Abstract
Children with attention-deficit/hyperactivity disorder (ADHD) are at increased risk of problematic alcohol and other substance use in adolescence. This study used data from an ongoing, prospective, population-based twin study of Swedish children and adolescents to evaluate the extent to which the association between ADHD symptoms and alcohol problems reflects a unique source of genetic or environmental risk related to ADHD versus a broader predisposition to youth externalizing behavior. We used all available data from same-sex MZ and DZ twins on ADHD symptoms in childhood (age 9/12; N = 15,549) and alcohol problems in late adolescence (age 18; N = 2,564). Consistent with prior longitudinal studies, the phenotypic association between hyperactive/impulsive ADHD symptoms and alcohol problems was small in magnitude, whereas the association for inattentive symptoms was even weaker. Additive genetic influences explained 99.8% of the association between hyperactive/impulsive symptoms and alcohol problems. Furthermore, we found that the genetic risk specifically associated with hyperactive/impulsive symptoms was attenuated when estimated in the context of externalizing behavior liability during childhood, of which ADHD symptoms were specific expressions. In sensitivity analyses exploring hyperactivity in mid-adolescence, we found a similar pattern of genetic associations. These results are consistent with previous findings of genetically driven overlap in the etiology of ADHD and problematic alcohol use. At least some of this co-occurrence may result from a general predisposition to externalizing behaviors in youth.
Keywords: Behavioral genetics, ADHD, substance misuse, externalizing, neurodevelopmental problems
Prospective and population-based studies have documented that youth with attention-deficit/hyperactivity disorder (ADHD) are at risk of developing alcohol and other substance use problems [Ameringer and Leventhal, 2013; Carragher et al., 2014; Charach et al., 2011; De Alwis et al., 2014b; Lee et al., 2011]. There are multiple possible mechanisms that might explain ADHD-related risk for alcohol problems, and consequently there is a need for research that can distinguish among underlying causal relations [Molina and Pelham, 2014]. In the present study, we used multivariate behavioral genetic methods to evaluate how genetic and environmental factors might account for the association between ADHD symptoms and alcohol problems.
Several patterns have emerged regarding the role of ADHD in the development of alcohol and other substance problems. First, the association has often been stronger for hyperactive/impulsive than for inattentive symptoms or subtypes [Chang et al., 2012; Edwards and Kendler, 2012; Elkins et al., 2007; Lee et al., 2015], although not always [Capusan et al., 2015; Derks et al., 2014]. Second, although the association has been consistently replicated for both symptom counts and diagnoses of ADHD [Biederman et al., 2008; Capusan et al., 2015; Derks et al., 2014; Edwards and Kendler, 2012; Elkins et al., 2007; Monuteaux et al., 2008; Sundquist et al., 2015], the magnitude may be modest, particularly when ADHD symptoms are measured by parental-report and substance use problems by self-report in adolescence [Chang et al., 2012]. Indeed, one study of adolescent female twins found no association between symptoms of ADHD and alcohol dependence [Knopik et al., 2009].
Third, twin and family studies have frequently shown that the association can largely be explained by shared familial or genetic influences on ADHD and alcohol and other substance use problems [Capusan et al., 2015; Chang et al., 2012; Derks et al., 2014; Edwards and Kendler, 2012; Monuteaux et al., 2008; Skoglund et al., 2015; Sundquist et al., 2015], yet some studies have failed to find genetic overlap among youth [Biederman et al., 2008; Rose et al., 2004]. That is, although some of the same genetic factors responsible for childhood ADHD symptomatology may also influence the emergence of alcohol problems later in adolescence or adulthood, the extent of shared etiology in adolescence remains somewhat uncertain. Moreover, evidence of genetic overlap between ADHD and alcohol problems does not necessarily provide information about whether the genetic overlap is ADHD-specific or more general. This issue is particularly salient given that models of the externalizing spectrum have demonstrated common genetic influences on a latent predisposition underlying multiple externalizing disorders and that ADHD may partly be an expression of this same predisposition [Carragher et al., 2014; Krueger et al., 2002; Tarter et al., 2003; Young et al., 2000].
Several studies, in fact, have found that the phenotypic association between ADHD symptoms and alcohol and other substance use problems is attenuated when accounting for their co-occurrence with conduct disorder [Chang et al., 2012; Palmer et al., 2013; Sibley et al., 2014]. These studies raise the possibility that, rather than resulting from ADHD-specific genetic influences, this risk for alcohol problems may instead be an expression of a common predisposition for ADHD and conduct disorder or other externalizing or disruptive behaviors. At the same time, other studies have shown persisting associations when controlling conduct disorder [De Alwis et al., 2014a; De Alwis et al., 2014b; Elkins et al., 2007]. Whereas few behavioral genetic analyses of ADHD and alcohol and other substance use problems have been able to consider other disruptive behaviors, one study of adult male twins assessed the genetic association between adolescent hyperactive/impulsive symptoms and alcohol dependence when controlling conduct problems and other ADHD symptoms [Edwards and Kendler, 2012]. In that study, the hyperactive/impulsive genetic association was attenuated by approximately 50% but remained significant, supporting the prospect of some ADHD-specific genetic risk. Further research is, therefore, needed to characterize childhood-ADHD-related risk for later alcohol problems.
The objective of the present investigation was to examine genetic and environmental factors that might account for the association between ADHD symptoms and subsequent alcohol problems, particularly considering the rates of comorbidity between ADHD and other childhood disruptive behaviors. In order to do so, we used a multivariate behavioral genetic approach to distinguish between common externalizing liability and ADHD-specific risks for alcohol problems. Further, we capitalized on a population-based, prospective study of twins to focus on assessments during key developmental periods: caregiver-reported ADHD symptoms in childhood and self-reported alcohol problems in late adolescence, the period of greatest risk for the onset of alcohol and other substance use disorders [Grant et al., 2015; Li et al., 2004; Vergés et al., 2013].
Materials and Methods
Sample
We used data from monozygotic (MZ) and same-sex dizygotic (DZ) twins in the Child and Adolescent Twin Study in Sweden (CATSS), an ongoing, longitudinal, population-based study of twins [for details, see Anckarsäter et al., 2011]. Beginning in 2004, CATSS has been recruiting annually from all 9-year-old twins and their caregivers identified in the Swedish Twin Registry to prospectively examine relationships between neurodevelopmental problems and physical and mental health. CATSS participation begins with a phone interview of the caregiver (CATSS-9/12; twins born July 1, 1992 – June 30, 1995 were included at age 12). Subsequently, twins and their caregivers are invited to complete paper or web-based questionnaires after the twins reach age 15 (CATSS-15). Finally, twins and their caregivers complete an additional wave of web-based questionnaires at age 18 (CATSS-18). CATSS is approved by the Ethical Review Board at Karolinska Institutet.
The present analyses included same-sex twins who provided data at CATSS-9/12, CATSS-15, or CATSS-18. Not all birth cohorts have reached CATSS-15 and CATSS-18, and current response rates among those eligible to participate are approximately 75% at CATSS-9/12 and 50% at CATSS-15 and CATSS-18. In the interest of maximizing power and generalizability, we included all available data from participating individuals. We also included age-15 data from a subgroup of CATSS participants born 1993 – 1995 who completed the relevant measures as part of a broader assessment that included a clinical examination and maternal and paternal evaluations (n = 428 included individuals). This sub-study recruited families with same-sex twins who screened positive in CATSS-9/12 for neurodevelopmental problems (ADHD, autism spectrum disorders, and, in the first two birth years, other disorders) or control families and can be merged with other CATSS-15 data to ensure coverage of screen-positive families [Anckarsäter et al., 2011; Larson et al., 2013]. We excluded participants (n = 252) with brain damage, epilepsy, and chromosomal syndromes. We also excluded participants whose caregivers reported that the twins smoke[d], used snuff, or used alcohol by CATSS-9/12 (i.e., at the same time as the assessment of ADHD symptoms; n = 54 additional exclusions). The early alcohol and tobacco use items were taken from the Autism – Tics, ADHD, and other Comorbidities interview but were only available to identify individuals for exclusion prior to birth year 2002. In twin analyses, we made exclusions at the pair level. The final sample (N = 15,602) was 49% female. Sample sizes for specific analyses were smaller and are described below. Of the included CATSS-9/12 individuals, 73% were 9 years old.
Zygosity was determined on the basis of 48 single nucleotide polymorphisms [Anckarsäter et al., 2011]. For twins without available DNA, zygosity was determined on the basis of five questions concerning twin similarity. This alternative zygosity algorithm was developed using 571 pairs of known zygosity, and assignment was made only for those with a 95% probability of a given zygosity.
Measures
Autism – Tics, ADHD, and other Comorbidities (A-TAC) Inventory
The A-TAC is the primary measure of childhood neurodevelopmental problems in CATSS-9/12 [Anckarsäter et al., 2011]. The inventory was completed via telephone by one caregiver per twin pair; 86% of respondents were biological mothers. All A-TAC items have 3 response options: 0 = No, 0.5 = Yes, to a certain degree, and 1 = Yes. Summary scores were computed as the mean of all completed items, multiplied by the total number of possible items to produce sum-equivalent scores. See Table I for summary statistics.
Table I.
Summary Statistics and Associations between Childhood Symptoms and Adolescent Alcohol Problems
| Variable | Summary Statistics | Association with Alcohol Problems | ||||
|---|---|---|---|---|---|---|
| N | Range | M | SD | Continuous | Categorical | |
| CATSS-9/12 (A-TAC) | ||||||
| ADHD | ||||||
| Hyperactive/Impulsive | 15,549 | 0 – 9 | 0.80 | 1.47 | .12 [.05, .19] | .13 [.07, .19] |
| Inattentive | 15,549 | 0 – 9 | 1.01 | 1.71 | .04 [−.02, .10] | .06 [−.01, .12] |
| Disruptive behaviors | 15,545 | 0 – 10 | 0.53 | 1.03 | .09 [.01, .16] | .09 [.03, .16] |
| CATSS-15 (SDQ) | ||||||
| Hyperactivity | ||||||
| Caregiver-report | 4,061 | 5 – 15 | 6.96 | 2.06 | .11 [.03, .18] | .12 [.04, .21] |
| Self-report | 4,225 | 5 – 15 | 8.31 | 2.19 | .22 [.16, .29] | .28 [.20, .35] |
| Conduct problems | ||||||
| Caregiver-report | 4,061 | 5 – 15 | 5.94 | 1.25 | .17 [.10, .24] | .18 [.10, .26] |
| Self-report | 4,224 | 5 – 14 | 6.74 | 1.45 | .25 [.19, .32] | .30 [.22, .37] |
| CATSS-18 | ||||||
| Alcohol problems | 2,564 | 0 – 34 | 4.81 | 4.30 | -- | -- |
Note. CATSS-9/12 reports are provided by caregivers, and CATSS-18 reports are provided by twins. Values for associations with alcohol problems are standardized β [95% CI] from separate regression models with sex as a covariate. Possible range for alcohol problems is 0 – 40. Categorical indicates associations with latent alcohol problems liability indexed by the risk-zone cut-off thresholds. A-TAC = Autism – Tics, ADHD, and other Comorbidities Inventory; SDQ = Strengths and Difficulties Questionnaire.
The 10-item “Impulsiveness and Activity” A-TAC scale includes 9 items related to DSM-IV ADHD hyperactive/impulsive symptoms, and the 9-item “Concentration and Attention” scale consists of questions related to DSM-IV inattentive symptoms, all of which are largely unchanged in DSM-5 [American Psychiatric Association, 1994; American Psychiatric Association, 2013]. These scales have good reliability and have been clinically validated cross-sectionally and longitudinally [Hansson et al., 2005; Larson et al., 2010; Larson et al., 2013], and the nine hyperactive/impulsive and nine inattentive items have been used to assess ADHD symptoms in previous research [Pettersson et al., 2015]. Internal consistencies for hyperactive/impulsive and inattentive symptoms were good in the present sample, Cronbach’s αs = .86 and .90, respectively. The A-TAC also includes two five-item scales of “Defiance” and “Conduct.” Although these scales are not comprehensive assessments of oppositional defiant disorder or conduct disorder symptoms, they have content corresponding to disorder criteria (e.g., Does s/he often argue with adults? and Has s/he ever deliberately been physically cruel to anybody?, respectively) and have demonstrated validity [for additional information, see Kerekes et al., 2014].
Whereas a 2-factor measurement model fit the 10 Defiance and Conduct items better than did a single-factor model, Satorra-Bentler scaled Δχ2 (1) = 136.39, p < .001, the correlation between the two factors was very high (r = .86, 95% CI: [.84, .88]), suggesting that the items could be considered unidimensional. Moreover, the absolute fit of the single-factor model was good, χ2 (35) = 733.02, p < .001, CFI = .97, TLI = .96, RMSEA = .04, and its internal consistency was acceptable, α = .75. We therefore combined all Defiance and Conduct items into a single score for disruptive behaviors, except in the scale-level independent and common pathway models described below.
Strengths and Difficulties Questionnaire (SDQ)
Although the CATSS-15 assessments do not include the A-TAC, they include the caregiver- and child-report SDQ hyperactivity and conduct problems scales [Goodman, 1997; Goodman et al., 1998]. Each scale has 5 items with 3 response options (1 = Not true, 2 = Somewhat true, 3 = Certainly true). When clinical sub-study families provided maternal and paternal report, we averaged responses to create single caregiver scores. Internal consistencies were stronger for the hyperactivity scale (αs ranging from .69 – .85) than for the conduct problems scale (αs from .50 – .67).
As a test of the SDQ’s validity, we examined associations with the A-TAC scales. For both caregiver- and self-report, hyperactivity was more strongly associated with age-9/12 caregiver-reported hyperactive/impulsive (rs = .46 and .22, respectively) and inattentive (rs = .50 and .23) symptoms than with disruptive behaviors (rs = .39 and .19). Similarly, caregiver- and self-reported conduct problems were more strongly associated with age-9/12 caregiver-reported disruptive behaviors (rs = .40 and .19, respectively) than with hyperactive/impulsive (rs = .30 and .15) or inattentive (rs = .29 and .14) symptoms.
Alcohol Use Disorders Identification Test (AUDIT)
CATSS-18 includes the 10-item AUDIT as a measure of self-reported alcohol problems [Babor et al., 2001]. The AUDIT is a widely used screening measure. It assesses frequency and quantity of use and related problems, such as loss of control over drinking and alcohol-related injuries. Each of the first 8 items have 5 response options (e.g., from 0 = Never to 4 = Daily or almost daily), and the final 2 items have 3 options (0 = No, 2 = Yes, but not in the last year, 4 = Yes, during the last year). Participants who reported never drinking on the first item were scored as 0 for all items. We created a summary alcohol problems score by multiplying the mean of all completed items by 10 (α = .80). AUDIT scores also can be categorized into four risk-severity zones for screening purposes: 0 – 7 = Zone I (“low risk,” 80% of the present sample), 8 – 15 = Zone II (“in excess of low-risk guidelines,” 18%), 16 – 19 = Zone III (“harmful and hazardous drinking,” 1%), 20 – 40 = Zone IV (“possible … alcohol dependence,” 1%) [Babor et al., 2001, p. 21].
To evaluate the validity of the self-reported AUDIT, we merged CATSS data with substance use disorder (SUD) diagnoses through 2012 from the Swedish National Patient Register, which includes information on all inpatient and outpatient non-general-practitioner specialist visits in Sweden. We compared AUDIT scores of individuals who received SUD diagnoses (ICD-10 codes F10.x – F19.x, excluding tobacco use disorders [F17.x]) between the ages of 12 – 18 with those of individuals without diagnoses in those ages. We limited our analyses to individuals who had reached 18 years of age by 2012 (i.e., birth years 1992 – 1994) in order to ensure that all individuals had lived through the full at-risk period covered by their AUDIT self-reports. We used PROC SURVEYLOGISTIC in SAS to adjust for the non-independence of individuals in twin pairs. A total of 97 (3%) of 3,549 eligible individuals received an adolescent SUD diagnosis. Supporting the AUDIT’s validity as a measure of problematic substance involvement, a 1-point AUDIT increase was associated with a 20% increase in the odds of any SUD diagnosis (odds ratio [OR] = 1.20 [1.14, 1.26]) and an 18% increase in the odds of alcohol use disorder diagnosis specifically, OR = 1.18 [1.12, 1.25], analytic ns = 1,850. A corresponding 8-point increase (e.g., from 0 to zone II or from zone II to zone III) was associated with 416% (OR = 4.16 [2.79, 6.21] and 387% (OR = 3.87 [2.54, 5.90]) increases in the odds of the respective diagnoses.
Analytic Approach
We conducted preliminary data management and calculated summary statistics in SAS 9.4 (SAS Institute, Inc., Cary, NC). Unless otherwise noted, all other analyses were conducted in Mplus version 7, using full-information maximum likelihood estimation to account for missing data on twin pairs and individuals within twin pairs, which enabled us to include all available data [Muthén and Muthén, 1998–2012; Schafer and Graham, 2002]. We controlled sex by regressing observed variables on pair-level sex in the Mplus analyses. Female participants were lower in CATSS-9/12 ADHD symptoms and other disruptive behaviors and CATSS-18 alcohol problems (standardized mean differences ranging from -.12 [−.21, -.03] for alcohol problems to -.23 [−.27, -.20] for inattentive symptoms). Our first analytic step was to examine phenotypic associations between ADHD symptoms and alcohol problems. For phenotypic analyses in Mplus, we adjusted for the non-independence of individuals in twin pairs by specifying TYPE = CLUSTER, with ESTIMATOR = MLR or, for categorical alcohol problems, WLSMV.
Our second step was to estimate univariate behavioral genetic parameters. These analyses treated the twin pair as the unit of analysis and included pairs in which only one twin provided data (total N = 7,686 pairs; 1,658 male-male MZ pairs, 1,773 female-female MZ pairs, 2,271 male-male DZ pairs, and 1,984 female-female DZ pairs). We used a robust estimator in Mplus to adjust for non-normality in the observed variables (ESTIMATOR = MLR). Classical twin models apportion variance in a measured phenotype into latent additive (A) and dominant (D) genetic and shared (C) and non-shared (E) environmental factors [Neale and Cardon, 1992; Prescott, 2004]. Factors A and D represent genetic influences on behavior and are estimated on the basis of differences between MZ and DZ twin correlations. C represents the extent to which environmental factors make twins similar to each other, regardless of zygosity. E represents environmental factors that make twins within pairs dissimilar from each other, including measurement error. D and C cannot be estimated simultaneously, so we estimated ADE models when MZ correlations were greater than twice the magnitude of DZ correlations, as was mostly the case. Additionally, caregiver ratings of their twins’ symptoms may be susceptible to rater contrast or sibling interaction effects (s), whereby reported differences in symptoms between siblings are amplified by their perceived contrast or by competitive sibling interactions. These effects can be detected when DZ correlations are decreased and DZ variances are increased relative to MZ correlations and variances [Carey, 1986; Rietveld et al., 2003a; Rietveld et al., 2003b]. We estimated paths a, d (or c), and e as main effects from the latent variance factors and path s from the twin-sibling’s phenotype, and we standardized the latent factors to means of 0 and variances of 1 to identify the models [Prescott, 2004]. Following genetic theory, in MZ twins, cross-twin A and D or C correlations were fixed to 1.0, whereas in DZ twins, A correlations were fixed to 0.5 and D to 0.25 or C to 1.0.
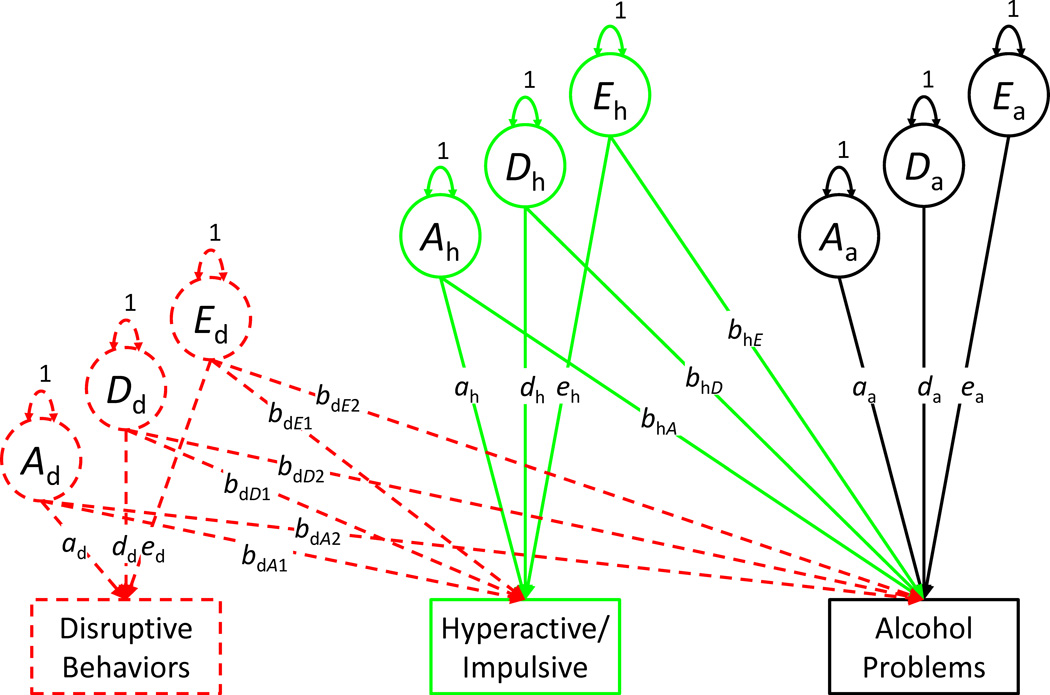
Third, we fit bivariate Cholesky decompositions to examine sources of covariation between ADHD symptoms and alcohol problems. Figure 1 displays the bivariate (and trivariate) Cholesky model for one twin per pair. Whereas the left-most phenotype in a Cholesky decomposition (e.g., hyperactive/impulsive symptoms) is regressed on its own ADE factors only, phenotypes farther to the right (e.g., alcohol problems) are regressed on their ADE factors as well as the ADE factors for the other phenotypes. As a result, the alcohol problems factors (Aa, Da, and Ea) represent genetic and environmental variance unique to alcohol problems. Crucially, the cross-trait regression paths (bhA, bhD, and bhE) estimate the extent to which genetic and environmental variance explains the association. The regression from E (bhE) is equivalent to a comparison between MZ twins and represents a strong test of an environmentally mediated effect of ADHD on alcohol problems [Turkheimer and Harden, 2014]. We compared the extent of genetic and environmental overlap between ADHD and alcohol problems across models using genetic and environmental correlations, which we calculated as the square root of the proportion of genetic or environmental variance explained using MODEL CONSTRAINT (e.g., rA = bhA / √ (bhA2 + aa2) or rA = bhA / √ (bdA22 + bhA2 + aa2) [Loehlin, 1996].
Figure 1.
Illustration of Cholesky decompositions (one twin per pair shown only). Dashed objects indicate components added in trivariate decompositions. A = additive genetic, D = dominant genetic, and E = non-shared environmental. Sibling contrast paths and pair-sex covariate not shown.
Fourth, we expanded the decompositions to include disruptive behaviors as an additional phenotype. As seen in Figure 1, disruptive behaviors were entered as the left-most phenotype in the decomposition. Although we cannot make inferences regarding causal ordering because disruptive behaviors and ADHD symptoms were assessed simultaneously, this approach has the benefit of producing residual ADE factors for ADHD symptoms that remove all variance shared with disruptive behaviors. The ADHD symptom regression paths in the model, therefore, estimate the extent to which genetic and environmental ADHD variance unique of disruptive behaviors was associated with alcohol problems. That is, in the context of this decomposition, the parameters estimate the extent to which the overlap between genetic and environmental influences on ADHD symptoms and alcohol problems is specific to ADHD or is an expression of a broader etiological overlap with a range of behavior problems in childhood.
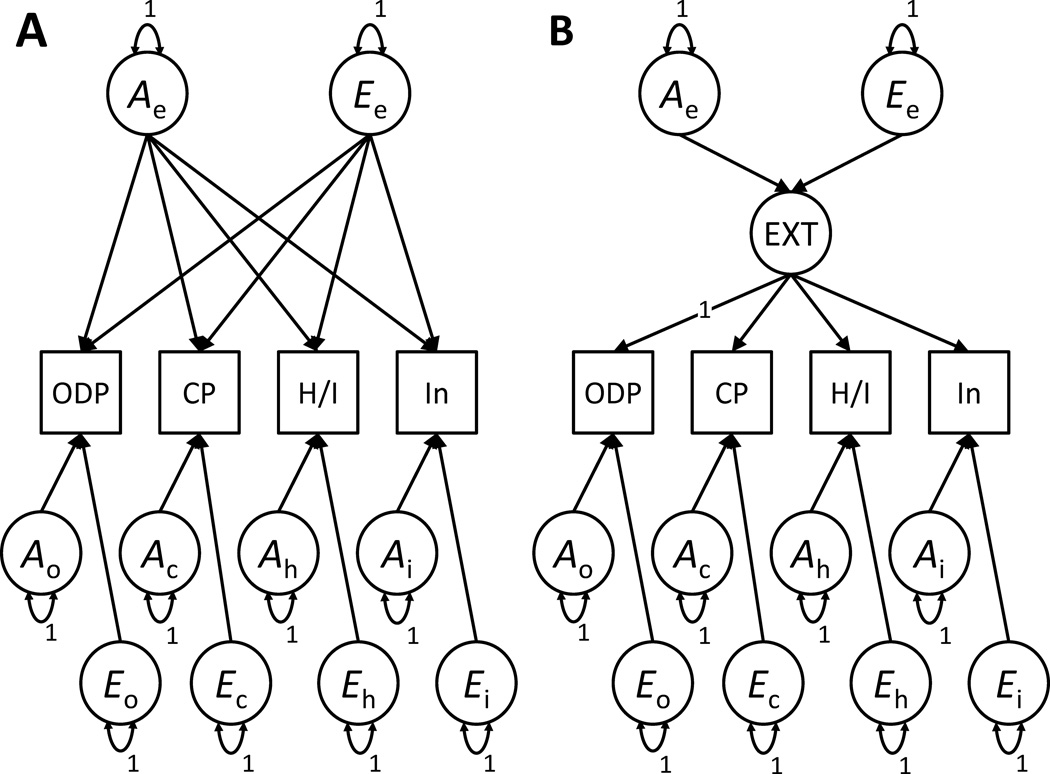
Fifth, more broadly, theoretical and empirical models place ADHD and other disruptive behaviors within an overarching childhood externalizing liability framework, such that a single, highly heritable predisposition drives at least some proportion of the expression of each phenotype [Young et al., 2000]. We compared independent and common pathway models to determine the best way to model the etiologic structure underlying symptoms of ADHD and other disruptive behaviors in childhood. The principal difference between the independent and common pathway models is that the common pathway specifies that common genetic and environmental influences are mediated through a phenotypic latent externalizing factor, which in turn affects each observed phenotype, whereas the independent pathway specifies that common genetic and environmental influences directly affect the phenotypes [Briley and Tucker-Drob, 2012; McArdle and Goldsmith, 1990]. As shown in Figure 2, we fit these models to the four childhood externalizing domains: hyperactive/impulsive symptoms, inattentive symptoms, oppositional/defiant problems, and conduct problems. After identifying the better-fitting structural model, we used that model to regress adolescent alcohol problems on common childhood externalizing genetic and environmental variance and ADHD-specific genetic and environmental variance not shared with other childhood externalizing behaviors. These tests asked the following question: In the context of a theoretically and empirically driven model of childhood externalizing behaviors, to what extents do ADHD-specific genetic and environmental factors influence adolescent alcohol problems?
Figure 2.
Illustrations of Independent Pathway (Panel A) and Common Pathway (Panel B) models (one twin per pair shown only). Sibling contrast paths and pair-sex covariate not shown. In the Common Pathway model, factors Ae and Ee are the common additive genetic and non-shared environmental factors, respectively, whereas factors Ao, Eo, Ac, Ec, Ah, Eh, Ai, and Ei are the specific factors. Paths are estimated but, for ease of reading, are not labeled. Common factor loading for ODP constrained to 1 to identify the externalizing (EXT) factor. CP = conduct problems, H/I = hyperactive/impulsive ADHD symptoms, In = Inattentive ADHD symptoms, ODP = oppositional/defiant problems.
Finally, previous research has demonstrated developmental continuity and change in the prevalence and etiology of ADHD symptoms [Larsson et al., 2006; Pingault et al., 2015]. We therefore conducted sensitivity analyses to examine the generalizability of the results across developmental periods as well as reporters. Specifically, we repeated steps one through four with caregiver- and self-reported SDQ hyperactivity and conduct problems from CATSS-15.
Results
Consistent with previous research, there was a small, positive association between hyperactive/impulsive ADHD symptoms and alcohol problems, regardless of whether alcohol problems were treated as continuous (β = .12 [.05, .19]) or as a latent liability indexed by the categorical risk-zone thresholds (β = .13 [.07, .19]). See Table I. Again consistent with prior findings, the association between inattentive ADHD symptoms and alcohol problems was very weak and did not significantly differ from zero. As a result, we focused our analyses on the association between hyperactive/impulsive symptoms and later (continuous) alcohol problems.
Previous studies have found that the AUDIT comprises distinct sets of alcohol consumption and alcohol-related problems items [Bush et al., 1998; Doyle et al., 2007; Maisto et al., 2000; Peng et al., 2012; Reinert and Allen, 2002; Rist et al., 2009]. Exploratory analyses suggested that the present associations were more strongly driven by alcohol-related problems than by alcohol consumption, supporting their clinical relevance. See Supplemental eTable I.
Univariate Behavioral Genetic Models
As shown in Table II, zygosity differences in twin correlations and variances for childhood symptoms were consistent with the possible presence of genetic influences and sibling contrast effects. The bottom portion of Table II displays estimates from the best-fitting twin models, all of which excluded dominant genetic variance. Most of the variance in ADHD symptoms could be explained by additive genetic influences, and we found substantial heritabilities for all phenotypes. We additionally found evidence of sibling contrasts for all childhood phenotypes, although these contrasts were most substantial for ADHD symptoms. Supplemental eTable II contains information about the univariate model comparisons.
Table II.
Twin Correlations and Variances and Univariate Twin Modeling Parameters
| Variable | N (pairs) | Twin Correlations | Twin Variances | Cross-Twin, Cross-Trait (Alcohol Problems) |
||||
|---|---|---|---|---|---|---|---|---|
| MZ | DZ | rMZ | rDZ | MZ | DZ | rMZ | rDZ | |
| CATSS-9/12 | ||||||||
| ADHD | ||||||||
| Hyperactive/ Impulsive |
3,421 | 4,253 | .70 [.66, .73] | .13 [.09, .16] | 1.77 [1.61, 1.94] | 2.36 [2.20, 2.52] | .11 [.04, .18] | .05 [−.02, .12] |
| Inattentive | 3,421 | 4,253 | .61 [.58, .65] | .11 [.07, .14] | 2.50 [2.30, 2.70] | 3.15 [2.97, 3.33] | .04 [−.03, .10] | .05 [−.02, .12] |
| Disruptive behaviors | 3,420 | 4,251 | .64 [.59, .68] | .30 [.26, .34] | 0.89 [0.79, 1.00] | 1.17 [1.06, 1.28] | .08 [.01, .16] | .08 [.003, .15] |
| CATSS-18 | ||||||||
| Alcohol problemsa | 726 | 748 | .69 [.62, .76] | .33 [.21, .46] | 18.92 [15.88, 21.96] | 18.69 [15.98, 21.39] | -- | -- |
|
Univariate Twin Modeling Parameter Estimates from Best-Fitting Models |
|||||
|---|---|---|---|---|---|
| Model | Additive (a2) | Dominant (d2) | Non-shared Environmental (e2) |
Sibling Contrast (s) | |
| CATSS-9/12 | |||||
| ADHD | |||||
| Hyperactive/ Impulsive |
AEs | .82 [.79, .85] | -- | .19 [.15, .22] | −.16 [−.19, −.14] |
| Inattentive | AEs | .76 [.72, .79] | -- | .24 [.21, .28] | −.15 [−.18, −.13] |
| Disruptive behaviors | AEs | .68 [.62, .75] | -- | .32 [.25, .38] | −.04 [−.08, −.003] |
| CATSS-18 | |||||
| Alcohol problems | AE | .69 [.63, .75] | -- | .31 [.25, .38] | -- |
Note. CATSS-9/12 reports are provided by caregivers, and CATSS-18 reports are provided by twins. Values are estimate [95% CI]. All parameter estimates control for pair sex. Twin correlations were estimated from models constraining intercepts and variances to equality across twins and zygosity groups. Variances were estimated from similar models without constraints for equal variances across zygosity groups. Unless otherwise noted, all MZ and DZ variances differed significantly from each other, Wald p < .05. Proportions of additive and non-shared environmental variance were calculated independent of the sibling contrast effects (i.e., a2 + e2 = 1).
Twin variances did not significantly differ from each other, Wald p = .91.
Genetic and Environmental Associations between ADHD Symptoms and Alcohol Problems
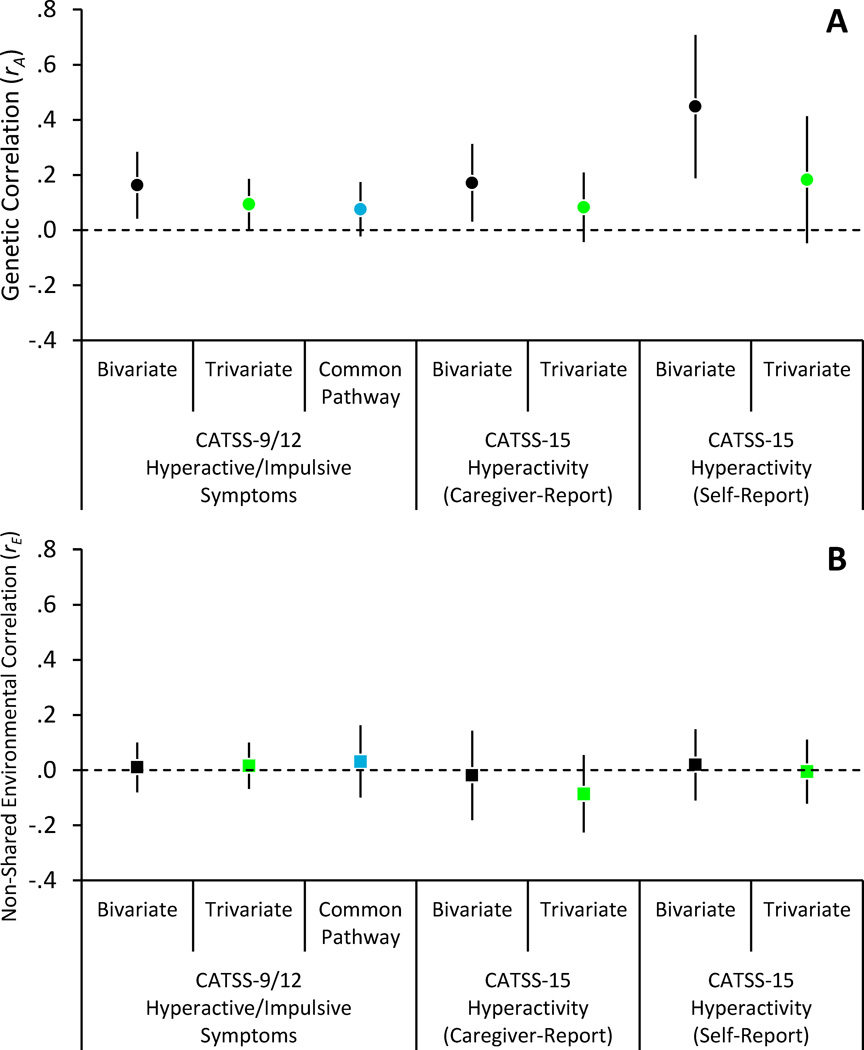
Our third analytic step was to determine the extent to which the association between hyperactive/impulsive ADHD symptoms and alcohol problems was explained by genetic or environmental influences. We used an AEs decomposition for hyperactive/impulsive symptoms to facilitate analyses on the basis of the univariate results (i.e., including sibling contrasts but not dominance). As shown in Table III, the association between hyperactive/impulsive symptoms and alcohol problems was virtually entirely (99.8%) explained by shared genetic influences on both outcomes. This proportion can be estimated by dividing the squared genetic regression path (.572) by the total shared variance (.572 + .022). As expressed in Figure 3 as a genetic correlation, the extent of the genetic overlap between the two phenotypes was relatively modest (rA = .16 [.04, .28]), indicating that a large proportion of genetic influences on alcohol problems were separate from genetic influences on hyperactive/impulsive symptoms. All parameter estimates from the Cholesky decompositions are available in Supplemental eTable III.
Table III.
Unstandardized Regression Coefficients from Bivariate and Trivariate Cholesky Decompositions
| Regression Coefficient | Bivariate Decomposition |
Trivariate Decomposition |
|---|---|---|
| Disruptive Behaviors → Hyperactive/Impulsive | ||
| Additive genetic path (bdA1) | -- | 0.89 [0.83, 0.95] |
| Non-shared environmental path (bdE1) | -- | 0.21 [0.17, 0.25] |
| Disruptive Behaviors → Alcohol Problems | ||
| Additive genetic path (bdA2) | -- | 0.52 [0.13, 0.90] |
| Non-shared environmental path (bdE2) | -- | 0.00 [−0.25, 0.25] |
| Hyperactive/Impulsive → Alcohol Problems | ||
| Additive genetic path (bhA) | 0.57 [0.22, 0.92] | 0.33 [0.01, 0.65] |
| Non-shared environmental path (bhE) | 0.02 [−0.19, 0.24] | 0.04 [−0.16, 0.24] |
Note. Values are estimate [95% CI]. Both decompositions control pair sex. Dominant genetic factors for disruptive behaviors and hyperactive/impulsive symptoms were omitted to facilitate modeling given results of univariate models (see Table II and eTable II). Hyperactive/impulsive symptoms coefficients from the trivariate decomposition represent genetic and non-shared environmental variance not shared with disruptive behaviors. N = 3,422 MZ pairs and 4,254 DZ pairs (4,255 DZ pairs for trivariate model).
Figure 3.
Additive genetic (Panel A) and non-shared environmental (Panel B) correlations between hyperactive/impulsive symptoms or hyperactivity and alcohol problems. In trivariate and common pathway models, correlations represent the variance shared between alcohol problems and the unique variance in hyperactive/impulsive symptoms (or hyperactivity), net of variance in common with other disruptive or conduct problems. Error bars are 95% CIs.
ADHD Symptoms, Other Disruptive Behaviors, and Alcohol Problems
Our fourth step was to add other disruptive behaviors to the decompositions. Here, we examined the extent to which genetic risk for alcohol problems was a) an expression of a broader liability for disruptive childhood behaviors (including hyperactive/impulsive symptoms) or b) specifically associated with hyperactive/impulsive symptoms. As shown in Table III and Figure 3, including disruptive behaviors attenuated but did not eliminate the genetic association between hyperactive/impulsive symptoms and alcohol problems, rA = .09 [.001, .19]. Comparing the genetic correlations, this association was attenuated by 42% relative to the magnitude of the association in the bivariate decomposition. The genetic association between disruptive behaviors and alcohol problems was also modest, rA = .15 [.01, .29].
Independent/Common Pathway Models
Our fifth step was to evaluate common and ADHD-specific genetic risks within a theoretically and empirically based model of childhood externalizing behaviors. We began by comparing independent and common pathway models of the structure of childhood externalizing liability (i.e., for hyperactive/impulsive symptoms, inattentive symptoms, conduct problems, and oppositional/defiant problems). Given the differences in ages and reporters and the small observed covariation with ADHD symptoms, we did not include alcohol problems in these structural models initially. On the basis of the univariate results, we report analyses from AEs models. Consistent with prior studies, a common pathway AEs model, χ2 (79) = 390.55, p < .001, RMSEA = .03, CFI = .97, AIC = 140,362.19, BIC = 140,535.84, scaled Δχ2 (3) = 0.19, p = .98, was a better fit to the childhood behavioral data than was an independent pathway AEs model, χ2 (76) = 515.22, p < .001, RMSEA = .04, CFI = .96, AIC = 140,364.27, BIC = 140,558.75. The common liability factor was highly heritable (.87 [.85, .90]), although there was specific genetic and environmental variance in all four behaviors. See Supplemental eTable IV for unstandardized parameter estimates from the common pathway model.
Drawing from this model, we tested whether common childhood externalizing and specific hyperactive/impulsive genetic influences were associated with adolescent alcohol problems. We regressed alcohol problems, which had not been initially included in the common pathway model, on the common and specific A and E factors. In the interest of limiting the number of estimated parameters, we included regression paths for the externalizing factor and the specific variance in hyperactive/impulsive symptoms only, χ2 (116) = 484.86, p < .001, RMSEA = .03, CFI = .97, N = 3,422 MZ and 4,255 DZ pairs. As shown in Figure 3, the specific genetic association (rA = .08 [−.02, .17]) was again attenuated (by a factor of 53% relative to the bivariate estimate), and we found virtually no non-shared environmental overlap, rE = .03 [−.10, .16]. In contrast, there was a small-to-moderate genetic (rA = .15 [.002, .29]) but not environmental (rE = −.01 [−.17, .15]) correlation between the common externalizing factor and alcohol problems. In sum, we found little evidence of hyperactive/impulsive-specific genetic risk for adolescent alcohol problems independent of common externalizing risk.
Sensitivity Analyses
Replacing CATSS-9/12 ADHD symptoms with CATSS-15 caregiver- or self-reported SDQ hyperactivity in analytic steps one through four produced comparable results (see Supplemental eTables V and VI). Figure 3 shows that the associations between hyperactivity and alcohol problems were driven by correlated genetic influences and minimal common non-shared environmental influences. Most important, the specific genetic risk for alcohol problems associated with hyperactivity was attenuated to non-significance (by factors of 51%-59%) when considering the overlap in genetic influences on conduct problems and hyperactivity. Thus, the pattern of results in our primary analyses could largely be generalized to mid-adolescence and to self-reported behavior.
It is important to note, however, that there were some differences between the self- and caregiver-reported results at CATSS-15. Caregiver-reported genetic influences on hyperactivity were greater than were self-reported genetic influences. Additionally, phenotypic and genetic alcohol problems associations with caregiver-reported hyperactivity were weaker than were associations with self-reported hyperactivity, perhaps because of shared self-report measurement variance with alcohol problems or parents’ difficulty reporting on adolescent hyperactivity.
Discussion
The current results extend previous research in four key domains. First, we found that hyperactive/impulsive ADHD symptoms conferred greater risk for alcohol problems than did inattentive symptoms, although neither association was large. The magnitude of hyperactive/impulsive risk was consistent across multiple tests and was similar to that found in a prior prospective Swedish twin study [Chang et al., 2012]. The current study therefore provides robust, population-based, prospective evidence of modest, hyperactive/impulsive-specific risk for alcohol problems. Previously identified differences in the strength of the association as a function of age and outcome (i.e., alcohol use vs. problems), as well as our supplemental analyses in eTable I, indicate that the association should be considered as potentially specific to alcohol problems in late adolescence [Lee et al., 2011; Molina et al., 2007].
Second, in best-fitting univariate models, variances for all study variables could be decomposed into additive genetic and non-shared environmental components. Our estimates of ADHD symptom heritabilities were comparable to those found elsewhere [Burt et al., 2012; Larsson et al., 2014; Rietveld et al., 2003a]. Similarly, Rhee and colleagues [2003] found that problem alcohol use in adolescents was highly heritable. It is worth noting that, like their associations with ADHD, the etiologies of alcohol phenotypes appear to vary by age and assessed outcome. For example, alcohol initiation and use, as well as rare early-adolescent AUD symptoms, have demonstrated substantial shared environmental variances [Rhee et al., 2003; Rose et al., 2004], and Knopik and colleagues [2009] found little heritability for dependence symptoms among female adolescents. Regarding other disruptive behaviors, our lack of shared environmental influences differs from meta-analytic estimates of 10% – 15% for these outcomes [Burt, 2009]. Previous results from CATSS suggest little shared environmental variance when these problems are measured dimensionally (except for conduct problems in girls) but substantial variance when they are measured with cut-off scores [Anckarsäter et al., 2011; Kerekes et al., 2014]. At least in this sample, it is possible that dimensional assessment yields greater sensitivity to genetic influences.
Third, we found that hyperactive/impulsive-symptom risk for alcohol problems was mostly explained by shared genetic influences. In bivariate decompositions, virtually all of their co-occurrence could be explained by shared genetic influences. Again, this result matches prior findings from Sweden [Chang et al., 2012], the US [Edwards and Kendler, 2012], and elsewhere [Derks et al., 2014]. However, it should be noted that a large proportion of the genetic influences on alcohol problems were independent of hyperactive/impulsive symptoms. That is, although genetic influences mostly explained the association, hyperactive/impulsive genetic influences could not explain the majority of genetic influences on alcohol problems.
Fourth, and most important, we found that approximately half of the genetic association between hyperactive/impulsive symptoms and alcohol problems resulted from influences common to ADHD and other disruptive (or externalizing) childhood behaviors. Our common pathway model replicated previous models of an externalizing spectrum that includes ADHD [Carragher et al., 2014; Young et al., 2000]. Evidence converged from Cholesky decompositions and the common pathway model that the association between hyperactive/impulsive symptoms and alcohol problems was in large part attributable to common childhood externalizing, rather than ADHD-specific, genetic influences. The extent of shared genetic influences on childhood externalizing behaviors has implications for future research on the genetics of childhood psychopathology. Despite increasing numbers of identified regions from genome-wide analyses, genomic associations cannot yet explain a substantial proportion of ADHD variance or functional mechanisms [Hawi et al., 2015]. Some have suggested that dimensional or endophenotype measures might aid in these efforts [Hawi et al., 2015]. Others have proposed using multiple behavioral phenotypes simultaneously in gene discovery given that genetic commonalities across childhood disorders raise the possibility that dysfunction of one or more neurobiological system might underlie a broader range of symptoms than those classified under a specific diagnostic category [Lahey et al., 2011; Rhee et al., 2015]. The present findings, notably including the high heritability of childhood externalizing (87%), support this second perspective.
In contrast, however, the extent of genetic overlap between childhood symptoms and adolescent alcohol problems was modest, with genetic correlations in the small-to-moderate range. Previous studies have found genetic correlations between ADHD- and alcohol-related phenotypes ranging from .01 to .50, the variability of which may be due to reporters (parent vs. self), development (adolescence vs. adulthood), design (cross-sectional vs. prospective), and/or outcomes (early alcohol use vs. problems or dependence) [Capusan et al., 2015; Chang et al., 2012; Derks et al., 2014; Edwards and Kendler, 2012; Knopik et al., 2009]. Genetic distinctions between childhood symptoms and later adolescent alcohol problems might be explained by the activation of novel genetic influences that drive both ADHD and alcohol problems in adolescence [Pingault et al., 2015]. At least some genetic influences on alcohol problems, though, such as those related to alcohol metabolism, may truly be unique of other externalizing behaviors [Bierut et al., 2012; Wall et al., 2013].
It is also important to note that our inferences are limited to the phenotypes we modeled. There is increasing interest in etiological commonalities not only within the externalizing spectrum but also across a broader range of psychiatric problems [Caspi et al., 2014; Lahey et al., 2011; Lahey et al., 2014]. In particular, CATSS data have revealed a common genetic factor underlying a seemingly diverse array of neurodevelopmental problems [Pettersson et al., 2013]. Future research should examine the role of this neurodevelopmental predisposition in the current findings and in the emergence of adolescent alcohol problems.
Our results raise the question of why there was negligible non-shared environmental overlap between childhood hyperactive/impulsive symptoms and adolescent alcohol problems. Prior studies have demonstrated non-shared environmental contributions to commonalities across the externalizing spectrum (including substance use problems), as did our model of childhood behavioral symptoms described in eTable IV [Krueger et al., 2002; Young et al., 2000]. This pattern suggests that the non-shared environment is involved in at least some externalizing comorbidity. However, it is possible that, because non-shared environmental influences tend to be more transient across development [Chang et al., 2013], only common genetic influences persist from childhood into late adolescence. Unique environmental influences on adolescent alcohol problems may therefore be more likely to be developmentally specific, perhaps including those associated with peer-group drinking [Cruz et al., 2012; Edwards et al., 2015].
The present study should be interpreted with an understanding of its strengths and limitations. First, classical and multivariate twin studies cannot identify the specific biological pathways through which genetic influences operate, and they are dependent upon several key assumptions, including those regarding equal environments, random mating, the extent to which twins share their segregating genes, and the absence of gene-environment correlation or interaction. For alcohol problems in particular, however, permissive environments have been found to promote genetic influences [Salvatore et al., 2014; Young-Wolff et al., 2011]. Future research should examine whether such gene-environment interactions are present in these data.
Second, we had difficulty estimating dominant genetic and sibling contrast effects simultaneously [Rietveld et al., 2003b]. Given that evidence of contrast effects has been found for ADHD-related behaviors when assessed by parents but not teachers or, as shown here, self-report, they appear to reflect rater biases rather than competitive sibling interactions [Eaves et al., 1997; Kuntsi et al., 2000; Saudino et al., 2005; Simonoff et al., 1998]. Some have suggested that the relative lack of objective criteria or norms for ADHD-related behaviors may lead caregivers to make greater rating contrasts for ADHD symptoms as compared to more overt disruptive behaviors [Eaves et al., 2000; Eaves et al., 1997; Kuntsi et al., 2000; Lahey et al., 2011; Saudino et al., 2005; Simonoff et al., 1998]. To facilitate multivariate analyses, we eliminated dominance from our childhood symptom models and retained contrast effects, meaning that we may have underestimated dominance. However, examination of the twin variances and univariate model comparisons reveals support for contrast effects and little evidence of dominance in caregiver-reported childhood behaviors, so this issue is unlikely to have impacted our results substantively.
Third, given the number of multivariate models reported here, we controlled sex rather than testing for sex differences. In support of this decision, several recent studies have found no sex differences in the phenotypic, genetic, and environmental covariation between ADHD and alcohol use or problems in early adolescence and adulthood [Capusan et al., 2015; Chang et al., 2012; Derks et al., 2014]. In contrast, at least two studies have failed to find phenotypic associations between ADHD symptoms and alcohol use disorders symptoms among female adolescents, although, as discussed above, it is possible that these null associations are attributable to developmental period rather than sex [Knopik et al., 2009; Moss and Lynch, 2001]. Nevertheless, this pattern of unclear findings suggests that further evaluation of sex differences in the association between ADHD symptoms and alcohol problems is warranted.
Finally, we measured alcohol problems with self-report and ADHD symptoms with caregiver-report, which has the potential to limit reliability or validity. Validating the AUDIT in the present sample, we found that it was meaningfully associated with clinical SUD diagnoses. Additionally, although the A-TAC is well validated and has been used in prior studies of ADHD, the scales were completed by caregivers rather than clinicians. They should be considered as DSM-IV proxies rather than clinical diagnoses, and their status remains uncertain under DSM-5. Further, the SDQ scales used in sensitivity analyses differed from their A-TAC counterparts. We stress, though, that the sensitivity analyses reproduced the pattern of findings for A-TAC hyperactive/impulsive symptoms in childhood with SDQ hyperactivity in adolescence, despite stronger phenotypic associations when hyperactivity and alcohol problems were both measured with self-report.
In summary, our results provide prospective, population-based, and robust evidence that the genetic association between childhood ADHD symptoms and adolescent alcohol problems is, to a large extent, an expression of a broader, genetically driven predisposition to childhood externalizing problems. They suggest that future behavioral genetic studies of ADHD and alcohol and other substance problems should incorporate other youth externalizing behaviors. Further, they support the notion that merely controlling for conduct problems does not adequately capture the causal relations underlying associations among conduct problems, ADHD, and alcohol problems [Molina and Pelham, 2014]. Rather, common genetic liability across childhood externalizing disorders should be considered as a potentially important mechanism in risk for alcohol problems. More broadly, our results highlight the value of multivariate behavioral genetic approaches to understanding the causes and consequences of ADHD and other childhood externalizing disorders.
Supplementary Material
Acknowledgments
The present study was supported by NIH grants MH102221 and TR001107 and the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM). The Child and Adolescent Twin Study in Sweden is supported by the Swedish Research Council for Health, Working Life and Welfare, the Swedish Research Council (Medicine and SIMSAM), funds under the ALF agreement, and the Söderström-Königska Foundation, and the research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no 602768.
Footnotes
Portions of the article were presented at the 2015 meeting of the Behavior Genetics Association in San Diego, CA and will subsequently be published in the annual meeting abstracts.
Conflicts of interest: Henrik Larsson has served as a speaker for Eli-Lilly and Shire and has received a research grant from Shire, all outside the submitted work.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fifth edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Ameringer KJ, Leventhal AM. Associations between attention deficit hyperactivity disorder symptom domains and DSM-IV lifetime substance dependence. Am J Addict. 2013;22(1):23–32. doi: 10.1111/j.1521-0391.2013.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckarsäter H, Lundström S, Kollberg L, Kerekes N, Palm C, Carlström E, Långström N, Magnusson PKE, Halldner L, Bölte S, Gillberg C, Gumpert C, Råstam M, Lichtenstein P. The Child and Adolescent Twin Study in Sweden (CATSS) Twin Res Hum Genet. 2011;14(06):495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for use in primary care. Geneva, Switzerland: World Health Organisation; 2001. [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, Mick E, Monuteaux MC, Faraone SV. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008;165(1):107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, Bucholz KK, Grucza R, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J, Porjesz B, Saccone NL, Schuckit M, Tischfield J, Wang JC, Foroud T, Rice JP, Edenberg HJ. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17(4):445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Broad bandwidth or high fidelity? Evidence from the structure of genetic and environmental effects on the facets of the Five Factor Model. Behav Genet. 2012;42(5):743–763. doi: 10.1007/s10519-012-9548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Larsson H, Lichtenstein P, Klump KL. Additional evidence against shared environmental contributions to attention-deficit/hyperactivity problems. Behav Genet. 2012;42(5):711–721. doi: 10.1007/s10519-012-9545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA for the Ambulatory Care Quality Improvement Project (ACQUIP) The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Capusan AJ, Bendtsen P, Marteinsdottir I, Kuja-Halkola R, Larsson H. Genetic and environmental contributions to the association between attention deficit hyperactivity disorder and alcohol dependence in adulthood: A large population-based twin study. Am J Med Genet Part B. 2015;9999B:1–9. doi: 10.1002/ajmg.b.32300. [DOI] [PubMed] [Google Scholar]
- Carey G. Sibling imitation and contrast effects. Behav Genet. 1986;16(3):319–341. doi: 10.1007/BF01071314. [DOI] [PubMed] [Google Scholar]
- Carragher N, Krueger RF, Eaton NR, Markon KE, Keyes KM, Blanco C, Saha TD, Hasin DS. ADHD and the externalizing spectrum: Direct comparison of categorical, continuous, and hybrid models of liability in a nationally representative sample. Soc Psychiatry Psychiatr Epidemiol. 2014;49(8):1307–1317. doi: 10.1007/s00127-013-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Asherson PJ, Larsson H. Developmental twin study of attention problems: High heritabilities throughout development. JAMA Psychiatry. 2013;70(3):311–318. doi: 10.1001/jamapsychiatry.2013.287. [DOI] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. 2012;40(3):425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Cruz JE, Emery RE, Turkheimer E. Peer network drinking predicts increased alcohol use from adolescence to early adulthood after controlling for genetic and shared environmental selection. Dev Psychol. 2012;48(5):1390–1402. doi: 10.1037/a0027515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alwis D, Agrawal A, Reiersen AM, Constantino JN, Henders A, Martin NG, Lynskey MT. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs. 2014a;75(2):211–221. doi: 10.15288/jsad.2014.75.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alwis D, Lynskey MT, Reiersen AM, Agrawal A. Attention-deficit/hyperactivity disorder subtypes and substance use and use disorders in NESARC. Addict Behav. 2014b;39(8):1278–1285. doi: 10.1016/j.addbeh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks EM, Vink JM, Willemsen G, van den Brink W, Boomsma DI. Genetic and environmental influences on the relationship between adult ADHD symptoms and self-reported problem drinking in 6024 Dutch twins. Psychol Med. 2014;44(12):2673–2683. doi: 10.1017/S0033291714000361. [DOI] [PubMed] [Google Scholar]
- Doyle SR, Donovan DM, Kivlahan DR. The factor structure of the Alcohol Use Disorders Identification Test (AUDIT) J Stud Alcohol Drugs. 2007;68(3):474–479. doi: 10.15288/jsad.2007.68.474. [DOI] [PubMed] [Google Scholar]
- Eaves L, Rutter M, Silberg J, Shillady L, Maes H, Pickles A. Genetic and environmental causes of covariation in interview assessments of disruptive behavior in child and adolescent twins. Behav Genet. 2000;30(4):321–334. doi: 10.1023/a:1026553518272. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff E, Pickles A, Rutter M, Reynolds CA, Heath AC, Truett KR, Neale MC, Erikson MT, Loeber R, Hewitt JK. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. J Child Psychol Psychiatry. 1997;38(8):965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Twin study of the relationship between adolescent attention-deficit/hyperactivity disorder and adult alcohol dependence. J Stud Alcohol Drugs. 2012;73(2):185–194. doi: 10.15288/jsad.2012.73.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Maes HH, Prescott CA, Kendler KS. Multiple mechanisms influencing the relationship between alcohol consumption and peer alcohol use. Alcohol Clin Exp Res. 2015;39(2):324–332. doi: 10.1111/acer.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: A research note. J Child Psychol Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R, Meltzer H, Bailey V. The Strengths and Difficulties Questionnaire: A pilot study on the validity of the self-report version. Eur Child Adolesc Psychiatry. 1998;7(3):125–130. doi: 10.1007/s007870050057. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson SL, Svanström Röjvall A, Rastam M, Gillberg C, Gillberg C, Anckarsäter H. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): Preliminary reliability and validity. Br J Psychiatry. 2005;187(3):262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Cummins TDR, Tong J, Johnson B, Lau R, Samarrai W, Bellgrove MA. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015;20(3):289–297. doi: 10.1038/mp.2014.183. [DOI] [PubMed] [Google Scholar]
- Kerekes N, Lundström S, Chang Z, Tajnia A, Jern P, Lichtenstein P, Nilsson T, Anckarsäter H. Oppositional defiant- and conduct disorder-like problems: Neurodevelopmental predictors and genetic background in boys and girls, in a nationwide twin study. PeerJ. 2014;2:e359. doi: 10.7717/peerj.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PAF, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology Biochemistry and Behavior. 2009;93(3):313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Kuntsi J, Gayán J, Stevenson J. Parents’ and teachers’ ratings of problem behaviours in children: genetic and contrast effects. Twin Res Hum Genet. 2000;3(04):251–258. doi: 10.1375/136905200320565229. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Hulle CAV, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry. 2011;68(2):181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Zald DH, Hakes JK, Krueger RF, Rathouz PJ. Patterns of heterotypic continuity associated with the cross-sectional correlational structure of prevalent mental disorders in adults. JAMA Psychiatry. 2014;71(9):989–996. doi: 10.1001/jamapsychiatry.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T, Anckarsäter H, Gillberg C, Ståhlberg O, Carlström E, Kadesjö B, Råstam M, Lichtenstein P, Gillberg C. The Autism - Tics, AD/HD and other Comorbidities inventory (A-TAC): Further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T, Lundstrom S, Nilsson T, Selinus E, Rastam M, Lichtenstein P, Gumpert C, Anckarsater H, Kerekes N. Predictive properties of the A-TAC inventory when screening for childhood-onset neurodevelopmental problems in a population-based sample. BMC Psychiatry. 2013;13(1):233. doi: 10.1186/1471-244X-13-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014;44(10):2223–2229. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson J-O. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2006;45(8):973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Lee C-T, Clark TT, Kollins SH, McClernon FJ, Fuemmeler BF. Attention deficit hyperactivity disorder symptoms and smoking trajectories: Race and gender differences. Drug Alcohol Depend. 2015;148(0):180–187. doi: 10.1016/j.drugalcdep.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31(3):328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T-K, Hewitt BG, Grant BF. Alcohol use disorders and mood disorders: A National Institute on Alcohol Abuse and Alcoholism perspective. Biol Psychiatry. 2004;56(10):718–720. doi: 10.1016/j.biopsych.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behav Genet. 1996;26(1):65–69. [Google Scholar]
- Maisto SA, Conigliaro J, McNeil M, Kraemer K, Kelley ME. An empirical investigation of the factor structure of the AUDIT. Psychol Assess. 2000;12(3):346–353. doi: 10.1037//1040-3590.12.3.346. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behav Genet. 1990;20(5):569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Attention-deficit/hyperactivity disorder and risk of substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annu Rev Clin Psychol. 2014;10(1):607–639. doi: 10.1146/annurev-clinpsy-032813-153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol Clin Exp Res. 2007;31(4):643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuteaux MC, Faraone SV, Hammerness P, Wilens TE, Fraire M, Biederman J. The familial association between cigarette smoking and ADHD: A study of clinically referred girls with and without ADHD, and their families. Nicotine Tob Res. 2008;10(10):1549–1558. doi: 10.1080/14622200802326137. [DOI] [PubMed] [Google Scholar]
- Moss HB, Lynch KG. Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug Alcohol Depend. 2001;64(1):75–83. doi: 10.1016/s0376-8716(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles: Muthén & Muthén; 1998–2012. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht. The Netherlands: Kluwer; 1992. [Google Scholar]
- Palmer RHC, Knopik VS, Rhee SH, Hopfer CJ, Corley RC, Young SE, Stallings MC, Hewitt JK. Prospective effects of adolescent indicators of behavioral disinhibition on DSM-IV alcohol, tobacco, and illicit drug dependence in young adulthood. Addict Behav. 2013;38(9):2415–2421. doi: 10.1016/j.addbeh.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C-Z, Wilsnack RW, Kristjanson AF, Benson P, Wilsnack SC. Gender differences in the factor structure of the Alcohol Use Disorders Identification Test in multinational general population surveys. Drug Alcohol Depend. 2012;124(1–2):50–56. doi: 10.1016/j.drugalcdep.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Anckarsäter H, Gillberg C, Lichtenstein P. Different neurodevelopmental symptoms have a common genetic etiology. J Child Psychol Psychiatry. 2013;54(12):1356–1365. doi: 10.1111/jcpp.12113. [DOI] [PubMed] [Google Scholar]
- Pettersson E, Sjölander A, Almqvist C, Anckarsäter H, D’Onofrio BM, Lichtenstein P, Larsson H. Birth weight as an independent predictor of ADHD symptoms: A within-twin pair analysis. J Child Psychol Psychiatry. 2015;56(4):453–459. doi: 10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault J-B, Viding E, Galéra C, Greven CU, Zheng Y, Plomin R, Rijsdijk F. Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry. 2015;72(7):651–658. doi: 10.1001/jamapsychiatry.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behav Genet. 2004;34(1):17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): A review of recent research. Alcohol Clin Exp Res. 2002;26(2):272–279. [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60(12):1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Lahey BB, Waldman ID. Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child Development Perspectives. 2015;9(1):26–31. doi: 10.1111/cdep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld MJH, Hudziak JJ, Bartels M, van Beijsterveldt CEM, Boomsma DI. Heritability of attention problems in children: I. Cross-sectional results from a study of twins, age 3–12 years. Am J Med Genet Part B. 2003a;117B(1):102–113. doi: 10.1002/ajmg.b.10024. [DOI] [PubMed] [Google Scholar]
- Rietveld MJH, Posthuma D, Dolan CV, Boomsma DI. ADHD: Sibling interaction or dominance: An evaluation of statistical power. Behav Genet. 2003b;33(3):247–255. doi: 10.1023/a:1023490307170. [DOI] [PubMed] [Google Scholar]
- Rist F, Glöckner-Rist A, Demmel R. The Alcohol Use Disorders Identification Test revisited: Establishing its structure using nonlinear factor analysis and identifying subgroups of respondents using latent class factor analysis. Drug Alcohol Depend. 2009;100(1–2):71–82. doi: 10.1016/j.drugalcdep.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin Exp Res. 2004;28(10):1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Edwards A, Evans D, Macleod J, Hickman M, Lewis G, Kendler K, Loukola A, Korhonen T, Latvala A, Rose R, Kaprio J, Dick D. Polygenic scores predict alcohol problems in an independent sample and show moderation by the environment. Genes. 2014;5(2):330–346. doi: 10.3390/genes5020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Ronald A, Plomin R. The etiology of behavior problems in 7-year-old twins: Substantial genetic influence and negligible shared environmental influence for parent ratings and ratings by same and different teachers. J Abnorm Child Psychol. 2005;33(1):113–130. doi: 10.1007/s10802-005-0939-7. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Jr, Molina BSG, Coxe S, Kipp H, Gnagy EM, Meinzer M, Ross JM, Lahey BB. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol. 2014;123(2):362–374. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Hervas A, Silberg JL, Rutter M, Eaves L. Genetic influences on childhood hyperactivity: Contrast effects imply parental rating bias, not sibling interaction. Psychol Med. 1998;28(04):825–837. doi: 10.1017/s0033291798006886. [DOI] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H. Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry. 2015;77(10):880–886. doi: 10.1016/j.biopsych.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Attention-deficit/hyperactivity disorder and risk for drug use disorder: A population-based follow-up and co-relative study. Psychol Med. 2015;45(05):977–983. doi: 10.1017/S0033291714001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Harden KP. Behavior genetic research methods: Testing quasi-causal hypotheses using multivariate twin data. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. 2nd. Cambridge: Cambridge University Press; 2014. pp. 159–187. [Google Scholar]
- Vergés A, Haeny AM, Jackson KM, Bucholz KK, Grant JD, Trull TJ, Wood PK, Sher KJ. Refining the notion of maturing out: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Public Health. 2013;103(12):e67–e73. doi: 10.2105/AJPH.2013.301358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Luczak SE, Orlowska D, Pandika D. Differential metabolism of alcohol as an intermediate phenotype of risk for alcohol use disorders: Alcohol and aldehyde dehydrogenase variants. In: MacKillop J, Munafò MR, editors. Genetic infuences on addiction: An intermediate phenotype approach. Cambridge: MIT Press; 2013. pp. 41–63. [Google Scholar]
- Young-Wolff KC, Enoch M-A, Prescott CA. The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clin Psychol Rev. 2011;31(5):800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96(5):684–695. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.