Abstract
Objective:
We sought to determine 30-day survival trends and prognostic factors following surgery for acute subdural hematomas (ASDHs) in England and Wales over a 20-year period.
Summary of Background Data:
ASDHs are still considered the most lethal type of traumatic brain injury. It remains unclear whether the adjusted odds of survival have improved significantly over time.
Methods:
Using the Trauma Audit and Research Network (TARN) database, we analyzed ASDH cases in the adult population (>16 yrs) treated surgically between 1994 and 2013. Two thousand four hundred ninety-eight eligible cases were identified. Univariable and multiple logistic regression analyses were performed, using multiple imputation for missing data.
Results:
The cohort was 74% male with a median age of 48.9 years. Over half of patients were comatose at presentation (53%). Mechanism of injury was due to a fall (<2 m 34%, >2 m 24%), road traffic collision (25%), and other (17%). Thirty-six per cent of patients presented with polytrauma. Gross survival increased from 59% in 1994 to 1998 to 73% in 2009 to 2013. Under multivariable analysis, variables independently associated with survival were year of injury, Glasgow Coma Scale, Injury Severity Score, age, and pupil reactivity. The time interval from injury to craniotomy and direct admission to a neurosurgical unit were not found to be significant prognostic factors.
Conclusions:
A significant improvement in survival over the last 20 years was observed after controlling for multiple prognostic factors. Prospective trials and cohort studies are expected to elucidate the distribution of functional outcome in survivors.
Keywords: craniotomy, neurosurgery, trauma, traumatic brain injury
Trauma is the most common cause of death for those under the age of 45. Traumatic brain injury (TBI) remains the cause of approximately half of the deaths secondary to trauma.1 One of the important early sequelae of TBI is the development of intracranial hematomas. These can be extradural, subdural, intraparenchymal, or a combination thereof. It has long been recognized that acute subdural hematomas (ASDHs) are often associated with intraparenchymal injuries and brain swelling. Hence, outcomes have historically been worse for patients with ASDH with mortality rates as high as 68%.2
Temporal evidence from the 1990s suggested that outcome following head injury had failed to improve to the same degree as other trauma.3–5 As a result, the UK National Institute for Health and Care Excellence (NICE) introduced guidelines for the management of head injury in 2003, subsequently revised in January 2014 (NICE CG176).6 Indeed, a study presenting data from 2003 to 2009 identified a reduction in the odds of death following head injury.7 There was, however, no specific analysis performed for ASDH, the focus of this study. Research utilizing the Nationwide Inpatient Sample in the USA identified an improvement in traumatic subdural hematoma mortality from 16.4% in 1996 to 11.6% in 2006.8 However, the authors did not to distinguish acute vs chronic subdural hematomas, as it was based on International Classification of Diseases, Ninth Revision (ICD)-9 codes. As the pathophysiology, patient populations, management strategies, and outcomes differ significantly between acute and chronic subdural hematomas, the findings of this study cannot be extrapolated to ASDH.8
Previous retrospective cohort studies have found age, Glasgow Coma Scale (GCS), Injury Severity Scale (ISS), and pupil reactivity as independent prognostic factors.9–11 Following research from the 1980s investigating the effect of time to surgery on outcome after ASDH,12 and studies supporting this finding thereafter,13 it has been advised that “life-saving decompressive surgery must be available within four hours”.14 However, more recent studies have challenged this conclusion.9,11,15–19
The aim of this study therefore was to identify trends in survival for surgical management of ASDH across England and Wales, leveraging multiple logistic regression analysis to control for case mix changes over the last 20 years. Through this, we also sought to identify prognostic factors relating to outcome in ASDH and examine the relationship between time interval from injury to craniotomy and survival.
METHODS
Study Design
This is an observational cohort study with a retrospective analysis of prospectively recorded data. The study is reported in accordance with the STROBE statement for cohort studies. We analyzed anonymized data on all patients with a diagnosis of subdural hematoma from the Trauma Audit and Research Network (TARN) database over a 20-year period (1994 to 2013). A 20-year period starting in 1994 was selected to provide data 10 years before and after the introduction of 2003 NICE head injury guidelines. The TARN database was established in 1989. The objective of TARN is to support trauma service development and inform the research agenda by collecting information on patients admitted with major trauma.20 The TARN database is now one of the largest trauma registries in Europe, with more than 500,000 cases. Initially covering 13 hospitals, the database now covers 100% of trauma-receiving hospitals in England and Wales.
Outcome was recorded as a dichotomous variable (alive or dead) based on assessment at 30 days or at discharge if the latter happened before 30 days. This will be referred to as 30-day survival for economy of words. No further outcome measures were collected for identification of neurological sequelae or other disability following injury. Cases over the age of 16 who underwent craniotomy within 48 hours of injury were selected, in order to focus on acute operative cases in the adult.
Statistical Analysis
Descriptive statistics were performed on the basis of 5-year intervals (1994 to 1998, 1998 to 2003, 2004 to 2008, 2009 to 2013). We investigated 30-day survival as a dependent variable. Variables hypothesized to be related to survival were modeled using univariate logistic regression, retained as continuous variables where possible to maximize statistical utility.21,22 The date of injury was converted to a decimal, such that the exact point in the year in which the injury occurred could be retained. Subdural hematoma severity was coded using the Abbreviated Injury Scale (AIS), ranging from 4 (Severe) to 5 (Critical) in this study. Additional injuries were combined with the subdural hematoma AIS to create the ISS.23 Other variables analyzed included GCS, age, sex, day of the week of injury, time interval from injury to craniotomy, location of admission, presence of polytrauma, pupil reactivity, and mechanism of injury. Pupil reactivity and GCS were recorded on arrival in the emergency department; the values at the scene of the accident were recorded for patients arriving intubated. This is a commonly employed approach in the TBI literature. Variables were progressed into a multiple logistic regression model. Interaction terms were tested and included if significant. Nonlinear variables in this study were all modeled using restricted cubic splines.
Following selection of operative cases in adults within 48 hours, where pupil reactivity data were missing but GCS was 14 or 15, we assumed that no significant brainstem compression/uncal herniation would exist and pupils were considered to be both reactive. Multiple imputation was then applied in the updated dataset. Sensitivity was tested under multiple logistic regression analysis in 3 ways, firstly, using an offset ranging between the maximum and minimum of the variable in question, with restrictions such that any imputed data did not exceed the highest and lowest possible values.24,25 For example, with an offset of +12 on GCS (ranging from 3 to 15), all imputed values would be 15 regardless of their initial value. Secondly, multiple logistic regression was repeated using only original sites to account for bias as a result of the addition of new sites over time. Finally, we performed propensity score matching as a further sensitivity test of modifiable risk factors found to be significant in the multivariable logistic regression model. Propensity score matching involves the construction of a conditional probability for a patient chosen at random to be exposed to the risk factor in question, after controlling for other known risk factors. This methodology was undertaken to minimize bias as a result of nonrandomization, and was performed through a one-to-one nearest neighbor method matching process.26
A single P value was obtained by computing a Wald χ2 pooled statistic of all coefficients of the variable of interest. Significance threshold for all variables and interaction terms was set at P < 0.05. All analyses were performed using package rms in R, version 2.15.2.27,28 Logistic regression output was converted to a probability of survival for graphical presentation. Graphical production was completed using the ggplot2 package in R, version 2.15.2.29 Further details to the statistical methodology can be found in the Supplemental Digital Content.
ETHICS
The UK Department of Health's Patient Information Advisory Group governs the use of patient information and has given ethical approval for research using anonymized TARN data.
RESULTS
Sample Characteristics
A total of 29,643 cases were identified between 1994 and 2013. Figure 1 demonstrates the process for selection of the final sample of 2498 patients (8.4% of total sample). Sample descriptive statistics through 5-year intervals and for the total cohort are summarized in Table 1.
FIGURE 1.
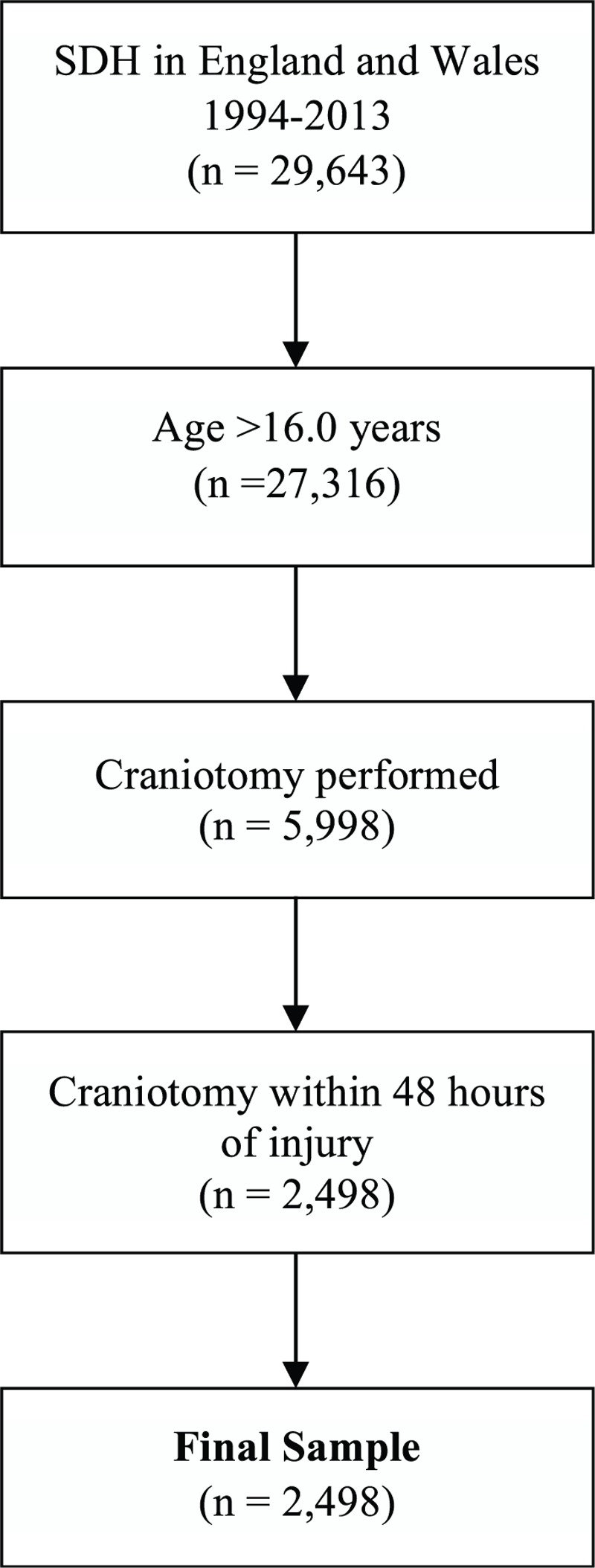
Process for selection of final sample.
TABLE 1.
Sample Description across 5-year Intervals
| Measure | 1994–1998 | 1999–2003 | 2004–2008 | 2009–2013 | Total | |
| Number of patients (n) | 217 | 347 | 398 | 1,536 | 2,498 | |
| Age (years, median, IQR) | 47.0 (33.0–62.0) | 49.1 (35.2–64.5) | 43.7 (30.9–57.3) | 50.7 (34.8–66.6) | 48.9 (33.6–64.8) | |
| Male % | 77% | 73% | 76% | 74% | 74% | |
| ISS (median, IQR) | 25 (25–29) | 26 (25–29) | 25 (24.3–29) | 25 (25–29) | 25 (25–29) | |
| GCS (median, IQR)* | 6 (3–11) | 7 (3–11) | 7 (3–13) | 9 (5–14) | 8 (4–13) | |
| LOS/days (median, IQR) | Overall | 12 (4–24) | 13 (5–30) | 20 (7–37.8) | 17 (7–39) | 16 (6–36) |
| Critical | 4 (1–8) | 6 (2–12) | 7 (3–13) | 6 (1–14) | 6 (2–13) | |
| Mechanism / % | Fall <2 m | 26% | 22% | 28% | 39% | 34% |
| Fall >2 m | 24% | 31% | 25% | 21% | 24% | |
| Other | 13% | 17% | 19% | 17% | 17% | |
| RTC | 37% | 31% | 27% | 22% | 25% | |
| Shooting | No Data | No Data | 1% | 1% | 1% | |
| SDH severity | 4 | 35% | 43% | 51% | 49% | 47% |
| 5 | 65% | 57% | 49% | 51% | 53% | |
| Polytrauma/% | 31% | 37% | 33% | 37% | 36% | |
| Reactive pupils/%* | Both | No Data | No Data | 23% | 58% | 39% |
| One | No Data | No Data | 7% | 10% | 7% | |
| None | No Data | No Data | 6% | 10% | 7% | |
| No Data | 100% | 100% | 64% | 22% | 46% | |
| Time from injury to craniotomy in hrs/% | 0–2 | 4% | 5% | 4% | 4% | 4% |
| 2–4 | 32% | 24% | 24% | 21% | 23% | |
| 4–6 | 26% | 30% | 19% | 21% | 22% | |
| 6–8 | 17% | 16% | 14% | 12% | 13% | |
| 8–10 | 7% | 7% | 6% | 7% | 7% | |
| 10–12 | 3% | 2% | 5% | 6% | 5% | |
| 12–24 | 7% | 9% | 17% | 15% | 14% | |
| 24–48 | 5% | 6% | 12% | 16% | 13% | |
| Direct NSU admission/% | 39% | 48% | 47% | 48% | 47% | |
| 30-day survival/% | 59% | 62% | 74% | 73% | 70% |
GCS indicates Glasgow Coma Scale; ISS, Injury Severity Scale; LOS, length of stay (overall and critical care); NSU, neurosurgical unit.
*Values for GCS and pupil reactivity were missing in 14% and 46% of cases, respectively.
The number of patients in the database increased dramatically over the 20-year period, such that 61% presented in the period 2009 to 2013 (Supplemental Digital Content Figure S1). The age and sex of the patient was stable throughout, as was the ISS at presentation (Table 1). GCS was categorized in accordance with the Head Injury National Institute for Health and Care Excellence Guideline CG176 (Fig. 2).6 The most common GCS at presentation was 3 (20%), followed by 15 (12%). The most common mechanism of injury for patients aged 16 to 45 years was a road traffic collision (RTC, 37%), compared with a fall of less than 2 m in patients aged over 45 years (47%) (Supplemental Digital Content Figure S2).
FIGURE 2.
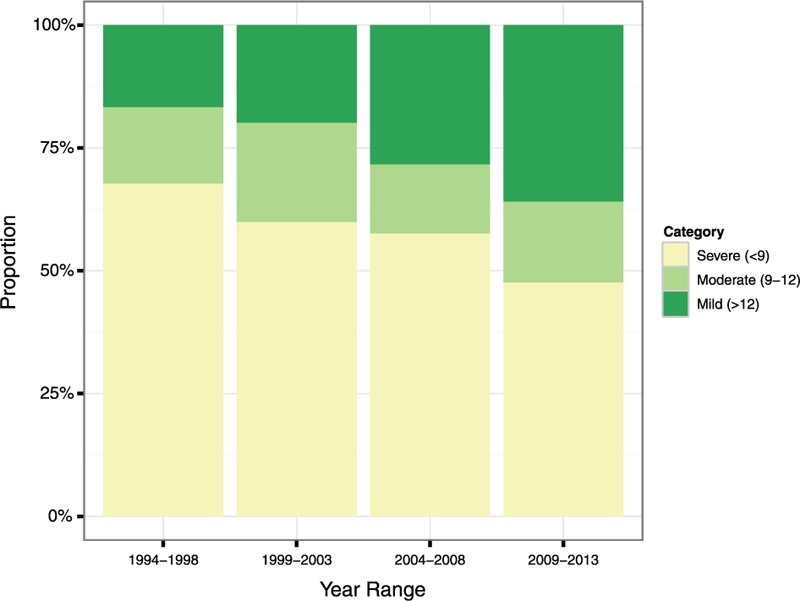
GCS category for ASDH across 5-year intervals. Values were missing in 14% of cases.
Statistical Analyses
Results from univariate logistic regressions for all variables are shown in the Supplemental Digital Content. Restricted cubic splines were fitted to model the year of the injury, time interval from injury to craniotomy, age, GCS, and the ISS. Pupil reactivity was modeled as a multinomial variable. All variables demonstrated significant associations to 30-day survival with the exception of sex and the day of the week of the injury (Supplemental Digital Content Tables S1 and S2).
All variables were included for multiple logistic regression. Despite a significant increase in GCS over the 20-year period, the interaction between these terms was not significant (χ2 < 4.33, P < 0.8885). All other interactions were also not found to be significant (P > 0.05) and were thus omitted. Full multiple logistic regression results were calculated with odds ratios for pupil reactivity, mechanism of injury, and day of the week given relative to their respective modal values (pupil reactivity was both reactive pupils; mechanism was a fall of less than 2 m; weekday was Sunday) (Tables 2 and 3). Median variables of continuous variables and mode values of discrete variables were imputed to produce plots of the results from the model.
TABLE 2.
Multiple Logistic Regression Results
| Variable | x2 | Survival OR (95% CI)* | P |
| Year of injury | 16.19 | 0.0010 | |
| Age | 63.44 | <0.0001 | |
| Time interval injury to craniotomy | 3.90 | 0.4203 | |
| Pupil reactivity | 169.21 | See Table 3 | <0.0001 |
| ISS | 34.68 | <0.0001 | |
| GCS | 10.31 | 0.0161 | |
| Mechanism | 9.14 | See Table 3 | 0.0577 |
| Direct NSU Admission = Yes | 2.59 | 0.82 (0.64–1.05) | 0.1078 |
| Polytrauma = Yes | 2.57 | 1.31 (0.94–1.82) | 0.1088 |
| SDH Severity = 4 | 0.17 | 1.05 (0.82–1.36) | 0.6837 |
| Sex = Female | 3.12 | 1.25 (0.98–1.60) | 0.0772 |
| Day of the Week | 6.67 | See Table 3 | 0.3527 |
GCS indicates Glasgow Coma Scale; ISS, Injury Severity Scale; NSU, neurosurgical unit.
*Odds ratios were not calculated for nonmonotonic continuous and ranked variables modeled using restricted cubic splines.
TABLE 3.
Multiple Logistic Regression Results: Relative Odds Ratios of Pupil Reactivity, Mechanism of Injury, and Day of the Week
| Variable | Survival OR (95% CI) | |
| Pupil reactivity relative to both reactive | No reactive pupils | 0.09 (0.06–0.14) |
| One reactive pupil | 0.19 (0.13–0.26) | |
| Mechanism relative to a Fall <2 m | Fall >2 m | 1.02 (0.74–1.40) |
| Other | 1.72 (1.15–2.56) | |
| Road Traffic Collision | 1.23 (0.84–1.79) | |
| Shooting/Stabbing | 3.10 (0.31–31.04) | |
| Day of injury relative to Sunday | Monday | 0.92 (0.61–1.38) |
| Tuesday | 0.94 (0.60–1.47) | |
| Wednesday | 0.67 (0.44–1.02) | |
| Thursday | 1.16 (0.75–1.77) | |
| Friday | 0.85 (0.57–1.25) | |
| Saturday | 0.90 (0.62–1.31) |
The multiple logistic regression results demonstrated a significant improvement in survival following surgery for ASDH between 1994 and 2013 (χ2 < 16.19, P < 0.0010). Age (χ2 < 63.44, P < 0.0001), pupil reactivity (χ2 < 169.21, P < 0.0001), ISS (χ2 < 34.68, P < 0.0001), and GCS (χ2 < 10.31, P < 0.0161) were also significant under multivariable analysis. Median values of continuous variables and modal values of discrete variables were imputed to produce plots of the results from the model for an example patient by year of injury, age, time interval from injury to craniotomy, GCS, and ISS (Figs. 3 and 4). The patient imputed was a 48.9-year-old male who suffered a grade 5 SDH after a fall of less than 2 meters, without polytrauma, presenting to a hospital without a dedicated neurosciences unit on a Sunday with both reactive pupils, an ISS of 25 and GCS of 8.
FIGURE 3.
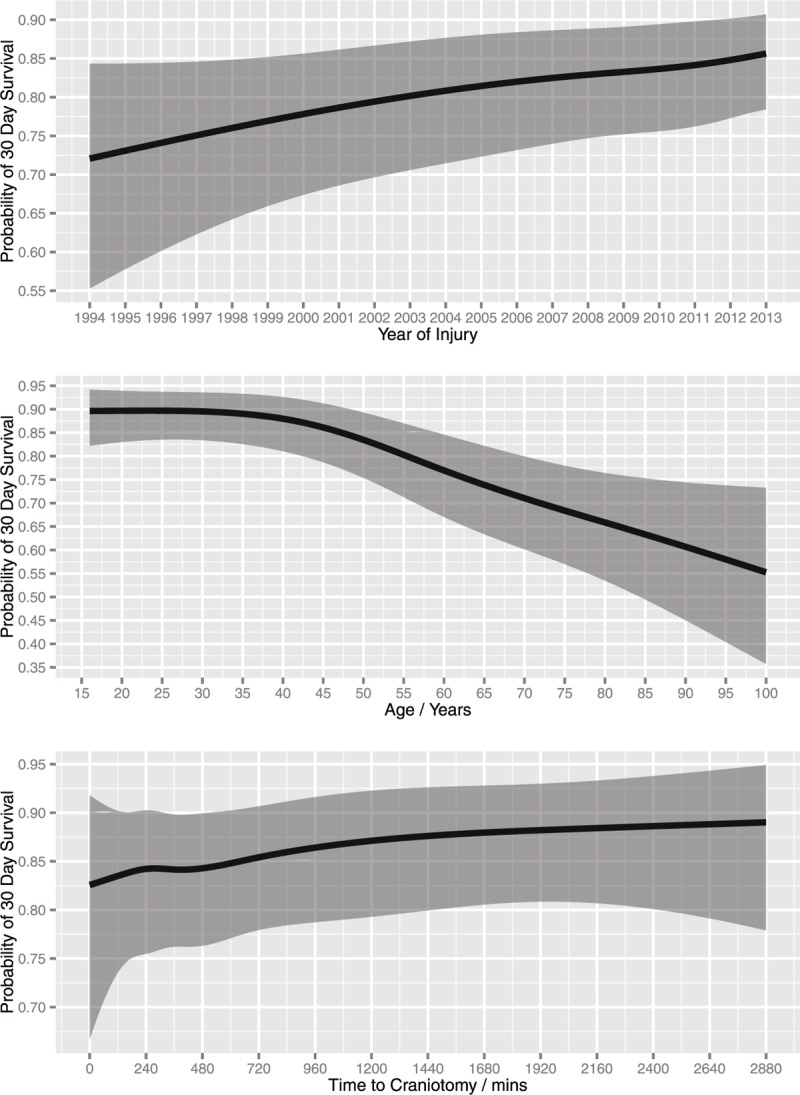
Effect of year of injury, age and time interval from injury to craniotomy on probability of survival following ASDH after controlling for mechanism of injury, location of admission, GCS, ISS, pupil reactivity, severity of SDH, existence of other trauma, sex, and day of the week. Shaded area represents a 95% confidence interval for the trend. GCS indicates Glasgow Coma Scale; ISS, Injury Severity Scale.
FIGURE 4.
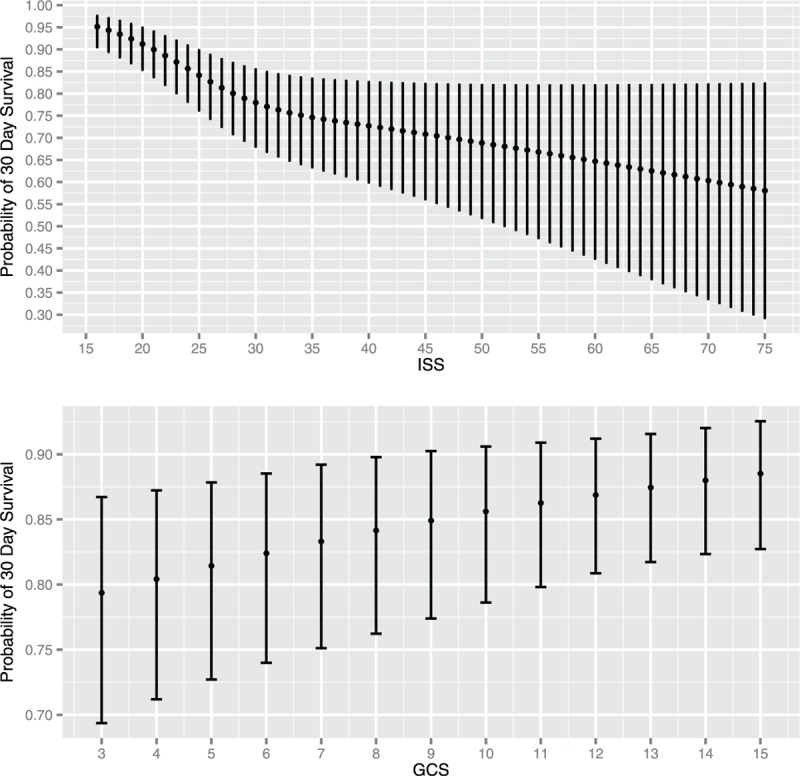
Effect of ISS and GCS on probability of survival following ASDH after controlling for year of injury, age, time interval from injury to craniotomy, mechanism of injury, location of admission, pupil reactivity, severity of SDH, existence of other trauma, sex, and day of the week. Error bars represent a 95% confidence interval for the trend. GCS indicates Glasgow Coma Scale; ISS, Injury Severity Scale.
With regard to age, the greatest fall in probability of survival was seen in the age range 45 to 70 years [45 years 30-day survival probability = 0.86; 95% confidence interval (95% CI) 0.78–0.91, 70 years 30-day survival probability = 0.71; 95% CI 0.59–0.80], in contrast to a steady decline elsewhere (Fig. 3). The time from injury to craniotomy showed no significant increase in probability of survival the earlier the procedure was performed (χ2 = 3.90, P = 0.4203). In addition, mechanism of injury (χ2 = 9.14, P = 0.0577), direct admission to a neurosurgical unit (NSU) (χ2 = 2.59, P = 0.1078), presence of polytrauma (χ2 = 2.57, P = 0.1088), SDH severity (χ2 = 0.17, P = 0.6837), sex (χ2 = 3.12, P = 0.0772), and day of the week (χ2 = 6.67, P = 0.3527) were not found to be significant.
With regard to sensitivity analysis, a range of offset values were added to the imputed data separately for pupil reactivity and GCS. The multiple logistic regression was repeated and probability of survival recalculated with 95% CIs at the median and mode values for all continuous and discrete variables, respectively. The probability of survival did not statistically significantly change with any offset of either variable (Supplemental Digital Content Figures S3 and S4). Furthermore, significant improvement in survival over time was observed in additional sensitivity analysis of original sites (χ2 = 9.47, P = 0.0088) and propensity score analysis of matched cohorts (χ2 = 21.63, P < 0.0001) at the beginning and end of the 20-year period (Supplemental Digital Content Tables S3 and S4).
DISCUSSION
The descriptive statistics identified a substantial improvement in survival following ASDH in England and Wales from 59% in 1994 to 1998 to 73% in 2009 to 2013. Furthermore, multiple logistic regression analysis showed a significantly improved survival over that period after controlling for all identified prognostic factors. This improvement in survival is consistent with other studies of TBI published from the TARN database.7,30 The significant improvement in gross survival may have been a consequence of improvements in pre-hospital and neurointensive care management of head-injured patients. The observed increase in baseline GCS over the 20-year period points toward improved pre-hospital management. However, one also needs to take into account that the introduction of the NICE head injury guidelines in 2003 has led to cranial computed tomographic (CT) imaging more often being performed in patients with higher GCS in recent years.6
Time interval from injury to craniotomy was not found to be predictive of survival under multivariable logistic regression analysis. These results are consistent with cohort studies from Canada, Poland, the USA, and the UK finding the time from injury to operative management was not predictive of outcome.15,16,18,19 Two studies have paradoxically found that increasing time to surgery had a significant effect in decreasing mortality. Dent et al9 evaluated 211 patients, finding a strong trend toward reduced survival for patients with hematoma evacuated within 4 hours (P = 0.07). Walcott et al11 identified a greater mortality associated with faster time to surgery, but the study was limited to patients with ASDH but limited additional structural brain injury. A 2006 systematic review by Bullock et al2 found that most studies focusing on the time between injury and surgery did not show a correlation with outcome but also noted that “the relationship between time from injury to operation and outcome is difficult to study because patients who are operated on soon after TBI tend to have more severe injuries than those who undergo delayed surgery.”
Similar to the approach in Walcott et al,11 our model controlled for ISS, GCS, and pupil reactivity. The current study also presents 2498 cases, in excess of the maximum of 211 patients previously published on this topic.9 Although it is still feasible that more severely injured people were operated on sooner, thus creating a selection bias, the design of this study significantly reduces this likelihood. However, perhaps the time interval that can have an impact on outcome is the one from the time of neurological deterioration (deterioration in GCS or new pupil abnormality), as previously suggested.2
The significance of age, GCS, ISS, and pupil reactivity identified in multiple logistic regression analysis is consistent with previous findings.9–11 In light of the aging population, our results suggest that the age range of 45 to 70 years is a critical range with a significant reduction in the probability of survival above the age of 70.
The proportion of patients presenting to NSUs on initial admission (33%) is consistent with the literature.6,7,9 Several studies in TBI literature have provided conflicting results of the effect of NSU direct admission. Raj et al31 identified no significant difference in 6-month mortality for direct and indirect admission to a neurosurgical trauma centre. However, Härtl et al32 identified a 50% higher risk of mortality for patients transported indirectly to a center with neurosurgical services. This study did not find a significant association between direct NSU admission and survival (χ2 = 2.59, P = 0.1078). Interaction terms between direct NSU admission and mechanism of injury, GCS, ISS, and pupil reactivity were also not found to be significant, suggesting that direct NSU admission is also not associated with more severely injured patients or improved pre-operative resuscitation and stabilization.
LIMITATIONS
There are some limitations to the results presented here. Firstly, we defined an ASDH as a subdural hematoma requiring a craniotomy within 48 hours of the injury. Radiological diagnosis of ASDH was not available. Our results may have therefore excluded some cases. However, overall, we feel that the cut-off of 48 hours is reasonable, as this paper aimed to focus on the more severe end of the spectrum of ASDH following acute trauma.
Secondly, the model did not adjust for comorbidities due to limited data in the TARN database in early years. However, as comorbidities are likely to be more prevalent with increasing age and the mean age increased over the study period, the improvement in risk-adjusted mortality over time may have been underestimated. Over the 20-year period, there was also a substantial increase in the number of hospitals covered in the TARN database. Sensitivity analysis of sites present throughout the 20-year period provided consistent results to the total cohort. Although the case mix of patients was controlled, their location and hence potential disparities in their management was not. However, none of the NSUs were outliers on TARN mortality monitoring over the time period studied.
Furthermore, there was a substantial proportion of data with missing values as previously identified in Table 1. This is particularly pertinent for pupil reactivity, which was missing throughout the period 1994 to 2003. Where pupil reactivity data were missing but GCS was 14 or 15, we assumed that no significant brainstem compression/uncal herniation would exist and pupils were considered to be both reactive. This way, the number of missing pupils was further reduced. Multiple imputation was then applied in the updated dataset, with statistical methods employed to minimize the potential risk to internal validity.33
Finally, the available data do not allow us to investigate the role of primary decompressive craniectomy in the management of ASDH.34 This is clearly an important aspect of surgical management and will hopefully be answered by an ongoing randomized trial (RESCUE-ASDH).35
CONCLUSION
This study has reviewed TARN data of 20 years of operative management of ASDH in England and Wales from 1994 to 2013. A significant improvement of survival was observed over the 20-year period after controlling for all identified prognostic factors. The analysis identified several prognostic factors of survival consistent with previously published data, notably age, GCS, ISS, and pupil reactivity. Direct admission to a hospital with an onsite NSU and a shorter time interval from injury to craniotomy were not associated with improved survival. Prospective trials and cohort studies are expected to elucidate the distribution of functional outcome in survivors.
Supplementary Material
Acknowledgment
We would like to thank all TARN member hospitals that submitted data over the time period studied and TARN co-ordination center injury coders.
Footnotes
DMF and AGK are joint first authors.
AGK is supported by a Royal College of Surgeons of England Research Fellowship, a National Institute for Health Research (NIHR) Academic Clinical Fellowship, and a Raymond and Beverly Sackler Studentship. PJH is supported by a NIHR Research Professorship and the NIHR Cambridge Biomedical Research Centre.
There are no conflicts of interest.
REFERENCES
- 1.Wilson MH, Kolias AG, Hutchinson PJ. Neurotrauma: a multidisciplinary disease. Int J Clin Pract 2014; 68:5–7. [DOI] [PubMed] [Google Scholar]
- 2.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery 2006; 58:16–24. [PubMed] [Google Scholar]
- 3.Lecky FE, Woodford M, Bouamra O, et al. Lack of change in trauma care in England and Wales since 1994. Emerg Med J 2002; 19:520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecky F, Woodford M, Yates DW. Trends in trauma care in England and Wales 1989-97. UK Trauma Audit and Research Network. Lancet 2000; 355:1771–1775. [DOI] [PubMed] [Google Scholar]
- 5.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma 1995; 38:185–193. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Head Injury – Triage, Assessment, Investigation and Early Management of Head Injury in Children, Young People and Adults. London, UK: National Clinical Guideline Centre; 2014. [PubMed] [Google Scholar]
- 7.Fuller G, Bouamra O, Woodford M, et al. Temporal trends in head injury outcomes from 2003 to 2009 in England and Wales. Br J Neurosurg 2011; 25:414–421. [DOI] [PubMed] [Google Scholar]
- 8.Kalanithi P, Schubert RD, Lad SP, et al. Hospital costs, incidence, and inhospital mortality rates of traumatic subdural hematoma in the United States: clinical article. J Neurosurg 2011; 115:1013–1018. [DOI] [PubMed] [Google Scholar]
- 9.Dent DL, Croce MA, Menke PG, et al. Prognostic factors after acute subdural hematoma. J Trauma Inj Infect Crit Care 1995; 39:36–43. [DOI] [PubMed] [Google Scholar]
- 10.Phuenpathom N, Choomuang M, Ratanalert S. Outcome and outcome prediction in acute subdural hematoma. Surg Neurol 1993; 40:22–25. [DOI] [PubMed] [Google Scholar]
- 11.Walcott BP, Khanna A, Kwon C-S, et al. Time interval to surgery and outcomes following the surgical treatment of acute traumatic subdural hematoma. J Clin Neurosci 2014; 21:2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seelig JM, Becker DP, Miller JD, et al. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med 1981; 304:1511–1518. [DOI] [PubMed] [Google Scholar]
- 13.Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien) 1988; 90:111–116. [DOI] [PubMed] [Google Scholar]
- 14.The Royal College of Surgeons of England. Report of the Working Party on the Management of Patients with Head Injuries. London: Royal College of Surgeons of England; 1999. [Google Scholar]
- 15.Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 1991; 74:212–218. [DOI] [PubMed] [Google Scholar]
- 16.Sergides IG, Whiting G, Howarth S, et al. Is the recommended target of 4 hours from head injury to emergency craniotomy achievable? Br J Neurosurg 2006; 20:301–305. [DOI] [PubMed] [Google Scholar]
- 17.Zafrullah Arifin M, Gunawan W. Analysis of presurgery time as a prognostic factor in traumatic acute subdural hematoma. J Neurosurg Sci 2013; 57:277–280. [PubMed] [Google Scholar]
- 18.Tien HCN, Jung V, Pinto R, et al. Reducing time-to-treatment decreases mortality of trauma patients with acute subdural hematoma. Ann Surg 2011; 253:1178–1183. [DOI] [PubMed] [Google Scholar]
- 19.Kotwica Z, Brzeziński J. Acute subdural haematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir (Wien) 1993; 121:95–99. [DOI] [PubMed] [Google Scholar]
- 20.TARN. The Trauma Audit & Research Network: An Overview. Salford, UK: The Trauma & Audit Research Network; 2006. [Google Scholar]
- 21.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2014; 332:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies. New York: Springer Science & Business Media; 2001. [Google Scholar]
- 23.Yates DW. ABC of major trauma. Scoring systems for trauma. BMJ 1990; 301:1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999; 18:681–694. [DOI] [PubMed] [Google Scholar]
- 25.Héraud-Bousquet V, Larsen C, Carpenter J, et al. Practical considerations for sensitivity analysis after multiple imputation applied to epidemiological studies with incomplete data. BMC Med Res Methodol 2012; 12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team. R. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. Available at: http://www.R-project.org/ (accessed August 12, 2015). [Google Scholar]
- 28.Harrell Jr FE. Rms: Regression Modeling Strategies. R Package Version 4.2-1. 2014. Available at: http://CRAN.R-project.org/package=rms (accessed August 12, 2015). [Google Scholar]
- 29.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 30.Ruff LM, Mendelow AD, Lecky FE. Improving mortality after extradural haematoma in England and Wales. Br J Neurosurg 2013; 27:19–23. [DOI] [PubMed] [Google Scholar]
- 31.Raj R, Siironen J, Kivisaari R, et al. Factors correlating with delayed trauma center admission following traumatic brain injury. Scand J Trauma Resusc Emerg Med 2013; 21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Härtl R, Gerber LM, Iacono L, et al. Direct transport within an organized state trauma system reduces mortality in patients with severe traumatic brain injury. J Trauma 2006; 60:1250–1256. discussion 1256. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 34.Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nat Rev Neurol 2013; 9:405–415. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson PJ, Kolias AG. Randomised Evaluation of Surgery with Craniectomy for patients Undergoing Evacuation of Acute Subdural Haematoma (RESCUE-ASDH). ISRCTN registry. BioMed Central. 2014. Available at: http://www.isrctn.com/ISRCTN87370545. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


