as the larger portion of the stomach is removed, whereas LRYGB is (at least to some extent) reversible. Furthermore, long-term outcome results after LSG are still scarce. A few randomized-controlled studies have compared outcome of LRYGB versus LSG, unfortunately with only a small number of patients or short follow-up.7–12 Up to now there is no clear evidence showing that 1 of these 2 procedures is superior to the other in long-term outcome.
The Swiss Multicentre Bypass Or Sleeve Study (SM-BOSS) is the first, prospective randomized trial comparing outcomes of LSG to LRYGB with an adequate number of patients and a nearly complete follow-up rate of 97% after 3 years.
METHODS
Study Design
The rationale, design, and methods of this study have been reported previously.13 The study protocol was reviewed and approved by the local ethical committees of each participating hospital and conducted in accordance with the principles of the Declaration of Helsinki and the trial was registered at the clinical trials registry of the National Institutes of Health (NCT 00356213). To summarize, the trial was a 2-group, randomized, controlled, multicenter study involving 217 morbidly obese patients, in which the outcomes of LSG were compared to those of LRYGB. Four bariatric centers in Switzerland participated in this trial, each with at least 10 years of bariatric experience and at least 200 bariatric procedures performed per year. Only surgeons with a personal experience of at least 400 bariatric interventions participated in this trial. On a regular basis research meetings were held, to enhance adherence to the protocol and guarantee high data quality.
Patients were assigned to either the LSG or LRYGB group, using a computer-based randomization with sealed envelopes. Eligibility criteria included an age of 18 to 65 years, a body mass index (BMI, the weight in kilograms divided by the square of the height in meters) >40 or >35 kg/m2 with the presence of at least 1 comorbidity, and failure of conservative treatment over 2 years. Exclusion criteria were: symptomatic gastro-esophageal reflux disease (GERD) despite medication (severe GERD), large hiatal hernia (para-esophageal hernia or axial hernia >4 cm), expected dense adhesions at the level of the small bowel, the need for endoscopic follow-up of the duodenum, and patients with inflammatory bowel disease. All patients gave written informed consent.
This investigator-initiated trial was financially supported by the Swiss National Science Foundation (SNF grants 32003B-120020 and 320030-138439) and Ethicon Endo Surgery USA. The authors declare no conflicts of interest. The sponsors had no role in study design, data accrual, data analysis, or manuscript preparation. The first author wrote the first draft of the manuscript. All the authors had full and independent access to all the data and vouch for the integrity and the accuracy of the analysis and its adherence to the protocol.
Study Outcomes
The primary end point of the study was weight loss defined by excessive BMI loss (EBMIL) over a period of 5 years. Secondary end points were the rate of perioperative and long-term morbidity and mortality, the remission rates of the associated comorbidities, the change in quality of life, and metabolic effects in subgroup analyses.14–18 This report provides the 3-year interim outcomes in the study patients, including measures of weight loss, course of comorbidities (remission, improved, unchanged, worsened) including glycemic control and lipid profiles, adverse events (including micronutrient deficiencies), and quality of life (as evaluated with the use of the Gastrointestinal Quality of Life Index (= GIQLI) and the BAROS Quality of Life Score).19,20
Definition of Comorbidities
Preoperative Comorbidities
Hypertension: systolic blood pressure 140 mm Hg or more and/or diastolic blood pressure ≥90 mm Hg or antihypertensive drug therapy; diabetes mellitus type 2 (T2DM): fasting plasma glucose ≥126 mg/dL or 2-hour plasma glucose ≥200 mg/dL during oral glucose tolerance test or antidiabetic drug with or without insulin therapy; dyslipidemia: fasting high-density lipoprotein <40 mg/dL for men, <50 mg/dL for women, and/or triglycerides >150 mg/dL and/or low-density lipoprotein >100 mg/dL or the use of statins; obstructive sleep apnea syndrome: in all patients, the Epworth Sleepiness questionnaire was applied; in case the score was >10 and/or signs of obstructive sleep apnea (eg, snoring, pauses in breathing) were present, pulse oximetry was performed followed by polysomnography21; Gastro-esophageal reflux (GERD): all patients received upper GI series, gastroscopy and manometry. GERD defined as: need for proton pump inhibitor agents (PPI) and/or esophagitis diagnosed on endoscopy and/or abnormal manometry. Patients with severe GERD (preexisting symptomatic GERD despite PPI medication) were excluded from the study; arthralgia: clinical and radiological findings; depression: all patients were seen by a psychiatrist. Depression as diagnosed by the psychiatrist and/or intake of antidepressants; hyperuricemia: plasma uric acid concentrations >476 mmol/L and/or intake of antihyperuricemic drugs (Table 1).
TABLE 1.
Baseline Demographic Data
| LSG | LRYGB | P Value | |
| Age (yrs; mean ± SD) | 43.0 ± 11.1 | 42.1 ± 11.2 | NS |
| Female (n (%) | 77 (72) | 79 (72) | NS |
| Weight (kg; mean ± SD) | 123.5 ± 19.4 | 124.8 ± 19.8 | NS |
| BMI (kg/m2; mean ± SD) | 43.6 ± 5.3 | 44.2 ± 5.3 | NS |
| QoL (GIQLI score; mean ± SD) | 99.0 ± 20.5 | 98.8 ± 17.4 | NS |
| Hypertension (%) | 63 | 59 | NS |
| Diabetes (%) | 24 | 26 | NS |
| Dyslipidemia (%) | 67 | 51 | NS |
| OSAS (%) | 48 | 42 | NS |
| GERD (%) | 44 | 46 | NS |
| Back/joint pain (Arthralgia) (%) | 61 | 68 | NS |
| Hyperuricemia (%) | 15 | 10 | NS |
| Depression (%) | 20 | 11 | NS |
GERD indicates gastro-esophageal reflux disease; GIQLI, Gastrointestinal Quality of Life Index; NS, nonsignificant; OSAS, obstructive sleep apnea syndrome.
Postoperative Comorbidities
In hypertension, dyslipidemia, GERD, OSAS, arthralgia, depression, and hyperuricemia the following definitions were applied: remission: defined as lack of symptoms and discontinuation of treatment; improved: reduction in treatment; unchanged: no difference to baseline; worsened: new treatment necessary or treatment intensified; new onset: disease diagnosed postoperatively. The remission of T2DM was defined according to the American Diabetes Association criteria for complete remission with HbA1c <42 mmol/mol (6.0%), fasting glucose <5.6 mmol/L (100 mg/dL), and at least 1 year's duration in the absence of active pharmacologic therapy or ongoing procedures (Table 2).22
TABLE 2.
Improvement in Comorbidities
| OP Type (%: Preoperative Prevalence of Comorbidity) | Remission (%) | Improved (%) | Unchanged (%) | Worsened (%) | |
| Hypertension | LSG (63%) | 65.2 | 34.8 | 0.0 | 0.0 |
| LRYGB (59%) | 71.2 | 25.0 | 3.8 | 0.0 | |
| Dyslipidemia* | LSG (67%) | 43.8 | 35.4 | 16.7 | 4.1 |
| LRYGB (51%) | 71.7 | 26.1 | 2.2 | 0.0 | |
| T2DM | LSG (24%) | 60.0 | 35.0 | 0.0 | 5.0 |
| LRYGB (26%) | 77.0 | 23.0 | 0.0 | 0.0 | |
| OSAS | LSG (48%) | 90.2 | 9.8 | 0.0 | 0.0 |
| LRYGB (42%) | 82.2 | 17.8 | 0.0 | 0.0 | |
| Back/joint pain | LSG (61%) | 44.2 | 37.2 | 14.0 | 4.6 |
| LRYGB (68%) | 42.5 | 47.5 | 7.5 | 2.5 | |
| GERD† | LSG (44%) | 61.0 | 5.0 | 14.6 | 19.4 |
| LRYGB (46%) | 77.6 | 14.3 | 6.1 | 2.0 | |
| Hyperuricemia | LSG (15%) | 81.8 | 9.1 | 0.0 | 9.1 |
| LRYGB (10%) | 100 | 0.0 | 0.0 | 0.0 | |
| Depression | LSG (20%) | 26.7 | 40.0 | 33.3 | 0.0 |
| LRYGB (11%) | 33.4 | 22.2 | 44.4 | 0.0 |
Comparing improvement of comorbidities between the 2 groups, the only statistically significant differences seen were in remission of dyslipidemia (*) and worsening of preexisting GERD (†), where LRYGB was superior to LSG 3 years after surgery. In brakes: preoperative prevalence.
Cholecystolithiasis: patients with preexisting gallstones received concomitant cholecystectomy. Patients without gallstones routinely received gallstone prophylaxis with ursodeoxycholic acid during the first 6 months after surgery.
Operation Techniques
In all patients standardized operation techniques were used. LSG: for calibration of the gastric tube, a 35-Fr bougie along the lesser curvature was used and the longitudinal resection of the stomach was done from approximately 3 to 6 cm orally of the pylorus to the angle of His. The staple line was oversewn with an absorbable running suture. Hiatal hernias were explored and repaired with posterior closure of the crura. LRYGB was performed with a 50-cm long biliopancreatic limb and a 150-cm antecolic Roux-limb using either a linear stapled or circular stapled (25-mm) gastrojejunostomy. To guarantee standardization of the 2 procedures, prior to inclusion of the first patient, all participating surgeons were invited to an instruction session by the principal investigator (RP), where the standardized operation techniques were demonstrated.
Statistical Analysis
To detect a 10% difference in EBMIL between the 2 procedures, we calculated a study size of 200 patients to reach 94% power at 5 years. Data analysis was performed using IBM SPSS for Windows (version 21; IBM, Armonk, NY). Values are reported as means ± SD. Descriptive statistics were used for demographic variables such as age, weight, and BMI. Analysis was performed on the intention-to-treat population using the last observation carry forward imputational approach to deal with missing follow-up values. Student t, chi-square, and Fisher exact 2-sided tests were used where appropriate. Longitudinal comparisons were done using repeated measure analysis of variance with Šidak multicomparison test. Treatment group was used as a covariate. A P value of less than 0.05 was considered statistically significant.
RESULTS
Of approximately 4000 patients, who underwent primary bariatric surgery in the 4 study centers, 225 agreed to participate in this randomized, controlled trial. Of the 225 patients who underwent randomization from January 2007 through November 2011, a total of 8 patients were excluded after randomization; 1 patient crossed over from the LRYGB to the LSG group because of unexpected dense adhesions of the jejunum, which were detected intraoperatively, and 7 patients were operated on after November 2011 when the recruitment phase was closed. This resulted in a total of 217 patients who were included in the study and assigned to either the LSG or LRYGB group. After 3 years, 6 patients were lost to follow-up: in the LRYGB group 1 patient died within 30 days, another died due to lymphoma 2.5 years postop, 2 patients moved away; in the LSG group, 2 patients moved away. The remaining 211 patients (97.2%) were evaluated in the 3-year assessment of efficacy and safety (Fig. 1). The baseline characteristics of the 217 patients were reported previously and are described in Table 1.13 There were no significant differences between the study groups at baseline. Analysis was performed on the intention-to-treat population. Therefore, the 2 patients in the LSG group who were converted to LRYGB remained in the LSG group for analysis.
FIGURE 1.
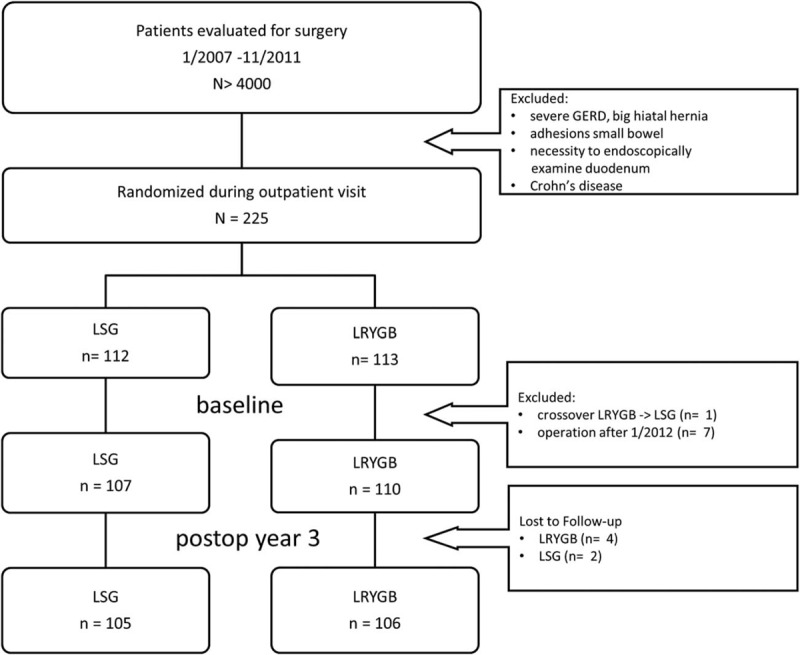
Flow diagram (study overview).
Primary End Point
Body weight and BMI significantly decreased for both treatments from baseline compared with 1, 2, and 3 years postop (P <0.001). In the LRYGB group, weight increased slightly but significantly from year 2 to year 3 (P = 0.01) and in both treatment groups, BMI increased slightly but significantly from year 2 to year 3 (P = 0.01). There were no significant differences between the treatments in BMI loss or weight loss. Reduction in body weight expressed as EBMIL was also similar between LSG and LRYGB at each time point (at 1 year: 72.3 ± 21.9% vs. 76.6 ± 20.9%, P = 0.139; at 2 years: 74.7 ± 29.8% vs. 77.7 ± 30%, P = 0.513; and at 3 years: 70.9 ± 23.8% vs. 73.8 ± 23.3%, P = 0.316) (Fig. 2).
FIGURE 2.
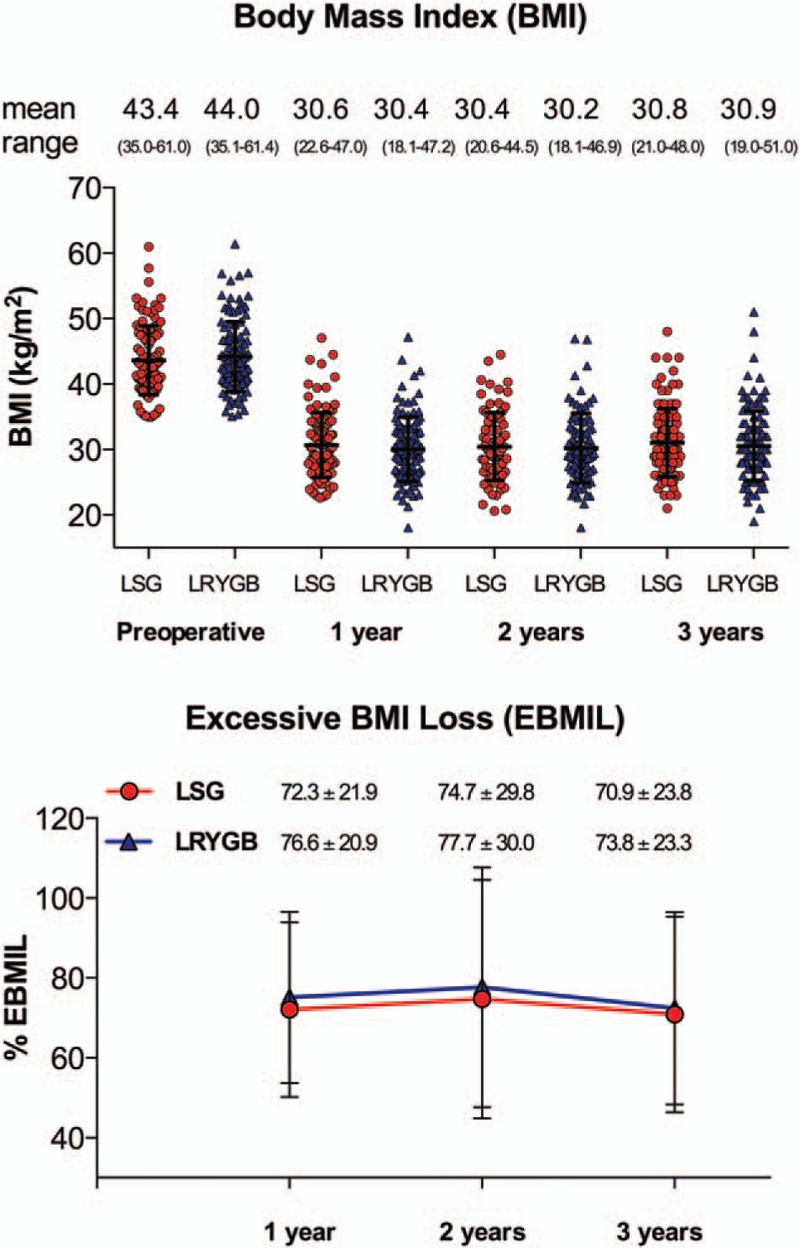
Weight loss: BMI and EBMIL, BMI significantly decreased for both treatments from baseline at all 3 years postop (P <0.001). In both treatment groups, BMI increased slightly but significantly from year 2 to year 3 (P = 0.01). There were no statistically significant differences between the 2 groups. EBMIL was also similar between LSG and LRYGB at each time point (at 1 year: 72 ± 22% in LSG group vs. 75 ± 22% in LRYGB group, P = 0.14; at 2 years: 75 ± 30% vs. 78 ± 30%, P = 0.51; and at 3 years: 71 ± 24% vs. 73 ± 23%, P = 0.29 respectively). Scatter plot: red dots: LSG, blue triangles: LRYGB.
The percentage of patients with EBMIL >50% was 80% in the LSG and 85% in the LRYGB group, and EBMIL >75% was observed in 46% of LSG patients and 50% of patients in the LRYGB group. There was no statistically significant difference between the 2 treatments.
Comorbidities
After 3 years, comorbidities (glycemic control, hypertension, OSAS, arthralgia, depression, and hyperuricemia) improved significantly after both procedures with no statistically significant difference between the 2 procedures. In contrast, GERD and dyslipidemia were better treated with LRYGB (Tables 2 and 3).
TABLE 3.
Secondary Endpoints
| A: Diabetic Patients (LSG: n = 26; LRYGB: n = 28) | |||||||
| P Values | |||||||
| End Point | Group | Baseline | Year 1 | Year 3 | Baseline Vs. Year 1 | Baseline Vs. Year 3 | Between Groups |
| Fasting Glucose (mmol/L) | LSG | 7.7 ± 0.75 | 5.8 ± 0.38 | 6.0 ± 0.32 | 0.115 | 0.211 | 0.141 |
| LRYGB | 6.7 ± 0.47 | 5.4 ± 0.23 | 5.6 ± 0.22 | 0.040 | 0.080 | ||
| HbA1C | LSG | 7.62 ± 0.38 | 6.12 ± 0.21 | 6.51 ± 0.31 | 0.005 | 0.035 | 0.129 |
| LRYGB | 7.25 ± 0.37 | 5.76 ± 0.15 | 5.97 ± 0.16 | <0.001 | 0.001 | ||
| B: Patients With Preexisting Dyslipidemia (LSG: n = 72; LRYGB: n = 56) | ||||||
| End Point | Group | Baseline | 3 Yrs | P Values Baseline to Year 3 | Between Groups | Rate of Decline Between Groups |
| Total Cholesterol | LSG | 5.67 ± 0.15 | 5.11 ± 0.93 | 0.001 | 0.053 | 0.033 |
| LRYGB | 5.42 ± 0.18 | 4.65 ± 0.14 | <0.001 | |||
| HDL | LSG | 1.13 ± 0.04 | 1.55 ± 0.06 | <0.001 | 0.661 | 0.423 |
| LRYGB | 1.13 ± 0.04 | 1.6 ± 0.06 | <0.001 | |||
| Chol/HDL ratio | LSG | 5.39 ± 0.26 | 3.9 ± 0.34 | <0.001 | 0.031 | 0.070 |
| LRYGB | 4.92 ± 0.19 | 3.01 ± 0.09 | <0.001 | |||
| LDL | LSG | 3.45 ± 0.14 | 3.16 ± 0.12 | 0.043 | 0.202 | 0.018 |
| LRYGB | 3.41 ± 0.17 | 2.78 ± 0.1 | <0.001 | |||
| TG | LSG | 2.22 ± 0.16 | 1.35 ± 0.11 | <0.001 | 0.327 | 0.368 |
| LRYGB | 2.07 ± 0.16 | 1.18 ± 0.10 | <0.001 | |||
| C: Quality of Life, All Patients (LSG: n = 107; LRYGB: n = 110) | |||||||
| End Point (Mean ± SD) | Group | Baseline | 1 Yr | 2 Yrs | 3 Yrs | Baseline Vs. Year 1 | Between Groups |
| GIQLI Score | LSG | 100.0 ± 20.6 | 126.3 ± 13.4 | 124.5 ± 16.9 | 117.4 ± 22.6 | 0.001 | 0.366 |
| LRYGB | 99.3 ± 17.5 | 128.5 ± 9.3 | 126.5 ± 14.8 | 121.2 ± 13.9 | 0.003 | ||
| BAROS QoL Score | LSG | 0.1 ± 1.2 | 1.9 ± 0.8 | 1.9 ± 0.9 | 1.7 ± 0.8 | <0.001 | 0.535 |
| LRYGB | 0.2 ± 1.3 | 1.9 ± 0.6 | 2.0 ± 0.8 | 1.7 ± 0.9 | <0.001 | ||
A: Improvement of glycemic control in diabetic patients. At 3 years there was no significant difference in fasting glucose or HbA1c between LSG and LRYGB. B: Dyslipidemia: significant improvements of lipid profiles in both groups. Rate of decline for LDL and total cholesterol was significantly higher in LRYGB group compared with LSG, adjusted for baseline values. Values are expressed as mean ± SD.
C: Quality of life measured by GIQLI score and BAROS QoL score. GIQLI score for healthy individuals = 121. After 1 year, quality-of-life scores were significantly higher than preoperatively and remained high. There was no difference between the 2 groups. Values are expressed as mean ± SD.
Glycemic Control
At baseline, 24% of LSG and 26% of LRYGB patients had type 2 diabetes, of which 23% in the LSG group and 21% in the LRYGB group were insulin-dependent, respectively. After 3 years, complete remission was seen in 60% of LSG patients and in 77% of LRYGB patients (P = 0.23). Marked amelioration of glycemic control was seen after 3 years: HbA1c decreased significantly for both treatments and median levels of fasting plasma glucose were significantly lower from baseline to year 3. There was no overall significant difference between the treatment groups (Tables 2 and 3).
Dyslipidemia
At baseline, 67% of the LSG patients and 51% of the LRYGB patients suffered from dyslipidemia. After 3 years, complete remission rate was 44% in LSG and 72% in LRYGB (P = 0.008). Significant improvement was seen in both groups; however, the rate of decline of total cholesterol and LDL was significantly higher in the LRYGB group compared with LSG, adjusted for baseline values.
Triglycerides decreased in both treatment groups from baseline to year 3 (P <0.001). The values of TG were not significantly different between the treatments, neither was the rate of decline when adjusted for baseline values (Tables 2 and 3).
Gastroesophageal Reflux Disease
At baseline, 44% had GERD in the LSG group, and 46% in the LRYGB group. After 3 years, in the LSG group, 61% experienced remission, 5% symptoms improved; in 15% symptoms were unchanged and in 20% worsened.
In the LRYGB group, 78% experienced remission, in 14% symptoms improved; in 6% symptoms were unchanged and in 2% worsened. At 3 years, the difference in remission rate between the groups did not reach significance (P = 0.09), but worsening of symptoms was more often seen in the LSG group (P = 0.01, Table 2). In addition, of the 66 LSG patients with no GERD at baseline, 18% developed de novo GERD symptoms whereas this was only seen in 2% of LRYGB patients (P = 0.002).
Quality of Life
The mean GIQLI score (GIQLI = Gastrointestinal Quality of Life Index) and BAROS quality of life (QoL) (BAROS = Bariatric Analysis and Reporting Outcome System) increased significantly in both groups after 1 year compared with baseline. Assessment of GIQLI score and BAROS QoL at 2 and 3 years demonstrates that patients experience a significant and sustainable improvement in quality of life compared with baseline with a slight decrease between years 2 and 3. Again, there was no statistically significant difference between the 2 groups at any time point (Table 3).
Adverse Events
Additional surgical interventions were required in 9 patients in the LSG group and in 16 patients in the LRYGB group from postoperative day 30 until the 3-year follow-up (8% vs. 15%, P = 0.15). In the LSG group, there were 2 patients converted to bypass due to severe GERD, 4 patients needed cholecystectomy due to newly developed, symptomatic gallstones, 2 patients suffered from insufficient weight loss (1 conversion into laparoscopic bilio-pancreatic diversion duodenal switch and 1 to LRYGB), and 1 patient had an umbilical hernia repair. In the LRYGB group 6 patients required cholecystectomy, 2 had a small bowel obstruction, 3 patients were treated for internal hernia by laparoscopy, in 1 patient a Fobi-ring was inserted to increase weight loss by adding restriction.23,24 In addition, 1 patient had an umbilical hernia repair, 1 patient underwent laparoscopy for gastroduodenoscopy, in 1 patient abdominal lavage had to be carried out due to an infectious early complication, and 1 patient had a resection of a Meckel diverticulum. In the 3-year follow-up there was no patient in either group with extreme weight loss (BMI ≤18 kg/m2) or hypoalbuminemia and no life-threatening complications or deaths associated with the intervention occurred. No statistically significant difference in complications treated conservatively such as peptic ulcer, stricture, kidney stones, and micronutrient deficiencies between the 2 groups was found (Table 4).
TABLE 4.
Complications (1 Month to 3 Yrs)
| Complication | LSG (n = 107) | LRYGB (n = 110) | P Values LSG Vs. LRYGB |
| Conservative treatment | |||
| General complications | |||
| Total | 9 | 11 | 0.67 |
| Peptic ulcer | 0 | 1 | |
| Stricture | 0 | 1 | |
| Kidney stones | 2 | 1 | |
| Other | 7 | 8 | |
| Deficiencies | |||
| Total: patients with ≥1 micronutrient deficiency | 39 | 45 | 0.59 |
| Vit. D | 34 | 26 | |
| Vit. B12 | 39 | 45 | |
| Iron | 24 | 29 | |
| Zink | 16 | 20 | |
| Folate | 10 | 5 | |
| Protein | 0 | 1 | |
| Operative treatment | |||
| Total | 9 | 16 | 0.15 |
| Conversion to LRYGB for GERD | 2 | NA | |
| Choleystectomy for newly acquired gallstones | 4 | 6 | |
| Revision for small bowel obstruction | 0 | 2 | |
| Internal hernia | 0 | 3 | |
| Insufficient weight loss | 2 | 1 | |
| Other (umbilical hernia, Meckel diverticulum, gastroduodenoscopy, abdominal lavage, etc.) | 1 | 4 | |
The reoperation rate was slightly higher in the LRYGB group. There was no statistically significant difference between the 2 groups.
DISCUSSION
The present study explored whether LSG compared with the current gold standard in bariatric surgery, laparoscopic gastric bypass (LRYGB) is equally effective in: 1) weight loss, 2) remission of comorbidities, 3) increase in quality of life, and 4) whether the 2 procedures are equally safe in long-term outcome.
Both procedures were highly effective in terms of weight loss with an excessive BMI loss at 3 years of 70.9% in the LSG group and 73.8% in the LRYGB group. The weight loss nadir was between 1 and 2 years postop with a discrete weight regain thereafter. LRYGB showed a nonsignificant better weight loss at each time point that is in line with most previously published series and meta-analyses.7–11,25,26
Differences in the amelioration of glycemic control in patients with T2DM between the 2 procedures are still controversially discussed. According to some study groups, LRYGB shows superiority to LSG in T2DM remission.27 Other groups found the opposite and even describe superiority of LSG in diabetes remission.28 However, only a few, prospective randomized-controlled studies have compared outcome of LRYGB versus LSG, unfortunately with either only a small number of patients or short follow-up.7–11 The STAMPEDE trial is doubtless the most important trial so far, comparing best medical treatment versus LSG and LRYGB with 50 diabetic patients in each arm over a period of 3 years.9 Regarding HbA1c <6.0% the 2 surgical arms were clearly superior to the conservative arm, but no statistically significant difference between the 2 surgical groups was found. In contrast, other endpoints (HbA1C <7% without medications, insulin dependency, and number of medications used) showed superiority of LRYGB over LSG.9 In our trial, no statistically significant difference in resolution of T2DM and amelioration of glycemic control could be shown, which is in line with a recently published trial by Yang et al.12 However, it should be noted that the SM-BOSS trial is not powered enough for this end-point.
Remission rates of other comorbidities showed equal effects, with the exception of GERD and dyslipidemia. Patients with severe, preexisting GERD (symptomatic GERD despite PPI medication) and big hiatal hernia (para-esophageal hernia or axial hernia >4 cm) were not included in the study, as in this case a LRYGB is clearly superior to LSG. Nevertheless, many morbidly obese suffer from intermittent reflux, which can exacerbate after LSG. Preexisting reflux was more efficiently treated with LRYGB and significantly more patients suffered from new onset reflux in the LSG group. In most cases, GERD could be treated conservatively with PPIs. However, in 2 patients, pharmaceutical treatment was insufficient and LSG had to be converted to LRYGB to treat refractory severe GERD. Dyslipidemia improved in both groups; however, decrease in total cholesterol and LDL was more pronounced in the LRYGB group compared with LSG and remission rate for dyslipidemia was higher in LRYGB compared with LSG. Quality of life improved significantly in both groups after surgery at 1 year compared with baseline, and remained stable up to 3 years postsurgery. There was no difference observed between the 2 groups, despite the difference seen in GERD. There was no statistically significant difference in complications within the first 3 years postop.
The rate of reoperations in the LRYGB group was slightly higher, but not statistically significant, which might probably be due to insufficient power for this secondary endpoint.
In the present study, we found no difference in vitamin deficiencies between the 2 groups, which is in contradiction to a previously conducted retrospective analysis, where we found a lower deficiency rate following LSG.29 Both groups had a rather high rate of deficiencies despite regular vitamin supplementation in all patients. The very high follow-up rate (97%) with regular laboratory investigations may explain the high prevalence. No severe malnutrition was observed. These results demonstrate the need for life-long vitamin supplementation and monitoring of deficiencies following LSG and LRYGB.
As a limitation of this study we would like to mention that the design of the study was powered at 5 years for the primary endpoint (weight loss) and is underpowered for T2DM remission (with only 26 vs. 28 diabetic patients in each treatment arm) and reoperation rate (with 9 vs. 16 reoperations in each arm). The results discussed are part of an interim analysis. Up-to-date SM-BOSS is the largest randomized controlled trial comparing LSG to LRYGB with a nearly complete follow-up rate at 3 years.
In conclusion, in this trial, LSG and LRYGB were equally efficient regarding weight loss, quality of life, and complications up to 3 years postsurgery. Improvement of comorbidities was similar except for GERD and dyslipidemia that appear to be more successfully treated by LRYGB. In our opinion, patients with preexisting symptomatic gastro-esophageal reflux disease (GERD) despite medication, or patients successfully treated with PPIs but refusing long-term acid-inhibitory medication, large hiatal hernia (para-esophageal hernia or axial hernia >4 cm), and severe gastroesophageal motility disorders are better treated with a LRYGB procedure. Concerning dyslipidemia, no conclusions can be drawn at this moment. However, LRYGB might be a better choice in patients with additional cardiovascular risk factors such as history of cardio-vascular event, smoking, arterial hypertension, or inherited predisposition for cardio-vascular disease.
Acknowledgments
The authors thank contributing clinicians Beatrice Kern, MD; Martina Gebhart, MD; Marc Slawik, MD; Dino Kröll, MD; Martin Thurnheer, MD; as well as Sandra Gagliardo & Philipp Hendrickson for data management and Helen Thurston for proof reading.
Footnotes
This investigator-initiated trial was financially supported by the Swiss National Science Foundation (SNF grants 32003B-120020 and 320030-138439) and Ethicon Endo Surgery USA.
The authors report no conflicts of interest.
REFERENCES
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273:219–234. [DOI] [PubMed] [Google Scholar]
- 3.Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015; 25:1822–1832. [DOI] [PubMed] [Google Scholar]
- 4.Wolnerhanssen BK, Peters T, Kern B, et al. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: results of a prospective study of 380 patients. Surg Obes Relat Dis 2008; 4:500–506. [DOI] [PubMed] [Google Scholar]
- 5.Peterli R, Wolnerhanssen BK, Peters T, et al. Prospective study of a two-stage operative concept in the treatment of morbid obesity: primary lap-band followed if needed by sleeve gastrectomy with duodenal switch. Obes Surg 2007; 17:334–340. [DOI] [PubMed] [Google Scholar]
- 6.Aghajani E, Jacobsen HJ, Nergaard BJ, et al. Internal hernia after gastric bypass: a new and simplified technique for laparoscopic primary closure of the mesenteric defects. J Gastrointest Surg 2012; 16:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008; 247:401–407. [DOI] [PubMed] [Google Scholar]
- 8.Kehagias I, Karamanakos SN, Argentou M, et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg 2011; 21:1650–1656. [DOI] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmio M, Victorzon M, Ovaska J, et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg 2014; 103:175–181. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg 2014; 24:1617–1624. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Wang C, Cao G, et al. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m(2). BMC Surg 2015; 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass Or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013; 258:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 2009; 250:234–241. [DOI] [PubMed] [Google Scholar]
- 15.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012; 22:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis 2011; 7:561–568. [DOI] [PubMed] [Google Scholar]
- 17.Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity 2013; 21:E660–E668. [DOI] [PubMed] [Google Scholar]
- 18.Schneider J, Peterli R, Gass M, et al. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis 2016; 12:563–570. [DOI] [PubMed] [Google Scholar]
- 19.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995; 82:216–222. [DOI] [PubMed] [Google Scholar]
- 20.Moorehead MK, Ardelt-Gattinger E, Lechner H, et al. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes Surg 2003; 13:684–692. [DOI] [PubMed] [Google Scholar]
- 21.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep 1997; 20:844–849. [DOI] [PubMed] [Google Scholar]
- 22.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009; 32:2133–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fobi MA. Placement of the GaBP ring system in the banded gastric bypass operation. Obes Surg 2005; 15:1196–1201. [DOI] [PubMed] [Google Scholar]
- 24.Himpens J, Coromina L, Verbrugghe A, et al. Outcomes of revisional procedures for insufficient weight loss or weight regain after Roux-en-Y gastric bypass. Obes Surg 2012; 22:1746–1754. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg 2016; 26:429–442. [DOI] [PubMed] [Google Scholar]
- 26.Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014; 149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yska JP, van Roon EN, de Boer A, et al. Remission of type 2 diabetes mellitus in patients after different types of bariatric surgery: a population-based cohort study in the United Kingdom. JAMA Surg 2015; 150:1126–1133. [DOI] [PubMed] [Google Scholar]
- 28.Pham S, Gancel A, Scotte M, et al. Comparison of the effectiveness of four bariatric surgery procedures in obese patients with type 2 diabetes: a retrospective study. J Obes 2014; 2014:638203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gehrer S, Kern B, Peters T, et al. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg 2010; 20:447–453. [DOI] [PubMed] [Google Scholar]


