Abstract
A 64-kDa hemagglutinin from a Phaseolus vulgaris cultivar, the northeast red bean, was purified by a protocol composed of three chromatographic steps involving affinity chromatography on Affi-gel blue gel, cation exchange chromatography on SP-Sepharose and FPLC-gel filtration on Superdex 75. The purified hemagglutinin appeared as a single 32-kDa band in SDS-PAGE indicating its dimeric nature. The N-terminal amino acid sequence of the hemagglutinin resembled the sequences of lectins and hemagglutinins from a number of Phaseolus species. The hemagglutinin manifested moderate thermostability and pH stability. It retained full activity up to 65 °C and in the pH range 2 – 12. It did not interact with simple sugars such as glucose, mannose and galactose. The hemagglutinin exerted immunostimulatory effects by upregulating the expression of cytokines like interferon-γ and tumor necrosis factor-α. It also exhibited antiproliferative activity on a number of tumor cells including MCF7 (breast cancer), HepG2 (liver cancer), CNE1 and CNE2 (nasopharyngeal cancer) cells, with stronger activity toward MCF7 and CNE1 cells. The hemagglutinin induced phophatidylserine externalization, mitochondrial depolarization and DNA condensation in MCF7 cells, indicating initiation of apoptosis. However, at high hemagglutinin concentrations, severe damage to the MCF7 cells was detected.
Keywords: Bean, hemagglutinin, isolation, immunomodulatory, anti-proliferative, apoptosis-inducing
INTRODUCTION
Lectins are produced by a variety of organisms. Humans [1, 2], animals [3, 4], plants [5, 6], fungi [7, 8] and bacteria [9, 10] produce their own lectins. Different lectins may display distinctly different molecular masses, number of subunits, as well as dissimilar biological activities. Some of the lectins exhibit immunomodulatory [11], antitumor [12], antifungal [13], antibacterial [14] and antiviral [15] activities, providing a certain degree of defense against pathogens.
Phaseolus vulgaris is a species of edible common beans rich in proteins. The species is cultivated worldwide, contributing to the development of many different cultivars. Most of the cultivars of P. vulgaris produce lectins or lectin-like hemagglutinins that belong to the group of phytohemagglutinins (PHAs) [16]. They are found in abundance in the beans, acting as storage proteins and protecting the beans from invasion of pathogens. They have similar molecular sizes of around 60 kDa [16], but PHAs produced by different cultivars of P. vulgaris may exhibit different biological activities. Some of them exhibit immunomodulatory effects. For example, lectins from red kidney bean and great northern bean evoke a mitogenic response [17,18], and lectin from blue tiger king bean upregulates expression of cytokines in murine splenocytes [19]. On the other hand, some PHAs exert antitumor effects. The PNA lectin reduces growth in Lovo and Sw387 colon cancer cell lines [20]. Hokkaido red bean lectin shows strong antiproliferative activity toward HepG2 hepatoma and MCF7 breast cancer cells [21]. Extralong autumn purple bean lectin also inhibits proliferation of HepG2 cells [22].
In this study, we have detected the presence of a hemagglutinin in the northeast red bean, a cultivar of P. vulgaris that has not been studied before. The hemagglutinin was isolated and tested for biological activities. It was found to exhibit cytokine-inducing activity toward splenocytes and antiproliferative activity in some tumor cells. These activities suggested the potential for the northeast red bean hemagglutinin to be applied for cancer treatment.
MATERIALS AND METHODS
Purification of Northeast Red Bean Hemagglutinin
Northeast red beans (Phaseolus vulgaris cv “northeast red bean) were a product of Northeast China. One hundred grams of the beans were soaked in 600 ml 10 mM Tris-HCl (pH 7.6) overnight, and homogenized using a Waring blender. The slurry was centrifuged at 30000 g, 4 °C for 25 min and the procedure was repeated. The supernatant was collected as the crude extract of the beans and the volume was adjusted to 1 L by adding 10 mM Tris-HCl, pH 7.6. The crude extract was loaded onto an 18 cm × 5 cm Affi-gel blue gel (Bio-Rad) column that had been pre-equilibrated with 10 mM Tris-HCl buffer (pH 7.6). After removal of unadsorbed materials, adsorbed proteins were eluted with 1M NaCl in 10 mM Tris-HCl (pH 7.6) and then dialyzed extensively against double distilled water. Then, the fraction was adjusted to 10 mM Tris-HCl (pH 7.6) by adding 2M Tris-HCl buffer (pH 7.6). The fraction was loaded onto an 18 cm × 5 cm SP-Sepharose (GE Healthcare) column pre-equilibrated with 10 mM Tris-HCl buffer, and the fraction containing unadsorbed proteins was collected. The fraction was dialyzed extensively against double distilled water and lyophilized into a powder form. Then, the powder was resuspended with 20 mM NaCl in 10 mM Tris-HCl buffer (pH 7.6) at a concentration of 5 mg/ml, and was subjected to FPLC-gel filtration on a Superdex 75 10/300 GL column (GE Healthcare) pre-equilibrated with the same buffer, using an AKTA Purifier (GE Healthcare). The purified hemagglutinin was collected as the major peak eluted from the Superdex 75 column.
Assay of Hemagglutinating Activity
Two-fold dilution of 50 μl of the protein sample was performed in a 96-well U plate using phosphate-buffered saline (PBS). Then, 50 μl of a 2% rabbit red blood cell suspension was added to each of the wells. The plate was incubated at room temperature until the red blood cells in the blank (without protein sample) had sedimented to the bottom of the well and appeared as a single red spot. Hemagglutinating activity was indicated by the presence of agglutinated red blood cells in the well. One hemagglutination unit is the reciprocal of the highest dilution of the protein sample exhibiting hemagglutination. Specific activity is the number of hemagglutination units per mg protein [23].
Molecular Mass Determination
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a 15% separating gel and a 5% stacking gel at a constant voltage of 120 V. Then the gel was stained with Commassie brilliant blue for 1 hour, and destained with 10% acetic acid overnight [24]. FPLC-gel filtration was performed using a Superdex 75 10/300 GL column (GE Healthcare) previously calibrated with molecular-mass standards including phosphorylase b (97 kDa), bovine serum albumin (67 kDa), carhonic anhydrase (30 kDa), soybean trypsin inhibitor (20 kDa) and α- lactoalbumin (14.4 kDa) [25].
Effects of pH and Temperature on Hemagglutinating Activity of Northeast Red Bean Hemagglutinin
Effect of temperature on northeast red bean hemagglutinin was determined by exposing the hemagglutinin sample to different temperatures (20 °C to 100 °C) for 30 minutes. The hemagglutinin sample was immediately cooled on ice to terminate the incubation. The assay of hemagglutinating activity was performed as described above. Percentage of hemagglutinating activity remaining at a particular temperature was calculated by dividing the activity of the sample determined at that temperature by the activity observed at room temperature × 100% [26].
Effect of pH on northeast red bean hemagglutinin was tested by treating the hemagglutinin sample with solutions at different pH values (pH 0 – 1: HCl; pH 2 – 5: NH4OAc; pH 6 – 10: Tris-HCl; pH 11 – 12: NaHCO3, and pH 13 – 14: NaOH). Mixtures of equal volumes of hemagglutinin sample and pH solution were incubated at room temperature for 30 minutes, followed by neutralization of the mixture. Assay of hemagglutinating activity was performed as described above. Percentage of hemagglutinating activity remaining was calculated by dividing the hemagglutinating activity of the sample by the maximal hemagglutinating activity × 100% [26].
Effect of Carbohydrates on Hemagglutinating Activity of Northeast Red Bean Hemagglutinin
Effect of carbohydrates on northeast red bean hemagglutinin was tested by dissolving the hemagglutinin powder in different carbohydrate solutions (all at 500 mM concentration) in PBS. Assay of hemagglutinating activity was performed as described above, but carbohydrate solutions (500 mM) instead of PBS were used for serial dilution of the hemagglutinin. Percentage of hemagglutinating activity remaining was calculated by dividing the activity of the sample by the maximal hemagglutinating activity × 100% [27].
N-terminal Sequencing
Analysis of N-terminal amino acid sequence was performed by automated Edman degradation, using a Hewlett Packard 1000A protein sequencer equipped with an HPLC system [28].
Assay of Isolated Hemagglutinin for Cytokine Inducing Activity
Splenocytes from BALB/c mice (body weight: 20 to 25 g, age: 4 to 6 weeks) were isolated and treated with northeast red bean hemagglutinin in 90 mm culture dishes for 4 hours in a cell culture incubator at 37 °C under an atmosphere of 5% CO2. Total RNA from the splenocytes was extracted by phenol-chloroform using TRIZOL® reagent (Invitrogen). Isopropanol was used to precipitate the RNA. The pellet was washed with 75% ethanol, and resuspended in diethyl pyrocarbonate (DEPC)-treated water. The extracted RNA was converted into cDNA by reverse transcription (RT), using the GeneAmp® RNA PCR kit from Applied Biosystems Company [29].
Polymerase chain reaction (PCR) of the RT product was performed using the AmpliTag® Gold kit from Applied Biosystems Company. The sequences of the upper and lower primers are listed in Table 3. PCR was performed for 25 cycles. Agarose gel electrophoresis of the PCR products was performed using 1.5% gel containing 0.5 μg/ml ethidium bromide, at 120 V for 30 minutes [29].
Table 3.
Sequences of the upper and lower primers used in PCR
| Cytokine | Primer | Sequence (5′ to 3′) | Size (b. p.) |
|---|---|---|---|
| IFN-γ | Upper | AGGAACTGGCAAAGGATGGTG | 353 |
| Lower | GTGCTGGCAGAATTATTCTTATTG | ||
| TNF-α | Upper | TCCCCAAAGGGATGAGAAGTTC | 411 |
| Lower | TCATACCAGGGTTTGAGCTCAG | ||
| GAPDH | Upper | ACCACAGTCCATGCCATCAC | 452 |
| Lower | TCCACCACCCTGTTGCTGTA |
Assay of Isolated Hemagglutinin for Anti-proliferative Activity
Human breast cancer (MCF7), hepatoma (HepG2) and nasopharyngeal carcinoma (CNE1 and CNE2) cells from American Type Culture Collection were adjusted to a cell density of 5 × 104 cells/ml in RPMI medium. Cells (100 μl) were seeded in a 96-well plate, followed by treatment with 100 μl of northeast red bean hemagglutinin for 24 hours. After incubation, the medium was discarded, and the wells were washed with phosphate buffered saline (PBS), followed by addition of 25 μl of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (5 mg/ml in PBS) and incubation for 4 hours. The plates were centrifuged at 2500 rpm for 5 minutes, and the supernatant was carefully removed. Then, 150 μl of dimethyl sulfoxide (DMSO) was added into the wells to dissolve the MTT (formazan). OD 590 nm was measured using a microplate reader within 10 minutes. Percentage inhibition of the cells by hemagglutinin was calculated by: [(OD 590 nm of the control – OD 590 nm of a culture exposed to a particular hemagglutinin concentration)/OD 590 nm of the control] × 100% [30].
Flow Cytometry Analysis
For annexin V-FITC and propidium iodide (PI) staining, 5 × 105 MCF7 cells were treated with northeast red bean hemagglutinin in 6-well culture plates for 24 hours. The cells were trypsinized and washed with PBS. After centrifugation at 2000 g, the cell pellets were resuspended in 250 μl binding buffer (0.01 M HEPES, pH 7.4, containing 140 mM NaCl and 25 mM CaCl2) containing 2.5 μl Annexin V-FITC solution (BD Phamingen, CA, USA) and 0.5 μl PI (6 mg/ml) (Sigma), and kept in the dark for 20 min. The cells were analyzed using a FACSort flow cytometer (Becton Dickinson, Cowley, UK). The data were analyzed using the program WinMDI (Version 2.8, Joseph Trotter, La Jolla, CA, USA) [31].
For JC-1 staining, 5 × 105 MCF7 cells were treated with northeast red bean hemagglutinin in 6-well culture plates for 24 hours. The cells were trypsinized and washed with PBS. After centrifugation at 2000 g, the cell pellets were resuspended in 500 μl plain RPMI medium containing 2.5 μg/ml JC-1 dye, and kept in the dark in an incubator at 37 °C for 15 min. The cells were analyzed using a FACSort flow cytometer [32].
For cell cycle analysis, 2 × 106 MCF7 cells were treated with northeast red bean hemagglutinin in 90 mm culture dishes for 24 hours. The cells were trypsinized and washed with PBS. The cells were fixed in 1 ml of 75% ethanol in −20 °C for 2 hours. The cells were washed with PBS three times. After centrifugation, the cell pellets were resuspended in 250 μl PBS containing 5 μl propidium iodide (6 mg/ml) (Sigma). The cells were kept in the dark for 20 min. The cells were analyzed using a FACSort flow cytometer [33].
Hoechst 33342 Staining
MCF7 cells (2 × 105) were treated with northeast red bean hemagglutinin in a 12-well culture plate for 24 hours. The cells were washed with PBS three times, then 1 μM Hoechst 33342 in PBS was added and the mixture was kept in the dark for 10 min. The cells were observed under a NIKON TE2000 microscope (Nikon, Japan) [34].
RESULTS
Purification of northeast red bean hemagglutinin (NRBH) involved three chromatographic steps. In the first step, the hemagglutinin was adsorbed onto Affi-gel blue gel and was eluted with 1M NaCl. The hemagglutinin in the adsorbed fraction was thus purified about 6 folds (Table 1), and appeared as the major protein band in SDS-PAGE (Fig. 2). In the second step, the hemagglutinin did not adsorb onto the cation exchanger SP-Sepharose column, while some of the impurities were adsorbed and hence removed. In the last step, the hemagglutinin was eluted from the FPLC-gel filtration Superdex 75 10/300 GL column according to its molecular size. The major peak contained purified NRBH which appeared as a single 32-kDa band in SDS-PAGE (Fig. 2). Based on the calibration curve for the column, the molecular weight of the hemagglutinin was 64 kDa. Hence NRBH was a 64-kDa protein with two 32-kDa subunits.
Table 1.
Table Summarizing Purification of Hemagglutinin from 100 Grams of Dried Northeast Red Beans
| Step of purification | Yield (mg) | Specific hemagglutinating activity (units/mg) | Total hemagglutinating activity (106 units) | Recovery of hemagglutinating activity (%) | Fold of purification |
|---|---|---|---|---|---|
| Crude extract | 17600 | 1164 | 20.48 | 100 | 1 |
| Affi-gel blue gel | 2040 | 7027 | 14.34 | 70.0 | 6.04 |
| SP-sepharose | 553 | 17035 | 9.41 | 46.0 | 14.64 |
| Superdex 75 | 292 | 23805 | 6.95 | 33.9 | 20.46 |
Figure 2.
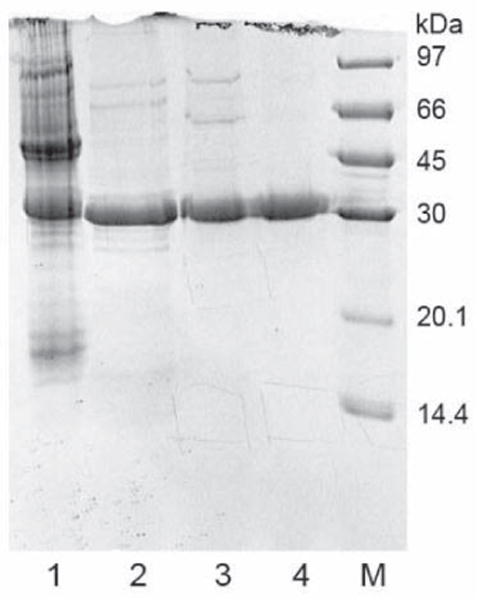
SDS-PAGE for purification of northeast red bean hemagglutinin. Lane 1: crude extract of northeast red beans. Lane 2: bound fraction from Affi-gel blue gel. Lane 3: unbound fraction from SP-Sepharose. Lane 4: Purified lectin from Superdex 75. M: protein markers from GE Healthcare including phosphorylase b (97 kDa), bovine serum albumin (67 kDa), carhonic anhydrase (30 kDa), soybean trypsin inhibitor (20 kDa), and α- lactoalbumin (14.4 kDa).
NRBH exhibited moderate temperature stability. Its hemagglutinating activity was preserved at temperatures up to 65 °C (Table 2A). The activity was halved at 70 °C, further reduced at 75 °C and 80 °C, and disappeared at and above 90°C. NRBH also had moderate pH stability. Full hemagglutinating activity was retained in the pH range 2 – 12 (Table 2B). The activity was reduced by 50% at pH 0 and 13, while it was totally abolished at pH 14. The hemagglutinating activity was not attenuated after addition of a variety of carbohydrates including glucose, mannose, galactose, lactose, glucosamine, glucuronic acid, galactonic acid, xylose, xylitol, raffinose, mannitol and arabinose (Table 2C), indicating that NRBH did not bind with any of them.
Table 2.
Effect of (A) Temperature, (B) pH and (C) Carbohydrates on Hemagglutinating Activity of Northeast Red Bean Hemagglutinin
| (A) | |
|---|---|
| Temperature (°C) | % hemagglutinating Activity Remaining |
| 20 | 100 |
| 30 | 100 |
| 40 | 100 |
| 50 | 100 |
| 60 | 100 |
| 65 | 100 |
| 70 | 50 |
| 75 | 25 |
| 80 | 12.5 |
| 90 | 0 |
| 100 | 0 |
| (B) | |
| pH | % hemagglutinating Activity Remaining |
| 0 | 50 |
| 1 | 100 |
| 2 | 100 |
| 3 | 100 |
| 4 | 100 |
| 5 | 100 |
| 6 | 100 |
| 7 | 100 |
| 8 | 100 |
| 9 | 100 |
| 10 | 100 |
| 11 | 100 |
| 12 | 100 |
| 13 | 50 |
| 14 | 0 |
| (C) | |
| Carbohydrate (250 mM) | Hemagglutinating Activity Remaining (%) |
| Glucose | 100 |
| Mannose | 100 |
| Galactose | 100 |
| Lactose | 100 |
| Glucosamine | 100 |
| Glucuronic acid | 100 |
| Galactonic acid | 100 |
| Xylose | 100 |
| Xylitol | 100 |
| Raffinose | 100 |
| Mannitol | 100 |
| Arabinose | 100 |
The N-terminal amino sequence of NRBH was ASQTSFSFQRFNE (Table 3). When the sequence was searched for similarity to other proteins using protein BLAST, a high degree of homology to a number of P. vulgaris lectins and phytohemagglutinins was found (Table 3).
NRBH exhibited immunomodulatory activity. Treatment of murine splenocytes with NRBH for 4 hours was shown to up-regulate the expression of the cytokines IFN-γ and TNF-α. Treatment of murine splenocytes with 2.5 μM NRBH resulted in a higher cytokine inducing effect than treatment with 0.5 μM NRBH (Fig. 3).
Figure 3.
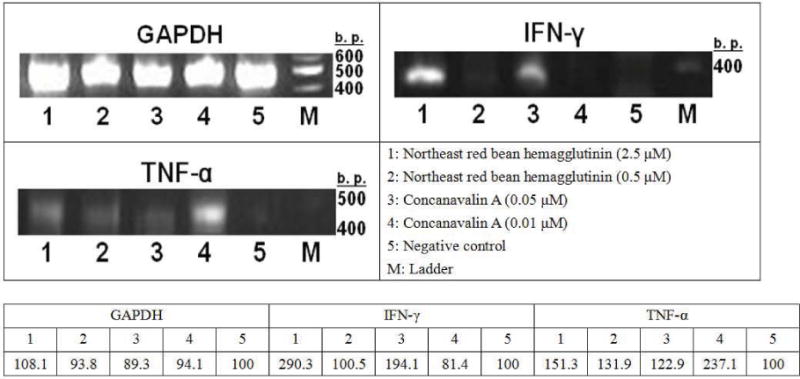
Induction of gene expression of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) of mouse splenocytes by northeast red bean hemagglutinin and concanavalin A. (GAPDH = glyceraldehydes-3-phosphate dehydrogenase, IFN-γ = interferon-γ, and TNF-α = tumor necrosis factor-α)
NRBH exhibited antiproliferative activity on some tumor cell lines. After treatment for 24-hour, NRBH strongly inhibited the growth of CNE1 cells with an IC50 of 1.63 μM, significantly suppressed MCF7 cell growth with an IC50 of 11.66 μM, and exerted anti-proliferative effects on CNE2 cells with an IC50 of 35.00 μM. However, it only slightly inhibited growth of HepG2 cells, with an IC50 beyond 50 μM (Fig. 4).
Figure 4.
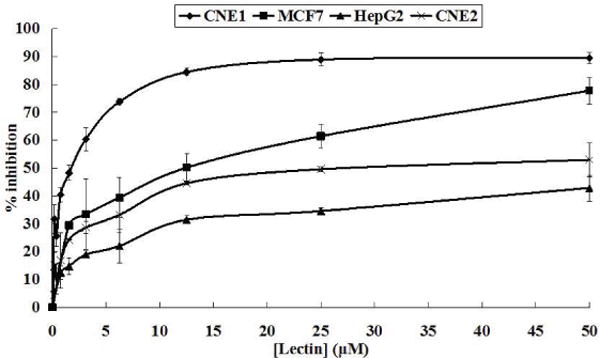
Results of MTT assay on inhibition of CNE1, MCF7, HepG2 and CNE2 cells after treatment with northeast red bean hemagglutinin for 24 hrs. Results represent mean±SD (n = 3).
To further study the anti-proliferative activity of NRBH, flow cytometry analysis was applied. In the study on phosphatidylserine (PS) externalization, upon Annexin V-FITC and propidium iodide (PI) staining, the proportion of MCF7 cells in the lower left region (low fluorescence intensity due to Annexin V-FITC and PI) decreased, while that in the lower right region (low intensity due to PI but high intensity from Annexin-FITC) was increased as NRBH concentration increased (Fig. 5A). Such shifting indicated PS externalization occurred in NRBH-treated MCF7 cells, which is a sign of early apoptosis. At higher NRBH concentrations, the proportion of MCF7 cells located in the upper right region (high intensity from Annexin V-FITC and PI) also increased (Fig. 5A), showing that an increasing proportion of MCF7 cells had entered the phase of late apoptosis. In the study on mitochondrial transmembrane potential, upon JC-1 staining, the MCF7 cells gradually shifted from the upper left region toward the lower right region as NRBH concentration increased (Fig. 5B). The shifting showed that mitochondrial depolarization took place in NRBH-treated MCF7 cells, and that the cells were undergoing cell death. In the cell cycle analysis, treatment with NRBH did not induce cell cycle arrest in MCF7 cells (Fig. 6). However, a rise in NRBH concentration led to an increase in the proportion of MCF7 cells with reduced DNA content (cells with weaker PI fluorescence intensity than those in G0/G1 phase) (Fig. 6). These cells had damaged or degraded DNA, and were undergoing cell death.
Figure 5.
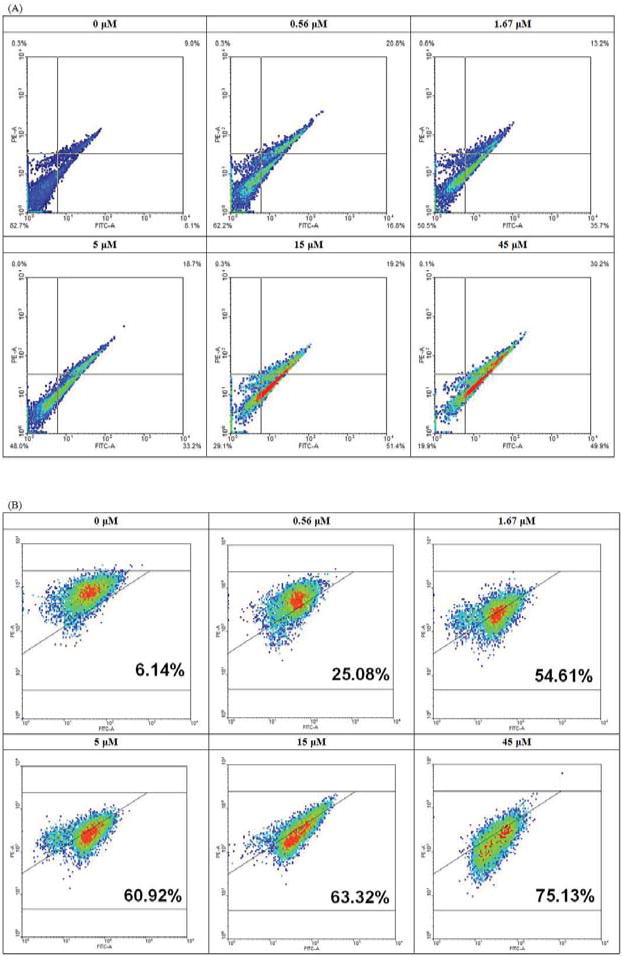
Flow cytometry analysis of northeast red bean hemagglutinin-treated MCF7 cells upon (A) Annexin V-PI staining and (B) JC-1 staining.
Figure 6.
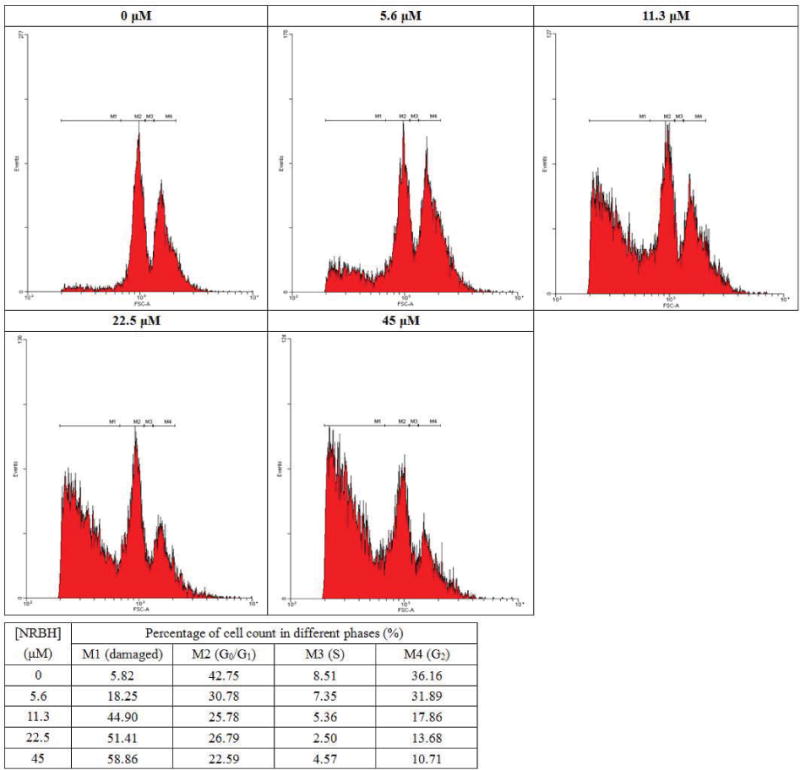
Cell cycle analysis of MCF7 cells treated with different concentrations of northeast red bean hemagglutinin.
When the NRBH-treated MCF7 cells were examined under a microscope, obvious morphological changes could be observed (Fig. 7). Upon Hoechst 33342 staining, some of the MCF7 cells showed DNA fragmentation and formation of apoptotic bodies (marked with white arrows in Fig. 7) after NRBH treatment, indicating induction of apoptosis by NRBH. At 15 μM NRBH, MCF7 cells with severe damage (indicated by grey arrows in Fig. 7) were observed. A higher proportion of damaged cells was detected after treatment with a high NRBH concentration (45 μM).
Figure 7.
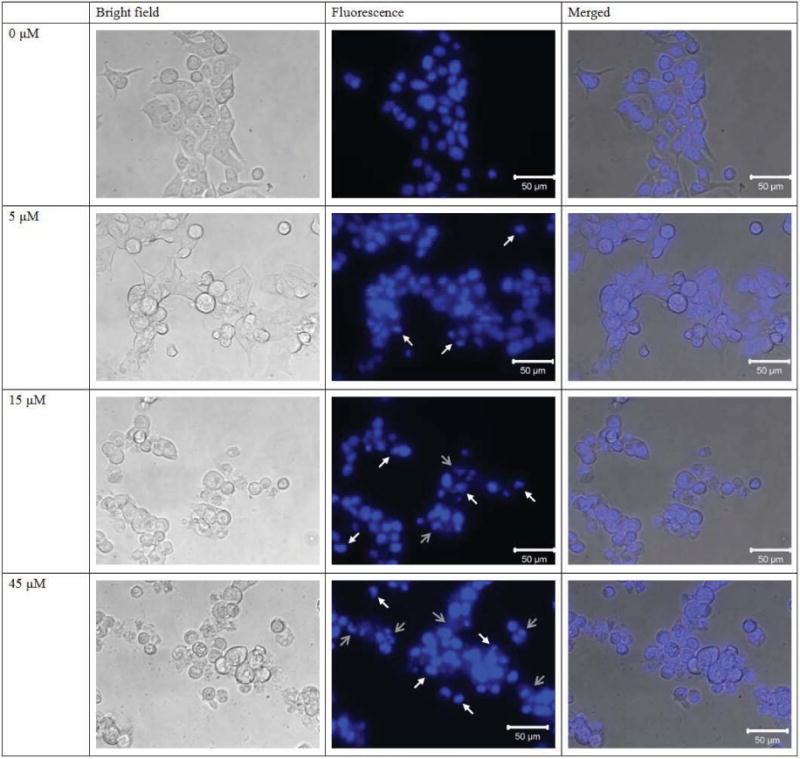
Hoechst 33258 staining of MCF7 cells treated with northeast red bean hemagglutinin.
DISCUSSION
For purification of northeast red bean hemagglutinin (NRBH), a three-step chromatographic protocol was used. This entailed affinity chromatography on Affi-gel blue gel, ion exchange chromatography on SP-Sepharose and FPLC-gel filtration on Superdex 75. Similar protocols were widely used in purification of other P. vulgaris lectins and hemagglutinins, such as lectins from French bean Indian cultivar and cultivar no. 12 and Anasazi beans [35–37], as well as those from other plant species such as the lectins from graviola seeds, white Bauhinia seeds and Luetzelburgia auriculata cotyledons [38–40]. The protocol used in present study allowed fast and simple purification of the target protein. From 100 grams of northeast red bean, about 292 mg NRBH was yielded, with about 33.9% recovery [Percentage recovery = (Total hemagglutinating activity of purified NRBH/Total hemagglutinating activity in crude extract) × 100%] (Table 1). The yield was satisfactory when compared to those obtained for some other P. vulgaris lectins such as French bean cultivar no. 12 (4.8 mg lectin/100 g beans, 18.1% recovery) [36], Anasazi beans (26 mg/100 g beans, 14% recovery) [37], and Hokkaido red bean (24 mg/100 g beans, 17% recovery) [21].
The N-terminal amino acid sequence of NRBH (ASQTSFSFQRFNE) closely resembled a number of P. vulgaris lectins and phytohemagglutinins (PHAs), as well as lectins from some other Phaseolus species, listed in Table 3. This suggested that NRBH is a PHA. PHA is found in abundance in most of the cultivars of P. vulgaris [41]. They may have the ability to agglutinate erythocytes (PHE-Es) and leukocytes (PHA-Ls). Most of them are carbohydrate-binding proteins, and are classified as lectins [42]. However, some of the PHAs like NRBH do not bind with carbohydrates are named as hemagglutinins. Similar to PHAs in other P. vulgaris cultivars, NRBH was found to be abundant in the northeast red beans. It was one of the major storage proteins in the beans as judged from the SDS-PAGE profile of the crude extract of the beans.
PHAs from P. vulgaris have different heat and pH stabilities. For example, lectins from Indian cultivar of French bean and Anasazi bean had pronounced thermostability (up to 90 °C and 80 °C, respectively) and pH stability (1 – 13 and 1 – 14, respectively) [35, 37], while lectin from French bean cultivar no. 35 was less heat stable (up to 50 °C) and pH stable (6 – 8) [43]. NRBH exhibited moderate heat stability (up to 65 °C) and pH stability (2 – 12) among different P. vulgaris PHAs.
The hemagglutinating activity of a number of PHAs is not inhibited by simple sugars. NRBH, the PHAs from cultivar 12, 35 and Indian cultivar of French beans [35, 36, 43], and Hokkaido red beans are other examples [21]. On the other hand, the sugar binding PHAs from different P. vulgaris cultivars exhibited a variety of carbohydrate-binding specificities. For example, lectin from cultivar no. 1 of French beans was glucuronic acid-specific [44], and lectins from extralong autumn purple beans and pinto beans were galactose-specific [22]. On the other hand, the hemagglutinating activity of some of them was inhibited by polysaccharides. For example, lectin from blue tiger king bean was polygalacturonic acid-binding [19], and lectin from red kidney bean was inhibited by a number of glycoproteins [45].
Some PHAs from P. vulgaris possessed anti-fungal activities. Lectins from red kidney bean lectin and French bean cultivar no. 35 inhibited the growth of a number of fungal species such as Fusarium oxysporum and Rhizoctonia solani [43, 45], while most of the PHAs could not affect fungal growth. For protection against infectious fungi, some of the P. vulgaris cultivars (e.g. French bean cultivar no. 12 [36]) produced defensins or defensin-like peptides as well. The presence of a defensin-like antifungal peptide was also detected in northeast red beans, but since the present study was focused on NRBH, details on the antifungal peptide are not covered here. Although NRBH did not exhibit antifungal activity, the defensin-like peptide in northeast red beans probably plays a role in resistance against pathogenic fungi.
Some P. vulgaris PHAs exhibit immunomodulatory activity while others do not. Lectins from French bean cultivar no. 35 and Indian cultivar did not affect the mitogenesis of murine splenocytes [35, 43], while lectins from French bean cultivar no. 1 and 12 enhanced proliferation in those cells [36, 44]. Lectin from blue tiger king beans could upregulate the expression of a number of cytokines in the splenocytes such as interleukin-2 (IL-2) and interferon-γ (IFN-γ) [19]. Similarly, NRBH evoked immunomodulatory effects by inducing expression of the pro-inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in murine splenocytes. IFN-γ is produced by T-helper (TH1) cells, cytotoxic T (TC) cells and NK cells. It is involved in macrophage activation and increase in macrophage antigen presentation and MHC expression in normal cells, resulting in stimulation of TH1 cell differentiation and cellular immunity, and suppression of TH2 differentiation and humoral response [46]. TNF-α is produced mainly by activated macrophages. It is involved in induction of fever, stimulation of acute phase response and activation of endothelial adhesion [47, 48].
Cellular immunity could be enhanced through upregulation of expression of several cytokines by treatment with 2.5 μM NRBH on splenocytes. On the other hand, NRBH also acted on tumor cells. It significantly inhibited the growth of nasopharyngeal cancer (CNE1 and CNE2) cells and breast cancer (MCF7) cells with IC50 values of 1.63 μM, 35.00 μM and 11.66 μM, respectively. Interestingly, treatment with 2.5 μM NRBH could induce cytokine expression in splenocytes, strongly inhibited proliferation of CNE1 cells, but did not affect the growth of hepatoma (HepG2) cells. It shows that NRBH can act differently on different types of cells.
NRBH exhibited anti-proliferative activity toward a greater variety of tumor cells than some other lectins. Lectin from blue tiger king bean was specific toward HepG2 cells [19] and lectin from French bean cultivar no. 35 was specific toward MCF7 cells [30]. Flow cytometric study on phosphatidylserine (PS) externalization, mitochondrial depolarization and cell cycle analysis was used for investigation of the anti-proliferative effect of NRBH. The shifting pattern of the MCF7 cells after Annexin V-PI staining indicated PS externalization in the cells, which is a feature of early apoptosis. The shifting pattern after JC-1 staining also showed mitochondrial depolarization which is often involved in apoptosis.
Results of cell cycle analysis disclosed that MCF7 cells did not experience cell cycle arrest in any phase after NRBH treatment. Many other apoptosis-inducing lectins trigger cell cycle arrest in their target cells [34]. NRBH treatment seemed not to affect the cell cycle of MCF7 cells. However, increase in NRBH concentration (e.g. >11.3 μM) caused severe DNA damage in the cells, as shown by the rise in the proportion of cells at the M1 region in (Fig. 6). Upon Hoechst 33342 staining, the untreated MCF7 cells appeared to be healthy, well adhered, and possessed a rigid, circular nucleus located at the center of the cell. In response to NRBH treatment, some of the MCF7 cells showed DNA condensation and formation of apoptotic bodies. However, following exposure to escalated concentrations of NRBH, a large proportion of MCF7 cells with reduced DNA content was recorded in cell cycle analysis. When observed under a fluorescence microscope after Hoechst 33342 staining, the cells possessed an irregular shape, and a broken cell membrane, with leakage of genetic materials. The cells appeared to be dying through necrosis. Based on the above observations, treatment with low concentrations of NRBH promoted apoptosis in the tumor cells. High concentrations of NRBH could generate more pro-apoptotic cells, but would also bring about necrosis in some of the cells.
Different P. vulgaris phytohemaglutinins performed differently toward tumor cells. The anti-proliferative activity of NRBH on tumor cells was quite different from other PHAs. Some Phaseolus hemagglutinins such as French bean lectins from cultivar no. 1 and an Indian cultivar did not exhibit any inhibitory effects on tumor cells [35, 44], and some others, for example, the lectins from blue tiger king bean and French bean cultivar no. 35, induced apoptosis in HepG2 and MCF7 cells, respectively [19, 30]. PHAs usually do not cause necrosis in cells unless at extremely high concentrations. NRBH induced apoptosis at lower concentrations, similar to those PHAs with potent anti-proliferative activity. However, NRBH could also trigger necrosis in MCF7 cells at higher concentrations (>15 μM). Thus NRBH is distinct from other Phaseolus hemagglutinins.
There were reports on the usage of lectins (e.g. mistletoe lectin [49, 50]) and phytohemagglutinins (e.g. PHA-L4 [51]) in treatment of cancers based on their immunostimulatory and antitumor activities. At some concentrations (e.g. 2.5 μM), NRBH exert both cytokine- inducing effects on splenocytes and anti-proliferative activity on several tumor cells. It is known that tumor necrosis factor (TNF) activates the NF-κB and AP-1 pathways crucial for expression of pro-inflammatory cytokines, and also the MLKL cascade essential to the generation of reactive oxygen species in response to TNF. TNF induces apoptosis and necrosis of cancer cells. TNF signaling plays an important role in cancer-related inflammation [52]. IFN-γ- and IFN-γ-induced type-1 chemokine IL-10 can directly inhibit tumor angiogenesis and cause death of tumor cells. This would then upregulate anti-tumor immunity because of augmented TAA release from dying tumor cells [53]. Thus NRBH exerts a direct antiproliferative action on tumor cells and also indirectly inhibit tumor growth by augmenting the production of TNF and IFN. It is a potential candidate to be developed into new drugs for cancer treatment.
Figure 1.
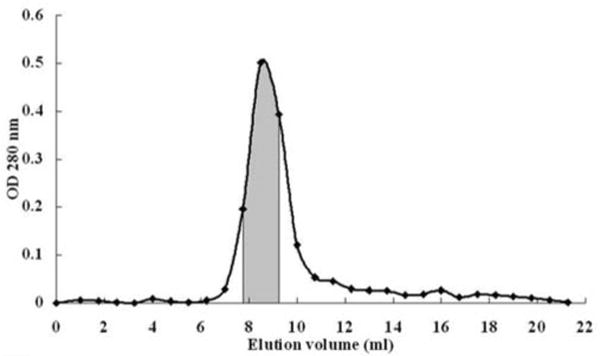
Elution profile of northeast red bean hemagglutinin from a Superdex 75 HR 10/300 gel filtration column. The shaded region contained purified hemagglutinin.
Table 4.
N-terminal Amino Acid Sequence of Northeast Red bean Hemagglutinin (NRBH) in Comparison with those of Related Lectins/hemagglutinins. Amino Acid Residues Different from those in NRBH are Underscored
| Accession | Description | Sequence | Percentage identity (%) |
|---|---|---|---|
| – | NRBH | 1 ASQTSFSFQRFNE 13 | 100 |
| P05088.1 | Erythroagglutinating phytohemagglutinin [P. vulgaris] | 22 ASQTSFSFQRFNE 34 | 100 |
| CAD28674.1 | phytohemagglutinin [P. vulgaris] | 22 ASETSFSFQRFNE 34 | 92 |
| CAD28675.1 | lectin [P. vulgaris] | 22 ASETSFSFQRFNE 34 | 92 |
| CAD29133.1 | lectin [P. vulgaris] | 22 ASETSFSFQRFNE 34 | 92 |
| AAB36314.1 | GNL-2 α subunit=lectin [P. vulgaris=Great Northern beans] | 1 ATETSFSFQRFXE 13 | 77 |
| CAD27654.1 | phytohemagglutinin [P. coccineus] | 22 ASETSFSFQRFNE 34 | 92 |
| CAH60212.1 | phytohemagglutinin-L precursor [P. costaricensis] | 22 ASETSFSFDRFNE 34 | 85 |
| AAA82181.1 | phytohemagglutinin [P. acutifolius] | 25 ANDISFNFQRFNE 37 | 69 |
Acknowledgments
Declared none.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Newlaczyl AU, Yu LG. Galectin-3–a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Ferreira M, Magalhães GS, Fernandez JH, Junqueira-de-Azevedo Ide L, Le Ho P, Lima C, Valente RH, Moura-da-Silva AM. Structural biological characterization of Nattectin a new C-type lectin from the venomous fish. Thalassophryne nattereri Biochimie. 2011;93:971–980. doi: 10.1016/j.biochi.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf GM, Banu N, Ahmad A, Singh LP, Kumar R. Purification, characterization, sequencing and biological chemistry of galectin-1 purified from Capra hircus (goat) heart. Protein J. 2011;20:39–51. doi: 10.1007/s10930-010-9300-2. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Zhou X, Wang J, Jiang P, Tang K. Purification and characterization of curcin, a toxic lectin from the seed of Jatropha curcas. Prep Biochem Biotechnol. 2010;40:107–118. doi: 10.1080/10826060903558588. [DOI] [PubMed] [Google Scholar]
- 6.Narahari A, Swamy MJ. Rapid affinity-purification and physicochemical characterization of pumpkin (Cucurbita maxima) phloem exudate lectin. Biosci Rep. 2010;30:341–349. doi: 10.1042/BSR20090117. [DOI] [PubMed] [Google Scholar]
- 7.Singh RS, Bhari R, Rai J. Further screening of Aspergillus species for occurrence of lectins and their partial characterization. J Basic Microbiol. 2010;50:90–97. doi: 10.1002/jobm.200900299. [DOI] [PubMed] [Google Scholar]
- 8.Singh RS, Sharma S, Kaur G, Bhari R. Screening of Penicillium species for occurrence of lectins and their characterization. J Basic Microbiol. 2009;49:471–476. doi: 10.1002/jobm.200800282. [DOI] [PubMed] [Google Scholar]
- 9.Mikcha JM, Freire MG, Macedo ML, Yano T, Piantino Ferreira AJ. Characterization of a nonfimbrial mannose-sensitive hemagglutinin (MSH) produced by Salmonella enterica serovar enteritidis. Comp Immunol Microbiol Infect Dis. 2006;29:301–314. doi: 10.1016/j.cimid.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Syed FB, Joshi BN, SivaRaman H, Khire JM, Khan MI. Purification and characterization of a cell-surface lectin (Lectin II) from Agrobacterium radiobacter NCIM 2443. Biochem Mol Biol Int. 1999;47:361–367. doi: 10.1080/15216549900201383. [DOI] [PubMed] [Google Scholar]
- 11.Dhirapong A, Lleo A, Leung P, Gershwin ME, Liu FT. The immunological potential of galectin-1 and -3. Autoimmun Rev. 2009;8:360–363. doi: 10.1016/j.autrev.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Singh RS, Bhari R, Kaur HP. Mushroom lectins: current status and future perspectives. Crit Rev Biotechnol. 2010;30:99–126. doi: 10.3109/07388550903365048. [DOI] [PubMed] [Google Scholar]
- 13.Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Zhang X, Chen G, Wei D, Chen F. Algal lectins for potential prevention of HIV transmission. Curr Med Chem. 2008;15:1096–1104. doi: 10.2174/092986708784221421. [DOI] [PubMed] [Google Scholar]
- 16.Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK. Plant lectins: targeting programmed cell death pathways as antitumor agents. Int J Biochem Cell Biol. 2011;43:1442–1449. doi: 10.1016/j.biocel.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Hou Y, Hou Y, Yanyan L, Qin G, Li J. Extraction and purification of a lectin from red kidney bean and preliminary immune function studies of the lectin and four Chinese herbal polysaccharides. J Biomed Biotechnol. 2010;2010:217342. doi: 10.1155/2010/217342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamemura K, Furuichi Y, Umekawa H, Takahashi T. Purification and characterization of novel lectins from Great Northern bean, Phaseolus vulgaris L. Biochim Biophys Acta. 1993;1158:181–118. doi: 10.1016/0304-4165(93)90012-w. [DOI] [PubMed] [Google Scholar]
- 19.Fang EF, Pan WL, Wong JH, Chan YS, Ye XJ, Ng TB. A new Phaseolus vulgaris lectin induces selective toxicity on human liver carcinoma Hep G2 cells. Arch Toxicol. 2011;85:1551–1563. doi: 10.1007/s00204-011-0698-x. [DOI] [PubMed] [Google Scholar]
- 20.Kiss R, Camby I, Duckworth C, De Decker R, Salmon I, Pasteels JL, Danguy A, Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997;40:253–261. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong JH, Wan CT, Ng TB. Characterisation of a haemagglutinin from Hokkaido red bean (Phaseolus vulgaris cv. Hokkaido red bean) J Sci Food Agric. 2010;90:70–77. doi: 10.1002/jsfa.3782. [DOI] [PubMed] [Google Scholar]
- 22.Fang EF, Lin P, Wong JH, Tsao SW, Ng TB. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J Agric Food Chem. 2010;58:2221–2229. doi: 10.1021/jf903964u. [DOI] [PubMed] [Google Scholar]
- 23.Yagi F, Iwaya T, Haraguchi T, Goldstein IJ. The lectin from leaves of Japanese cycad, Cycas revoluta Thunb. (gymnosperm) is a member of the jacalin-related family. Eur J Biochem. 2002;269:4335–4341. doi: 10.1046/j.1432-1033.2002.03127.x. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK, Favre M. Gel electrophoresis of proteins. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 25.Silva JA, Macedo ML, Novello JC, Marangoni S. Biochemical characterization and N-terminal sequences of two new trypsin inhibitors from Copaifera langsdorffii seeds. J Protein Chem. 2001;20:1–7. doi: 10.1023/a:1011053002001. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa R, Yasokawa D, Ikeda T, Nagashima K. Purification and characterization of two lectins from calllus of Helianthus tuberosus. Biosci Biotechnol Biochem. 1996;60:259–262. doi: 10.1271/bbb.60.259. [DOI] [PubMed] [Google Scholar]
- 27.Koike T, Beppu H, Kuzuya H, Maruta K, Shimpo K, Suzuki M. A 35 kDa mannose-binding lectin with hemagglutinating and mitogenic activities from “Kidachi Aloe” (Aloe arborescens Miller var. natalensis Berger) J Biochem. 1995;118:1205–1210. doi: 10.1093/oxfordjournals.jbchem.a125008. [DOI] [PubMed] [Google Scholar]
- 28.Edman P. Method for determination of amino acid sequences in peptides. Acta Chem Scand. 1950;28:283–293. [Google Scholar]
- 29.Chan YS, Wong JH, Ng TB. A cytokine-inducing hemagglutinin from small taros. Protein Pept Lett. 2010;17:823–830. doi: 10.2174/092986610791306742. [DOI] [PubMed] [Google Scholar]
- 30.Lam SK, Ng TB. First report of a haemagglutinin-induced apoptotic pathway in breast cancer cells. Biosci Rep. 2010;30:307–317. doi: 10.1042/BSR20090059. [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Li Y, Jiang Z, Sun Y, Zhu L, Ding Z. Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine. 2009;16:586–593. doi: 10.1016/j.phymed.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Chen X, Zhang Q, Chen L, Wang Y. Corydalis yanhusuo W.T. Wang extract inhibits MCF-7 cell proliferation by inducing cell cycle G2/M arrest. Am J Chin Med. 2011;39:579–586. doi: 10.1142/S0192415X11009044. [DOI] [PubMed] [Google Scholar]
- 33.Cao X, Huo Z, Lu M, Mao D, Zhao Q, Xu C. Purification of lectin from larvae of the fly, Musca domestica, and in vitro antitumor activity in MCF-7 cells. J Insect Sci. 2010;10:164. doi: 10.1673/031.010.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang EF, Zhang CZ, Ng TB, Wong JH, Pan WL, Ye XJ. Momordica Charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prev Res (Phila) 2012;5:109–121. doi: 10.1158/1940-6207.CAPR-11-0203. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Wong JH, Lin P, Chan YS, Ng TB. Purification and characterization of a lectin from the Indian cultivar of French bean seeds. Protein Pept Lett. 2010;17:221–227. doi: 10.2174/092986610790226067. [DOI] [PubMed] [Google Scholar]
- 36.Leung EH, Wong JH, Ng TB. Concurrent purification of two defense proteins from French bean seeds: a defensin-like antifungal peptide and a hemagglutinin. J Pept Sci. 2008;14:349–353. doi: 10.1002/psc.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma A, Ng TB, Wong JH, Lin P. Purification and characterization of a lectin from Phaseolus vulgaris cv. (Anasazi beans) J Biomed Biotechnol. 2009;2009:929568. doi: 10.1155/2009/929568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damico DC, Freire MG, Gomes VM, Toyama MH, Marangoni S, Novello JC, Macedo ML. Isolation and characterization of a lectin from Annona muricata seeds. J Protein Chem. 2003;22:655–661. doi: 10.1023/b:jopc.0000008730.50675.de. [DOI] [PubMed] [Google Scholar]
- 39.Lin P, Ng TB. Preparation and biological properties of a melibiose binding lectin from Bauhinia variegata seeds. J Agric Food Chem. 2008;56:10481–10486. doi: 10.1021/jf8016332. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira JT, Melo VM, Câmara MF, Vasconcelos IM, Beltramini LM, Machado OL, Gomes VM, Pereira SP, Fernandes CF, Nunes EP, Capistrano GG, Monteiro-Moreira AC. Purification and physicochemical characterization of a cotyledonary lectin from Luetzelburgia auriculata. Phytochemistry. 2002;61:301–310. doi: 10.1016/s0031-9422(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 41.Sathe SK. Dry bean protein functionality. Crit Rev Biotechnol. 2002;22:175–223. doi: 10.1080/07388550290789487. [DOI] [PubMed] [Google Scholar]
- 42.Sgarbieri VC, Whitaker JR. Physical, chemical, and nutritional properties of common bean (Phaseolus) proteins. Adv Food Res. 1982;28:93–166. doi: 10.1016/s0065-2628(08)60111-1. [DOI] [PubMed] [Google Scholar]
- 43.Lam SK, Ng TB. Isolation and characterization of a French bean hemagglutinin with antitumor, antifungal, and anti-HIV-1 reverse transcriptase activities and an exceptionally high yield. Phytomedicine. 2010;17:457–462. doi: 10.1016/j.phymed.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Chan YS, Wong JH, Ng TB. A glucuronic acid binding leguminous lectin with mitogenic activity toward mouse splenocytes. Protein Pept Lett. 2011;18:194–202. doi: 10.2174/092986611794475110. [DOI] [PubMed] [Google Scholar]
- 45.Ye XY, Ng TB, Tsang PW, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolusvulgaris) seeds. J Protein Chem. 2001;20:367–375. doi: 10.1023/a:1012276619686. [DOI] [PubMed] [Google Scholar]
- 46.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 47.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beuth J. Clinical relevance of immunoactive mistletoe lectin-I. Anticancer Drugs. 1997;8:53–55. doi: 10.1097/00001813-199704001-00012. [DOI] [PubMed] [Google Scholar]
- 50.Schink M. Mistletoe therapy for human cancer: the role of the natural killer cells. Anticancer Drugs. 1997;8:47–51. doi: 10.1097/00001813-199704001-00011. [DOI] [PubMed] [Google Scholar]
- 51.Wimer BM. Therapeutic activities of PHA-L4, the mitogenic isolectin of phytohemagglutinin. Mol Biother. 1990;2:74–90. [PubMed] [Google Scholar]
- 52.Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer Immunol Immunother. 2011;60:1529–1541. doi: 10.1007/s00262-011-1104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


