Abstract
Background
We evaluated clinical and functional outcomes following salvage total laryngectomy (STL).
Methods
Retrospective review of 218 patients who underwent STL between 1994 and 2014.
Results
Seventy percent of patients originally had T1–2, N0 tumors and 73% had definitive external beam radiation therapy (EBRT) alone. A majority utilized TEP (77%) and were gastrostomy free (80%) at last follow up. The 5-year disease control and overall survival rates were 65% and 57%. Patients with disease-free interval (DFI) following initial treatment < 2 years were more likely to develop recurrence (p=0.001) and die of disease (p=0.032) following STL. DFI following EBRT impacted disease control (p<0.001), with 5-year disease control of 92% for >5 year DFI and 60% for <2 year DFI.
Conclusion
Most patients remain disease-free following STL, achieve intelligible tracheo-esophageal speech and maintain an oral diet. Delayed recurrence following initial treatment portends better survival and may indicate a distinct biological profile.
Keywords: laryngectomy, squamous cell carcinoma, recurrence, salvage, complications, disease free interval, fistula, radiation, free flap
Introduction
Laryngeal squamous cell carcinoma (SCC) is diagnosed in approximately 20,000 patients annually in the United States.1 The 1991 Veterans Affairs cooperative study created a shift from primary surgery to non-surgical treatment regimens for advanced laryngeal cancer.2 Numerous retrospective studies from various institutions have demonstrated excellent clinical outcomes for early stage laryngeal cancer treated with definitive EBRT.3–5 The combination of these data sets has resulted in a widespread shift away from surgery toward radiation for most primary laryngeal tumors. As a result, surgery for laryngeal cancer is now most often performed in the setting of recurrent disease or incomplete tumor response to non-surgical treatment. Despite extensive research over the last 30 years, several studies have suggested that survival for patients with laryngeal cancer may be declining.6,7 and some have suggested the decline could be related to treatment of T4 laryngeal SCC’s with organ preservation protocols.8,9
Salvage total laryngectomy remains the standard treatment for advanced recurrent/residual local laryngeal disease following external beam radiation therapy.10–12 Previous studies have reported approximately 50–70% of patients alive and disease-free 5 years following STL.13–16 Initial and recurrent tumor staging have varyingly correlated with outcomes for patients undergoing STL.13 Our group has previously published data indicating that lack of a disease free interval (DFI) following definitive EBRT for oropharyngeal cancer is associated with poor clinical outcomes.17 We hypothesized that lack of DFI following initial treatment would also be associated with poor clinical outcomes in laryngeal cancer. To test this hypothesis and other factors which may influence outcomes following STL, we retrospectively reviewed all patients undergoing STL at the University of Texas MD Anderson Cancer Center between the years 1994 and 2014.
Materials and methods
Patients
Following approval by the University of Texas MD Anderson Cancer Center institutional review board, we reviewed the medical records of 259 patients with recurrent laryngeal SCC who underwent STL between 1994 and 2014. Patients with hypopharyngeal tumors (6 patients) at time of initial presentation were excluded from the study, as were patients who underwent surgical treatment prior to, or in addition to EBRT +/− chemotherapy (35 patients), such that the final study population was 218 patients. Demographics, tobacco and alcohol exposure, and patient clinical-pathologic history were comprehensively reviewed through the institutional electronic medical record. Tumor staging was conducted according to the American Joint Commission on Cancer staging system. Since many patients had their initial cancer treatment at outside institutions, precise T and N staging was unavailable for 69 patients, while precise tumor site (larynx versus hypopharynx) could not be determined for 2 patients. Patients were considered to have a smoking history if they were current smokers, or former smokers with greater than 10 pack/year history. All patients had biopsy proven recurrence prior to STL. Speech and swallowing data were censored at the time of last follow-up or disease-related functional deterioration. Disease free interval (DFI) was defined as the time period between completion of treatment for the initial tumor and the time of recurrence prior to STL.
Study endpoints and statistical analysis
Endpoints included time to second recurrence following STL and death. Time to second recurrence was calculated as date of STL to date of recurrence following STL. Overall survival (OS) following STL was calculated as date of STL to date of death. Actuarial survival rates were generated using the Kaplan-Meier method, and comparisons between groups were made using log-rank statistics. Univariate analysis was performed using Cox regression. All variables with p≤0.1 on univariate analysis were used for multivariate analysis. Statistical calculations were performed with SPSS (v16.0). For all statistics, p-values were considered to be statistically significant if below a threshold of 0.05 (two-sided).
Results
Patient and tumor characteristics
Patient demographics and smoking history are summarized in Table 1. Tumor characteristics are summarized in Table 2. Fifty-two patients (24%) reported continued smoking between their initial diagnosis of laryngeal cancer and STL. Mean and median age were 64 (standard deviation 10 years) and 64 (range 40–88 years) years respectively. At initial presentation prior to primary treatment, most patients were male (87%) current or former smokers (94%) with primary tumors of the glottic larynx (68%), early T stage (70%), and no neck disease (89%).
Table 1.
Patient characteristics
| Patient characteristics | N | % |
|---|---|---|
| Patients | 218 | |
| Mean age (yr) | 64 | |
| Male | 191 | 87 |
| Female | 27 | 13 |
| Smoker (>10packyr)* | 183 | 94 |
| Non-smoker (<10packyr)* | 11 | 6 |
Smoking data unavailable for 24 patients
Table 2.
Initial tumor characteristics
| Tumor characteristics | N | % | |
|---|---|---|---|
| Laryngeal Subsite* | 218 | ||
| Glottis | 149 | 75 | |
| Supraglottis | 51 | 25 | |
| T stage** | 167 | ||
| T1 | 58 | 35 | |
| T2 | 59 | 35 | |
| T3 | 40 | 24 | |
| T4 | 10 | 6 | |
| N stage*** | 167 | ||
| N0 | 148 | 89 | |
| N1 | 11 | 7 | |
| N2a | 0 | 0 | |
| N2b | 2 | 1 | |
| N2c | 6 | 4 | |
| N3 | 0 | 0 | |
Initial laryngeal subsite distinction unavailable for 18 patients
Initial T stage unavailable for 51 patients
Initial N stage unavailable for 51 patients
Primary treatment characteristics
One hundred sixty patients were treated with curative intent EBRT and 58 were treated with curative intent chemo-EBRT. Patients who were treated surgically prior to STL were excluded from the study.
Salvage treatment characteristics
All patients underwent STL. Patients were classified into one of 2 groups: STL for early recurrence (<2 years from end of previous treatment) (n=150) or STL for late recurrence (>2 years from end of previous treatment) (n=68). Mean and median disease free interval prior to STL were 806 days and 369 days respectively. Mean and median time from diagnosis of recurrent disease to STL were 42 and 42 days respectively. One hundred ten patients (50%) underwent a lateral neck dissection (unilateral or bilateral) and 106 patients (49%) underwent primary TEP placement. We evaluated the potential impact of elective neck dissection using pathologic data. Of 23 patients noted to have pathologically positive lymph nodes following STL, 17 patients (74%) had clinical or imaging evidence of nodal disease on pre-operative work up, while 6 patients (26%) had occult nodal disease. The rate of occult nodal disease in those without preoperative evidence of disease was 5% (6/110 patients).
Reinforcement of the pharyngeal closure using a local flap (sternocleidomastoid muscle or pectoralis major myofascial/myocutaneous) was performed in 19 (9%) patients, and free flap reconstruction was utilized in 42 (19%) patients. Treatment patterns changed over time with respect to primary TEP placement and free flap utilization. Prior to 2007, 76 of 127 STLs (59%) were accompanied by primary TEP placement, compared to only 30 of 91 STLs (33%) performed after 1/1/2007. Secondary TEP placement increased slightly over time [23 of 127 (18%) prior to 1/1/2007 and 27 of 91 (30%) after 1/1/2007]. Prior to 2007, only 3 of 127 STLs (2%) underwent free flap reconstruction, compared to 39 of 91 STLs (43%) performed after 1/1/2007. Ten patients underwent re-irradiation based on time interval from previous radiation, presence of new vascularized tissue in the radiation field (i.e. free flap reconstruction), and consideration of high risk features such as size and extent of the recurrent tumor, positive margins, perineural invasion, number of lymph node metastases, and lymph node extracapsular extension. Eight patients underwent adjuvant chemotherapy; of these, one patient refused adjuvant EBRT, one patient was an active participant on a chemoprevention trial, and one patient had active lung carcinoma. The remaining 5 patients underwent chemotherapy in the setting of adjuvant EBRT for high-risk features such as positive margins and extracapsular extension.
Complications
Twenty-nine (13%) patients required return to the operating room for post STL complications. Of these patients, 18 had fistula, 8 hematoma, and 1 revision of free flap anastomosis; 8 patients required multiple surgeries for complications. There was one peri-operative death, a 93 year old female patient who died of unknown causes in a skilled nursing facility 8 days following STL. Of the 18 patients who developed fistulas requiring re-operation, 4 of 42 had undergone free flap reconstruction (fistula rate of 10%), 2 of 19 had undergone pedicled flap reconstruction (fistula rate of 11%) and 14 of 157 had undergone primary closure (fistula rate of 9%). Patients who had pre salvage laryngectomy chemo-EBRT had higher rates of fistula formation (9/58, 16%) than patients treated with EBRT alone (9/160, 6%) (z-test, p<0.05). The higher rate of fistula formation for chemo-EBRT occurred despite the use of 29 free flap reconstructions utilized at time of STL for this patient group (23/58 patients, 40%), compared to 19 free flap reconstructions utilized at time of STL in the context of previous EBRT alone (19/160 patients, 12%). Eleven fistulas occurred in patients who underwent a lateral neck dissection along with STL (11/110, 10%) compared to 7 fistulas which occurred in patients who did not undergo a lateral neck dissection along with STL (7/108, 6%); this difference was not statistically significant.
Functional outcomes following STL
A total of 156 patients underwent TEP placement, of which 106 were primary TEPs at the time of STL, and 50 were secondary TEPs performed in a delayed fashion following STL. One patient underwent primary TEP placement with spontaneous closure, followed by secondary TEP placement. At last follow-up (and prior to any disease-related functional decline), 120 patients utilized TEP, 1 utilized esophageal speech, 74 utilized electrolarynx and 19 utilized writing as means of communication. Data were not readily available for 3 patients. Among patients who underwent TEP placement, 120/156 (77%) utilized TEP as the primary means of communication at last follow up. Forty five patients (20%) continued to utilize a gastrostomy tube, while 166 were gastrostomy free (data not available on 7 patients). Gastrostomy tube utilization at the time of last disease free follow up was more common among patients who underwent free flap reconstruction at the time of STL (19/42, 45%) compared to those patients which did not undergo free flap reconstruction at the time of STL (26/176, 15%) (p <0.05).
Recurrent disease characteristics
Among pathology reports that included perineural (PNI) or lymphovascular invasion (LVI), 32% of specimens were positive for PNI and 14% were positive for LVI. Positive margins were noted in 7% of specimens. Of the 23 specimens which demonstrated positive lymph nodes (11%), extracapsular extension was noted in 14 (61%).
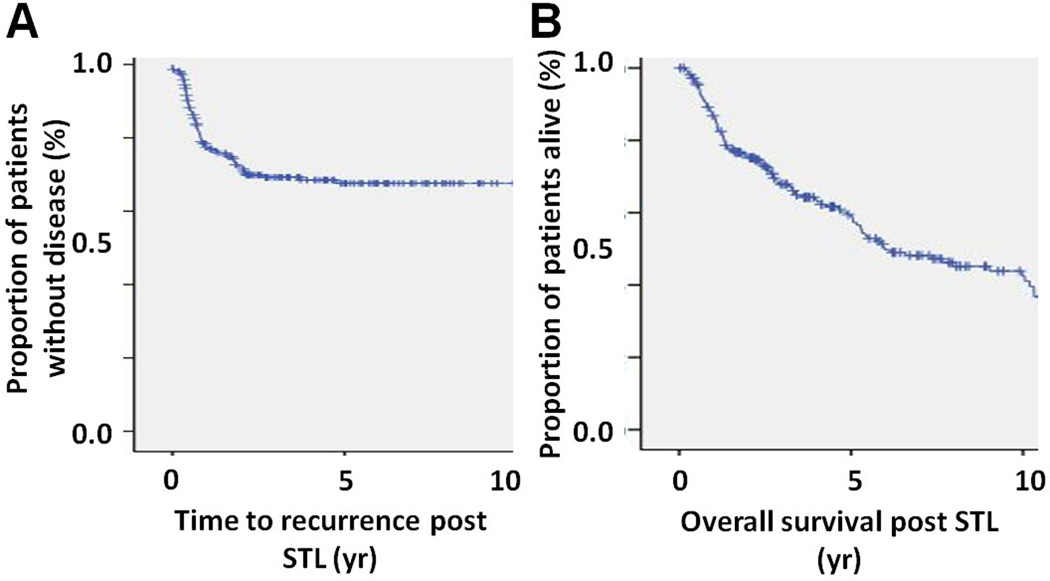
Recurrence and survival post STL
The overall mean and median follow-up time post STL for all 218 patients were 4.7 and 3.4 years, respectively. Seventy two percent and 65% of patients were recurrence free at 2 and 5 years post STL, respectively, while overall survivals at 2 and 5 years were 75% and 57% respectively (Figure 1). Sixty four patients (29%) demonstrated recurrence following STL, with mean and median time to recurrence of 344 and 247 days respectively (range 0 to 1737 days). Patterns of recurrence were as follows: 9 local only, 21 regional only, 21 distant only, 5 local and regional, 1 local and distant and 7 regional and distant. Among the 33 patients who experienced regional recurrence, 15 recurred along the internal jugular chain, 8 recurred in the paratracheal/paraesophageal chain, 4 had soft tissue peristomal recurrences and 6 patients recurred at multiple sites throughout the neck; only 5 patients recurred along the internal jugular chain after having undergone a lateral neck dissection on that side at the time of STL. Of the 23 patients who exhibited pathologically positive lymph nodes at time of STL, 14 had extracapsular extension, while 9 did not have extracapsular extension. Among the 14 patients with extracapsular extension, 5 (36%) developed regional recurrence, while 2 of 9 (22%) patients without extracapsular extension developed regional recurrence (p=NS).
Figure 1. Survival.
A) Time to recurrence following STL. B) Overall survival following STL.
Performing a lateral neck dissection had no statistically significant impact on regional recurrence; 17/110 (15%) of patients who underwent lateral neck dissection developed regional recurrence compared to 16/118 (14%) patients who did not undergo lateral neck dissection. Of the 23 patients with pathologically positive lymph nodes, 17 demonstrated pre-STL clinical or imaging evidence of nodal disease. After removing these 17 patients, we evaluated the impact of neck dissection on regional recurrence. In this subset of 201 patients without preoperative evidence of neck disease, 13 patients developed regional recurrence following STL and neck dissection (13/95, 14%) compared to 15 patients who developed regional recurrence following STL without neck dissection (15/106, 14%; p=NS).
Five patients developed a new primary in the upper aerodigestive tract, and all five of these patients received definitive treatment for their second primary malignancy. Among patients who recurred, 12 were alive at last contact and 52 were dead (mortality rate 83%). Among all patients at last follow up, 109 were alive and 109 were dead (mortality rate 50%). Of the patients who died, 47 died of recurrent/metastatic disease, while 22 died of other malignancies.
The impact of patient, tumor and initial treatment characteristics on recurrence and survival following total laryngectomy was ascertained via univariate analysis. Perineural or lymphovascular invasion did not significantly impact time to recurrence or overall survival. Only nodal status at time of STL and time between completion of initial treatment and STL (i.e. disease free interval) correlated with overall survival and time to recurrence on univariate analysis. These variables were confirmed to correlate with both time to recurrence and overall survival on multivariate analysis (Table 4).
Table 4.
Multivariate analysis
| TIME TO PROGRESSION | |||||
| Variable | Comparison | p-value | HR | 95.0% CI | |
| Lower | Upper | ||||
| pN | pN+ vs pN0 | 0.001 | 3.195 | 1.72 | 5.93 |
| DFI | DFI >24months vs DFI <24 months | 0.001 | 0.307 | 0.15 | 0.62 |
| OVERALL SURVIVAL | |||||
| Variable | Comparison | p-value | HR | 95.0% CI | |
| Lower | Upper | ||||
| pN | pN+ vs pN0 | 0.001 | 3.278 | 2.100 | 6.376 |
| DFI | DFI >24months vs DFI <24 months | 0.032 | 0.624 | 0.405 | 0.961 |
DFI denotes disease free interval following initial EBRT +/− chemotherapy
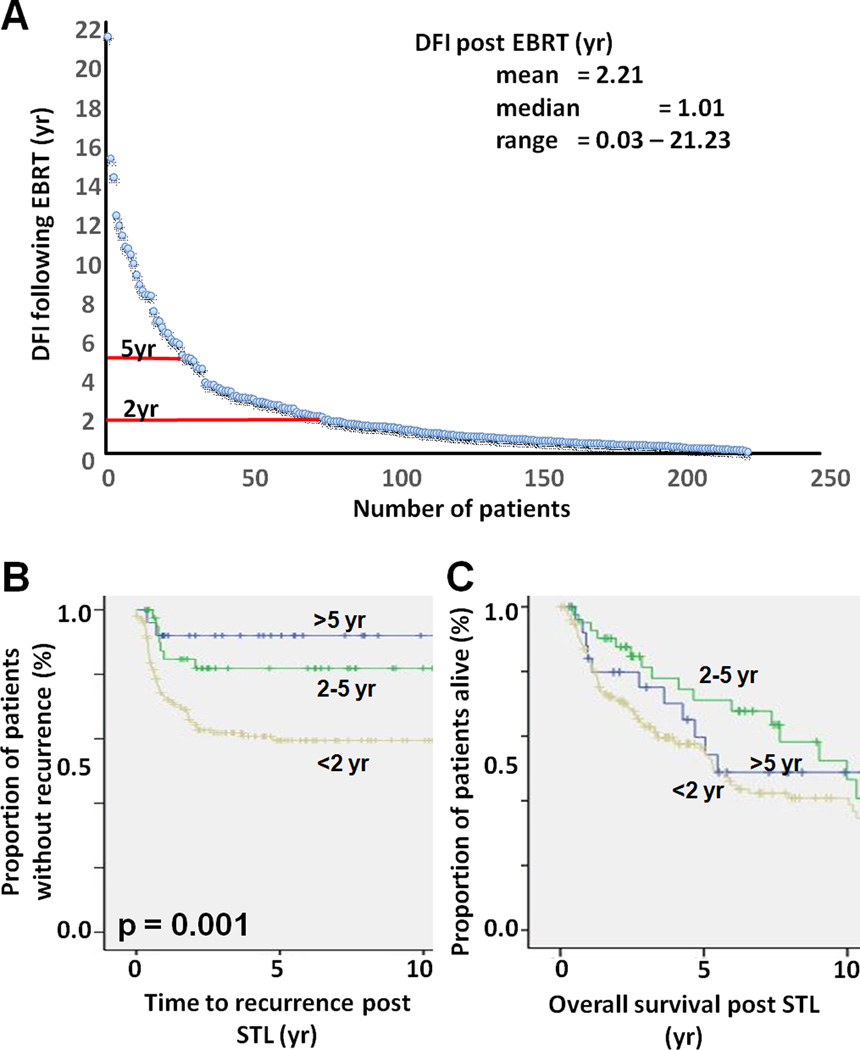
A total of 26 (12%) patients exhibited a DFI >5 years following initial EBRT. Among these patients, only two patients experienced recurrence following STL; both patients died of their disease. Among all 26 patients, 11 were dead at last follow up. Forty two patients (19%) exhibited a DFI following initial EBRT of 2–5 years. Among these patients, 7 experienced recurrence following STL; and 17 patients were dead at last follow up. Among 150 patients with a DFI <2 years, 55 patients experienced recurrence; and 81 were dead at last follow up. The overall recurrence rates for the three groups post STL (<2 years, 2–5 years, >5 years DFI prior to STL) were 36%, 17% and 8%, respectively. This is a linear relationship with an R2 value of 0.97. Kaplan Meier analysis of these three patient subgroups demonstrated a statistically significant impact of DFI following EBRT on clinical outcomes following STL (Figure 2). Pair-wise comparison demonstrated a statistically significant difference in overall survival between patients with DFI of 2–5 years compared to patients with DFI <2years (p=0.037).
Figure 2. Impact of DFI following EBRT on time to recurrence and overall survival post STL.
A) Most patients exhibited a DFI of <2 years post EBRT. B) DFI post EBRT impacts time to recurrence post STL. Trend analysis demonstrated a statistically significant relationship between the three groups (p-value = 0.001). C) DFI post EBRT impacts overall survival post STL (DFI <2years vs DFI 2–5 years p=0.037).
Discussion
In this large series of 218 patients with recurrent SCC larynx following previous external beam radiation therapy, STL provides acceptable disease control 2 (72%) and 5 (65%) years post-surgery. Disease control following STL further improves with length of DFI following initial EBRT completion, such that greater than 90% of patients with DFI beyond five years demonstrate long-term disease control. In contrast, overall survival after STL at 5 years drops to 57%, consistent with other studies of salvage total laryngectomy.13–16 The dichotomy between disease control and overall survival is not unexpected in a cohort of patients with older age, significant smoking history, multiple comorbidities, and second primary malignancies. Approximately 20% of patients who died in this cohort without recurrent disease succumbed to second primary malignancies, a statistic which likely significantly underrepresents the effect of second primary malignancy, given that many patients died of unknown causes.
Disease free interval following end of EBRT for primary disease was a major factor influencing both disease recurrence and overall survival. Among patients with disease-free interval greater than 5 years, only 8% developed recurrence following STL and 2-year overall survival was approximately 80%. It is quite possible that many of these patients with disease-free interval beyond 5 years represent those with second primary laryngeal tumors in contrast to true recurrences, which likely partially accounts for the improved survival in this subset of patients. The delayed recurrence would potentially portend a less aggressive tumor biology, whether a true recurrence of the same tumor clone, or a second primary tumor with more favorable tumor biology. Previous studies have similarly shown disease-free interval to be an important prognostic factor in other head and neck upper aerodigestive tract sites.17
The presence of neck disease generally decreases survival in laryngeal SCC by nearly 50%.18,19 The current data confirm the finding that patients with recurrent neck disease at the time of STL are nearly 4 times more likely to develop recurrent disease and over 3 times more likely to die following STL. Almost half of patients with disease recurrence in this cohort had regional disease recurrence, while less than 20% had local disease recurrence. Extracapsular extension was not significantly correlated with an increased rate of regional recurrence, although the numbers available for this analysis were very small.
The role of elective neck dissection during STL remains unclear. While some authors have illustrated improved survival outcomes with elective neck dissection,20–22 others have demonstrated no benefit.23,24 In our cohort, the rate of occult nodal disease among patients who underwent neck dissection in the absence of pre-operative clinically evident nodal burden was 5%. Among patients with no pre-STL clinical or radiographic neck disease, neck dissection with STL did not significantly impact the risk of regional recurrence when compared with patients who did not undergo neck dissection. However, even factoring out patients with pre-STL neck disease, there remains an obvious selection bias between those patients who underwent neck dissection at the time of STL versus those who did not. While we did not identify a clear benefit to elective lateral neck dissection in this study, it is difficult to account for this inherent selection bias in order to meaningfully determine whether the patients who developed regional recurrence in the absence of neck dissection with STL could have potentially benefited from an elective neck dissection.
The overall rate of regional recurrence in this cohort was 15%. Five patients (< 5% of patients who had a lateral neck dissection) developed recurrence in a previously dissected lateral neck, while 12 (6%) patients had paratracheal or peristomal soft tissue recurrences. Potential reasons for these recurrences include a missed regional node at the time of initial lateral neck or central compartment dissection; perineural spread; or soft tissue recurrence due to extranodal extension at the time of surgery, since very few of these patients were able to undergo further radiation for any potential microscopic disease after salvage STL. Additionally, there can be difficulty in distinguishing local versus regional recurrence with respect to central compartment/peristomal recurrences, and since these patients were considered to have regional recurrence, it is possible that this number may be slightly artificially high in terms of true regional recurrence. Finally, several of the central compartment recurrences occurred in superior mediastinal lymph nodes just inferior to the field of previous dissection. Overall these data are comparable with previous series which have reported local recurrence rates as high as 26% and locoregional control rates in the 74–90% range, although some smaller series have reported no isolated regional recurrences following STL.16,18,24
Although adjuvant re-irradiation with or without chemotherapy is a potential option following STL, relatively few patients undergo reirradiation in practice (only 6% reirradiation in the current cohort) due to morbidity/toxicity and equivocal impact on survival.25 For those that can undergo re-irradiation, the impact appears to be primarily limited to disease free survival and to those with high-risk pathologic features (perineural invasion, lymphovascular invasion, positive margins).26 In the current cohort, too few patients underwent adjuvant treatment to determine any potential impact on clinical outcomes.
Postoperative complications requiring reoperation occurred in 13% of patients in this cohort, most commonly for fistula, with an 8% rate of fistula formation requiring reoperation. Neck dissection with STL did not negatively impact the rate of fistula formation following STL. There was no difference in fistula rates in this cohort between patients closed primarily, those closed with the addition of a pectoralis muscle flap, and those closed with a free tissue transfer. However, the fistula rates do not take into account an inherent selection bias, in that patients with more advanced disease and more significant pharyngeal resections were presumably more likely to be closed with the addition of a pectoralis flap or free tissue transfer. One example of this inherent selection bias is illustrated by the finding that patients who were initially treated with chemo-EBRT were more likely to undergo free flap reconstruction compared to those initially treated with EBRT alone. Others have previously reported lower fistula rates with vascularized free tissue transfer or pedicled pectoralis flap when compared with primary closure.27–31
At this institution, free flap reconstruction has become increasingly utilized in pharyngeal reconstruction following STL, with 42% of STL’s since 2007 reconstructed with vascularized free tissue transfer, most commonly from the radial forearm or anterior lateral thigh. The primary indication for free flap reconstruction with STL at this institution is the extent of pharyngeal resection (partial or total pharyngectomy), with concurrent chemotherapy in the treatment of the primary disease as a secondary factor. In the current study, postoperative adjuvant chemotherapy was significantly correlated with higher rates of fistula formation requiring reoperation, despite a higher rate of free flaps in this population of patients. The addition of vascularized tissue should be considered for this patient population who undergo STL after chemoradiation therapy, as is the current practice of most head and neck surgeons at this institution.
Restoration of speech and swallowing function is essential to providing meaningful quality of life post STL.32 The current study demonstrates that a greater utilization of free flap reconstruction at the time of STL has been accompanied by a decrease in the rate of primary TEP placement and a concomitant decrease in the total number of TEP procedures among STL patients. Speech language pathologists at this institution generally prefer secondary TEPs in this patient population who undergo free flap pharyngeal reconstruction, as it potentially allows better assessment of tracheoesophageal speech potential and fewer ultimate failed TEP users, as well as potentially more accurate TEP placement and voice results following postoperative wound healing.
As organ preservation protocols have become more prevalent in the treatment of laryngeal SCC, tertiary referral centers may be receiving a greater proportion of patients with advanced recurrent laryngeal disease following EBRT. At the same time, the increasing reliability of free flap reconstruction over the last several decades has allowed surgeons to operate on patients who require greater amounts of pharyngeal mucosal resection.33,34 However, even with advancements in free flap reconstruction, inherent dynamic pharyngeal functionality may not be maintained with reconstruction, especially in the setting of previous radiation therapy. This loss of pharyngeal functionality contributes to lower rates of TEP procedures and tracheo-esophageal speech utilization among STL patients requiring free flap reconstruction. While the overall PEG tube rate in this study was 20%, the rate of PEG tube utilization among STL patients requiring free flap reconstruction was found to be 45%, over 3 times higher than that of patients who did not require free flap reconstruction. This subset of 42 (19%) free flap patients among the STL cohort included many patients who had total laryngopharyngectomy and/or concomitant esophageal or oropharyngeal resection, and 23 (55%) of these patients had history of concurrent chemotherapy along with EBRT for their primary disease. This subset therefore represented patients with the most advanced recurrent disease, the most significant surgical resections, and the most significant burden of treatment history. Smaller series evaluating dysphagia in laryngopharyngectomy populations have reported lower 13–16% gastrostomy tube rates, although these studies do not include exclusively salvage patients, and many of the larger series simply do not describe swallowing outcomes in detail.36,37 In an elderly population, pretreatment dysphagia and extensive treatment history with surgery and radiation have been associated with long term dysphagia.35 While the vast majority of patients who had a feeding tube in this study were able to tolerate some degree of oral diet, the modest 20% rate of at least partial feeding tube dependence related to pharyngeal dynamic dysfunction in the STL patient population should not be underestimated.
Conclusion
Approximately two-thirds of patients undergoing STL for recurrent SCC larynx remain disease-free and over half remain alive at 5 years post STL. Most patients undergo TEP and achieve intelligible tracheo-esophageal speech, while 20% of patients remain gastrostomy tube dependent. Factors which significantly impact recurrence and overall survival include disease-free interval following initial EBRT and recurrent nodal status. Patients with greater disease-free interval demonstrate progressively better disease control and overall survival, a factor which likely portends to progressively more favorable tumor biology.
Table 3.
Treatment characteristics
| Initial treatment characteristics | N | % |
|---|---|---|
| EBRT | 160 | 73 |
| EBRT + chemotherapy | 66 | 27 |
| Recurrent treatment characteristics | ||
| Surgery (STL) | 218 | 100 |
| Adjuvant EBRT* | 10 | 5 |
| Adjuvant chemotherapy | 8 | 4 |
indicates re-irradiation
Acknowledgments
Funding: none
Footnotes
None of the authors have any financial disclosures.
Conflict of interest: none
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Wang B, Wen S. Laser surgery versus radiotherapy for T1-T2N0 glottic cancer: a meta-analysis. ORL J Otorhinolaryngol Relat Spec. 2011;73:336–342. doi: 10.1159/000327097. [DOI] [PubMed] [Google Scholar]
- 4.Dey P, Arnold D, Wight R, MacKenzie K, Kelly C, Wilson J. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002027. CD002027. [DOI] [PubMed] [Google Scholar]
- 5.Abdurehim Y, Hua Z, Yasin Y, Xukurhan A, Imam I, Yuqin F. Transoral laser surgery versus radiotherapy: systematic review and meta-analysis for treatment options of T1a glottic cancer. Head Neck. 2012;34:23–33. doi: 10.1002/hed.21686. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 7.Chen AY, Schrag NM, Halpern MT, Ward EM. The impact of health insurance status on stage at diagnosis of oropharyngeal cancer. Cancer. 2007;110:395–402. doi: 10.1002/cncr.22788. [DOI] [PubMed] [Google Scholar]
- 8.Agra IM, Ferlito A, Takes RP, et al. Diagnosis and treatment of recurrent laryngeal cancer following initial nonsurgical therapy. Head Neck. 2012;34:727–735. doi: 10.1002/hed.21739. [DOI] [PubMed] [Google Scholar]
- 9.Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010;32:1–7. doi: 10.1002/hed.21294. [DOI] [PubMed] [Google Scholar]
- 10.Canis M, Ihler F, Martin A, Wolff HA, Matthias C, Steiner W. Organ preservation in T4a laryngeal cancer: is transoral laser microsurgery an option? Eur Arch Otorhinolaryngol. 2013;270:2719–2727. doi: 10.1007/s00405-013-2382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziegielewski PT, O'Connell DA, Klein M, et al. Primary total laryngectomy versus organ preservation for T3/T4a laryngeal cancer: a population-based analysis of survival. J Otolaryngol Head Neck Surg. 2012;41(Suppl 1):S56–S64. [PubMed] [Google Scholar]
- 12.Del Bon F, Piazza C, Mangili S, Redaelli De Zinis LO, Nicolai P, Peretti G. Transoral laser surgery for recurrent glottic cancer after radiotherapy: oncologic and functional outcomes. Acta Otorhinolaryngol Ital. 2012;32:229–237. [PMC free article] [PubMed] [Google Scholar]
- 13.Zafereo M. Surgical salvage of recurrent cancer of the head and neck. Current oncology reports. 2014;16:386. doi: 10.1007/s11912-014-0386-0. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin WJ., Jr Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Lorenz RR, Khan MJ, et al. Salvage laryngectomy in patients with recurrent laryngeal cancer in the setting of nonoperative treatment failure. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;149:245–251. doi: 10.1177/0194599813486257. [DOI] [PubMed] [Google Scholar]
- 16.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91–11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115:5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 18.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Mamelle G, Pampurik J, Luboinski B, Lancar R, Lusinchi A, Bosq J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am J Surg. 1994;168:494–498. doi: 10.1016/s0002-9610(05)80109-6. [DOI] [PubMed] [Google Scholar]
- 20.Hilly O, Gil Z, Goldhaber D, et al. Elective Neck Dissection during Salvage Total Laryngectomy - A Beneficial Prognostic Effect in Locally Advanced Recurrent Tumors. Clin Otolaryngol. 2014 doi: 10.1111/coa.12309. [DOI] [PubMed] [Google Scholar]
- 21.Hilly O, Stern S, Horowitz E, Leshno M, Feinmesser R. Is there a role for elective neck dissection with salvage laryngectomy? A decision-analysis model. Laryngoscope. 2013;123:2706–2711. doi: 10.1002/lary.24138. [DOI] [PubMed] [Google Scholar]
- 22.Koss SL, Russell MD, Leem TH, Schiff BA, Smith RV. Occult nodal disease in patients with failed laryngeal preservation undergoing surgical salvage. Laryngoscope. 2014;124:421–428. doi: 10.1002/lary.24005. [DOI] [PubMed] [Google Scholar]
- 23.Pezier TF, Nixon IJ, Scotton W, et al. Should elective neck dissection be routinely performed in patients undergoing salvage total laryngectomy? J Laryngol Otol. 2014;128:279–283. doi: 10.1017/S0022215114000425. [DOI] [PubMed] [Google Scholar]
- 24.Basheeth N, O'Leary G, Sheahan P. Elective neck dissection for no neck during salvage total laryngectomy: findings, complications, and oncological outcome. JAMA Otolaryngol Head Neck Surg. 2013;139:790–796. doi: 10.1001/jamaoto.2013.3995. [DOI] [PubMed] [Google Scholar]
- 25.Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol. 2014;32:2486–2495. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 27.Comert E, Tuncel U, Torun MT, et al. Pectoralis major myofascial flap in salvage laryngectomy. J Laryngol Otol. 2014;128:714–719. doi: 10.1017/S0022215114001479. [DOI] [PubMed] [Google Scholar]
- 28.Gendreau-Lefevre AK, Audet N, Maltais S, Thuot F. Prophylactic pectoralis major muscle flap in prevention of pharyngocutaneous fistula in total laryngectomy after radiotherapy. Head Neck. 2014 doi: 10.1002/hed.23742. [DOI] [PubMed] [Google Scholar]
- 29.Paleri V, Drinnan M, van den Brekel MW, et al. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. Laryngoscope. 2014;124:1848–1853. doi: 10.1002/lary.24619. [DOI] [PubMed] [Google Scholar]
- 30.Patel UA, Moore BA, Wax M, et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013;139:1156–1162. doi: 10.1001/jamaoto.2013.2761. [DOI] [PubMed] [Google Scholar]
- 31.Timmermans AJ, Lansaat L, Theunissen EA, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. Predictive factors for pharyngocutaneous fistulization after total laryngectomy. Ann Otol Rhinol Laryngol. 2014;123:153–161. doi: 10.1177/0003489414522972. [DOI] [PubMed] [Google Scholar]
- 32.Eadie TL, Day AM, Sawin DE, Lamvik K, Doyle PC. Auditory-perceptual speech outcomes and quality of life after total laryngectomy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;148:82–88. doi: 10.1177/0194599812461755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanasono MM, Weinstock YE, Yu P. Reconstruction of extensive head and neck defects with multiple simultaneous free flaps. Plast Reconstr Surg. 2008;122:1739–1746. doi: 10.1097/PRS.0b013e31818a9afa. [DOI] [PubMed] [Google Scholar]
- 34.Hanasono MM, Corbitt CA, Yu P, Skoracki RJ. Success of sequential free flaps in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2014;67:1186–1193. doi: 10.1016/j.bjps.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Gourin CG, Starmer HM, Herbert RJ, et al. Short- and long-term outcomes of laryngeal cancer care in the elderly. Laryngoscope. 2015;125:924–933. doi: 10.1002/lary.25012. [DOI] [PubMed] [Google Scholar]
- 36.Ho MW, Houghton L, Gillmartin E, et al. Outcomes following pharyngolaryngectomy reconstruction with the anterolateral thigh (ALT) free flap. Br J Oral Maxillofac Surg. 2012;50:19–24. doi: 10.1016/j.bjoms.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Clark JR, Gilbert R, Irish J, Brown D, Neligan P, Gullane PJ. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116:173–181. doi: 10.1097/01.mlg.0000191459.40059.fd. [DOI] [PubMed] [Google Scholar]