Summary
Purpose
In a phase I study, the combination of gemcitabine and imatinib was well tolerated with broad anticancer activity. This phase I trial evaluated the triplet of docetaxel, gemcitabine and imatinib.
Experimental Design
Imatinib was administered at 400 mg daily on days 1–5, 8–12 and 15–19. Gemcitabine was started at 600 mg/m2 at a rate of 10 mg/min on days 3 and 10 and docetaxel at 30 mg/m2 on day 10, on a 21-day cycle. Diffusion and dynamic contrast-enhanced perfusion MRI was performed in selected patients.
Results
Twenty patients with relapsed/ refractory solid tumors were enrolled in this IRB-approved study. The mean age was 64, and mean ECOG PS was 1. Two patients were evaluated by diffusion/perfusion MRI. After two grade 3 hematological toxicities at dose level 1, the protocol was amended to reduce the dose of imatinib. MTDs were 600 mg/ m2 on days 3 and 10 for gemcitabine, 30 mg/ m2 on day 10 for docetaxel, and 400 mg daily on days 1–5 and 8–12 for imatinib. Dose limiting toxicities after one cycle were neutropenic fever, and pleural and pericardial effusions. The best response achieved was stable disease, for six cycles, in one patient each with mesothelioma and non small cell lung cancer (NSCLC) at the MTD. Two patients with NSCLC had stable disease for four cycles.
Discussion
An unexpectedly low MTD for this triplet was identified. Our results suggest drug-drug interactions that amplify toxicities with little evidence of improved tumor control.
Keywords: Phase I Gemcitabine, Docetaxel and Imatinib, Diffusion and dynamic contrast-enhanced perfusion MRI
Introduction
Gemcitabine (GEM), docetaxel and imatinib are all active anticancer agents. In particular, imatinib has altered treatment of chronic myeloid leukemia (CML), gastrointestinal stromal tumor (GIST) and hypereosinophilic syndrome because of its inhibition of the BCR-ABL tyrosine kinase (TK), as well as the TK receptors for platelet-derived growth factor (PDGF), and c-kit [1–4]. Gemcitabine and docetaxel are used as single agents and in combination to treat wide variety of cancers including pancreatic cancer [5], non small cell lung cancer [6–9], metastatic breast cancer [10–13], ovarian cancer [14], and urothelial cancer [15]. The combination of gemcitabine and docetaxel at different dosing schedules has shown activity in multiple different malignancies including lung cancer, sarcoma, and breast cancer [16–19].
Combining TK inhibitors with cytotoxic chemotherapy may yield additional benefit as compared to cytotoxics alone. Pietras and colleagues have suggested that inhibition of the PDGFR signal transduction pathway with imatinib may decrease interstitial hypertension within the tumor stroma and allow for improved uptake of systemic therapy within the tumor [20]. Thus, combining the PDGFR inhibition of imatinib with chemotherapy may enhance tumor uptake of anticancer-therapy.
A Phase I trial of gemcitabine and imatinib mesylate for the treatment of patients with refractory solid tumor malignancy has shown substantial activity in solid tumors with little increase in toxicities [21]. Daily imatinib with low dose gemcitabine was prohibitively toxic [21]. However, the administration of gemcitabine with imatinib “bracketing” was more successful. Among 54 patients treated with gemcitabine on days 3 and 10, in combination with imatinib, given on days 1–5, 8–12, and 15–19, three patients had partial responses (laryngeal, renal, mesothelioma) and seventeen patients had stable disease for 6 months or longer. The maximum tolerated dose (MTD) and the proposed dose for phase II usage, was gemcitabine 1500 mg/m2/150 min and imatinib 400 mg/day on days 1–5, 8–12, and 15–19. For the combination of docetaxel and imatinib, an MTD of docetaxel 30 mg/m2 weekly with imatinib 600 mg daily has been reported [22].
Since gemcitabine and docetaxel are active in combination and gemcitabine and imatinib are as effective and well tolerated in combination, the triplet combination of gemcitabine, docetaxel and imatinib mesylate appeared promising and worthy of further investigation.
Therefore, we conducted an open label, dose escalation study of gemcitabine and docetaxel in combination with fixed dose imatinib mesylate in patients with refractory malignancies with the purpose of identifying the maximum tolerated dose of each component in the triple regimen. Based on the prior phase I study of gemcitabine/imatinib combination, the “bracketing” imatinib schedule was used. The study was conducted at the Cancer Institute of New Jersey and the University of Michigan.
As part of the study, dynamic contrast-enhanced (DCE) perfusion and diffusion magnetic resonance imaging was performed on a limited number of patients as a correlative study. Diffusion imaging has been shown to be an earlier predictor of response to therapy in patients with brain gliomas. In this study, we examined the applicability of this technique in other tumors and investigated, in a preliminary fashion, whether treatment-induced perfusion changes could be detected and whether they were predictive of response.
Patients and methods
Adult patients with refractory solid tumor malignancies with the following eligibility criteria were included in this study: ECOG performance status 0–2, estimated survival >3 months, measurable or evaluable disease, intact GI absorption; total leukocytes>3500/μl; neutrophil count>1,500/μl; platelet count > 125,000/μl; prothrombin time and partial prothromboplatin time within institutional upper normal limits (ULN), serum creatinine<1.5 mg/dl, total bilirubin<1.5 mg/dl; ALT, AST and alkaline phosphatase<2.5x the institutional ULN. Patients were also required to have had their last chemotherapy or radiotherapy at least 4 weeks prior to start of the trial and to have recovered from the toxicities of previous chemotherapy or radiotherapy. Prior therapy with gemcitabine and/or docetaxel was allowed.
Patients were excluded if they had more than 12 months of continuous cytotoxic therapy; more than 2 prior cytotoxic regimens for metastatic disease; history of bone marrow transplantation; radiation to more than 25% of bone marrow; known or suspected bone marrow infiltration by cancer; newly diagnosed or uncontrolled brain metastasis; chronic uncontrolled diarrhea and/or daily emesis; Grade 2 or higher neuropathy; or significant peripheral edema. Patients were instructed to not use acetaminophen or grapefruit juice during the trial.
The protocol was approved by the IRBs at the Cancer Institute of New Jersey and the University of Michigan.
All dose level cohorts consisted of at least three patients. No intra-patient dose escalation was allowed. Dose escalation could not occur until at least three patients had completed one cycle at the active dose level and were evaluable for toxicity. However, patients were added into a cohort to replace patients who, for reasons other than toxicity, did not receive a full cycle of all drugs.
If none of the first three patients in any cohort experienced dose-limiting toxicity (DLT), the next three patients were enrolled at the next higher dose level. If one of the first three patients in a dosing cohort had a DLT, two more patients were treated at that dose level. If neither of those additional patients had a DLT, the dose was escalated. If one of these additional patients had a DLT, the dose was considered as the MTD. If two of five patients at a given dose level had a DLT, two more patients were treated at the prior dose level (if only three patients were previously treated at that prior dose) to confirm tolerability.
DLT was defined as the occurrence of any of the following serious adverse events during the first cycle of therapy: grade 3–4 non-hematologic toxicity (including nausea and vomiting that could not be controlled with oral medication); grade 4 hematologic toxicity that occurred during treatment or within 1 week of treatment completion and lasted >7 days; omission or delay of treatment for toxicity by two or more doses in a cycle; or, delay in initiation of a treatment cycle beyond 2 weeks.
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 3.0. All patients receiving one dose of protocol therapy were evaluable for toxicity.
Pretreatment evaluations
Baseline evaluations included history, complete physical examination, ECOG performance status, CBC with platelet count, serum chemistries and electrolytes, chest x-ray, EKG, tumor markers, if indicated. In addition to these evaluations, imaging studies (computed tomography or magnetic resonance imaging) of the sites of measurable disease were performed.
Treatment schema
Cycles were repeated every 21 days. Imatinib mesylate was taken orally at 400 mg per day on days 1–5, 8–12, and 15–19, preferably with a meal. Gemcitabine was administered as a 10 mg/m2/min infusion, starting at 600 mg/m2 on days 3 and 10. Docetaxel was administered as a 60-min infusion starting at 30 mg/m2 on day 10. Premedications for docetaxel included dexamethasone 8 mg orally twice daily on the day prior to chemotherapy, on the day of chemotherapy and on the day after chemotherapy. After two cycles of docetaxel without hypersensitivity reaction, the dexamethasone schedule could be modified at the discretion of the treating physician.
Patients were assessed for toxicity weekly and for disease regression or progression every two cycles. All patients receiving any treatment were considered evaluable for response. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) [23].
Treatment at the second dose level was associated with unexpected myelosuppression. This was attributed to possible imatinib toxicity due to Days 15–19 of imatinib, and the protocol was amended to include dosage levels 2A, 2B as shown in Table 1. Cohort 2B was designed with increasing gemcitabine but fixed docetaxel dose to determine whether the toxicities were dependent on the other two drugs.
Table 1.
Dosing levels
| Regimen | Gemcitabine 10mg/m2/min on Days 3, 10 | Docetaxel IV 60min infusion on Day 10 | Imatinib Mesylate (mg) on Days 1–5, 8–12 | # patients |
|---|---|---|---|---|
| 1* | 600 mg/m2 | 30 mg/m2 | 400 mg (included days 15–19) | 8 |
| 2 | 600 mg/m2 | 45 mg/m2 | 400 mg (included days 15–19) | 4 |
| 2A | 600 mg/m2 | 45 mg/m2 | 400 mg | 5 |
| 2B | 600 mg/m2 | 30 mg/m2 | 400 mg | 2 |
Diffusion and perfusion magnetic resonance imaging (MRI)
Diffusion-weighted images (DWI) of one primary or metastatic lesion were acquired on a 3T human MRI system (Philips Medical Systems, Best, The Netherlands) using a 6-channel torso array coil. DWI was performed using a single-shot echoplanar imaging technique with parallel imaging (SENSE=2), an 200×172 acquisition matrix over a 350×300 mm field-of-view (FOV), and 5 mm axial slices. Three-axis diffusion sensitization at b-values 0 and 800 s/mm2 were acquired with fat suppression and 8 averages, TR/TE=3200/60 ms. Apparent diffusion coefficient (ADC) maps were compared to pre-therapy maps by inspection on a quantitative color scale display, and by region of interest analysis. Dynamic contrast-enhanced (DCE) perfusion sensitive MRI was performed using 3D T1-weighted fast field echo (3D T1-FFE) sequence at 20 s temporal resolution during injection of a body-weight dose of gadolinium contrast material (Magnevist at 0.1 mMol/kg). DCE acquisition parameters included: 364× 240×70 axial slices at 1×1×2mm acquired resolution; SENSE=2; TR/TE/Flip=4.6/2.2 ms/12°. The Ktrans maps were derived from the DCE image series using established Tofts 2-compartment model for contrast exchange.
Included participants signed informed consent for this portion of the protocol, did not require sedation for an MRI, did not have metal fragments within their body that might put them at increased risk for harm from the MRI, and were treated at the University of Michigan. MRIs were performed prior to therapy, on cycle 1, day 10 prior to infusion, and on cycle 2, day 3.
Results
Between February 2006 and January 2008, 20 patients were enrolled into the study. Patient characteristics are listed in Table 2.
Table 2.
Patient characteristics
| No. of patients | |
|---|---|
| Total | 20 |
| Median age, years (range) | 64.5(43–83) |
| Sex | |
| Male | 12 |
| Female | 8 |
| Performance status, ECOG | |
| 0–1 | 16 |
| 2 | 4 |
| Tumor type | |
| Lung Cancer | |
| NSCLC | 4 |
| SCLC | 1 |
| Sarcoma | 5 |
| Pancreatic/Biliary | 3 |
| Mesothelioma | 2 |
| Bladder | 2 |
| Other (colon, Breast, Cervical, Melanoma) | 4 |
| No. of prior chemotherapy regimens | |
| 0 | 4 |
| 1 | 7 |
| ≥ 2 | 9 |
| Prior Radiotherapy | 12 |
Of the first six patients, two patients had a delay in day 10 dosing. One patient at drug level 2 had day 10 dose held; at drug level 2A, day 10 doses were held for two patients. At least six patients had delays in second cycle initiation due to toxicities. The dose limiting toxicities are reported in Table 3.
Table 3.
Dose limiting toxicities
| Dose level | # patients | DLT |
|---|---|---|
| 1* | 8 | 2 Neutropenic Fever |
| 2 | 4 | 2 Neutropenic Fever |
| 2A | 5 | 2 Neutropenic Fever and Weakness |
| 2B | 2 | 1 Neutropenic Fever |
The MTD was determined to be 600 mg/m2 for gemcitabine, 30 mg/m2 for docetaxel, and 400 mg per day given on days 1–5 and 8–12 for imatinib. The dose limiting toxicities were neutropenic fever, and pleural and pericardial effusions. The best response achieved was stable disease, for six cycles, in one patient each with mesothelioma and non small cell lung cancer (NSCLC) at the MTD. Two other patients with NSCLC had stable disease for four cycles.
For all patients, the median number of cycles received was 2; and the median duration of therapy was 12 weeks with a range from 4 to 30 weeks.
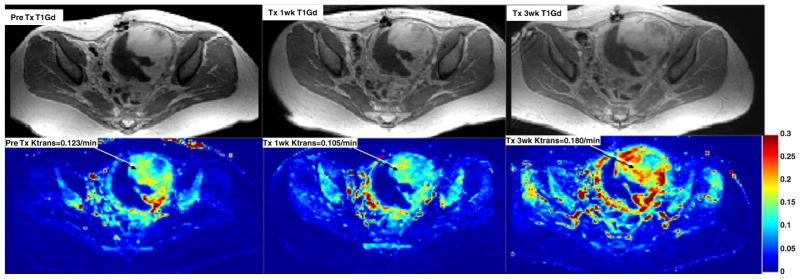
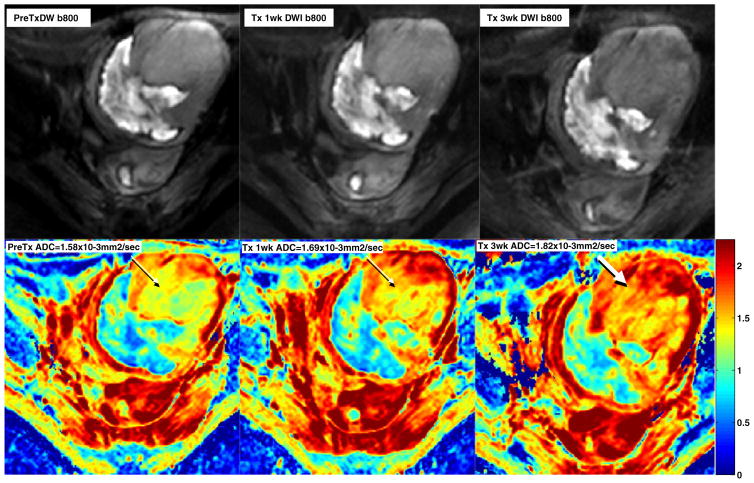
Functional MRI was performed on two patients. Changes in the perfusion and diffusion weighted images were demonstrated in both patients. Figure 1 shows minimal changes in Ktrans (microvascular permeability) indicating decreased perfusion at the middle of cycle 1 and increased perfusion at the initiation of cycle 2 in a patient with a uterine leiomyosarcoma. The clinical significance of these changes is unclear. Figure 2 illustrates changes in the tumor ADC or water mobility on diffusion MRI sequences in the same patient. Increased ADC has been shown to correlate with patient benefit from therapy in other malignancies.
Fig. 1.
MRI imaging with conventional (top row) and perfusion (bottom row) sequences in a patient with uterine leiomyosarcoma showing variable microvascular permeability (Ktrans) at baseline (left), cycle 1 day 10 (middle), and on cycle 2 day 3 (right) of therapy
Fig. 2.
MRI images in the same patient with uterine leiomyosarcoma showing conventional (top row) and diffusion sequences (bottom row) prior to(left), on cycle 1 day 10 (middle), and on cycle 2 day 3 (right) of therapy. Images reveal an increase in diffusion (ADC or water mobility) as therapy progresses
Discussion
This phase I study of docetaxel, gemcitabine, and imatinib yielded an unexpectedly low maximally tolerated dose for the combination of these drugs. The results are surprising, given that, in combination with bracketing imatinib, gemcitabine could be given at much higher doses (up to 1500 mg/m2 over 150 min) in our previous phase I study [21].
The potential causes for the higher toxicities observed in the current study include patient factors and possible drug interactions. Since this three drug combination has not been studied before, we reviewed experiences with each of the 2-drug combinations to determine possible etiological factors for these significant toxicities.
Host factors
Compared to the prior study of gemcitabine and imatinib, this patient population was comparable in terms of demographics (age, sex, performance status), specific diagnoses and number of prior therapies (45% of patients had more than two prior chemotherapies in this study vs. 41% in the gemcitabineimatinib study) [21]. In the present study, more patients had prior radiotherapy (60% v. 33%). Thus, a higher proportion of patients in this study had less bone marrow reserve.
In a previous phase I study of gemcitabine and docetaxel, the MTD was determined at 800 mg/m2 on days 1 and 8 for gemcitabine, and 40 mg/m3 on days 1 and 8 every 21 days for docetaxel, a regimen not too dissimilar from the one used in the present study [24]. The dose limiting toxicities were Grade 3/4 neutropenia in 62% of patients (8/13) who had received two or more prior chemotherapy regimens. However, no neutropenia was noted among fifteen patients who had received no more than one prior chemotherapy regimen [24]. In our study, among the patients who experienced neutropenic fever, two patients in Level 2 had received 2 prior therapies and two patients in level 2A had received 0 and 3 prior chemotherapies, respectively.
Thus, the number of prior therapies, including radiation therapy, may have played a role in the low MTD of the present study. Many studies of gemcitabine and docetaxel use filgrastim or pegfilgrastim routinely. This protocol did not utilize growth factors, likely contributing to higher rates of neutropenia and related toxicities.
Drug-drug interactions
Gemcitabine and docetaxel combination has been studied much more extensively than the combination of imatinib with gemcitabine or imatinib with docetaxel. Gemcitabine and docetaxel combination has been studied in different schedules including weekly gemcitabine for 3 weeks out of four with various dosing of docetaxel [25, 26]; gemcitabine on days 1 and 8 with docetaxel on day 8 [27–29] every 21 days, and gemcitabine and docetaxel on days 1 and 8 every 3 weeks.
In studies with similar schedule of gemcitabine and docetaxel, a broad range of dosages was tolerated (Table 4). There are several studies where toxicities were observed at lower dosages for each of the two drugs. In one phase I study, gemcitabine and docetaxel were safely administered weekly for three out of 4 weeks. The MTD for gemcitabine was 750 mg/m2 and docetaxel 35 mg/m2, and the dose-limiting toxicity (DLT) was neutropenia [30]. It has been suggested that specific sequencing of the drugs (docetaxel followed by gemcitabine) produces powerful cytocidal effect [31]. It is hypothesized that the gemcitabine attacks cells as they progress to the S phase after recovering rapidly from an M phase block induced by docetaxel [31]. In our study, the gemcitabine preceded docetaxel.
Table 4.
Combination of Gemcitabine and Docetaxel
| Study | Phase | Disease | Gemcitabine dose (mg/ m2) Days 1,8 | Docetaxel dose (mg/ m2) on Day 8 | Cycle duration | Commen |
|---|---|---|---|---|---|---|
| Matsui [38] | I/II | NSCLC | 1000 mg/m2 | 50 mg/m2 | 21 | |
| Hensley [39] | II | Uterine Leiomyosarcoma | 900 FDR mg/m2 | 100 mg/m2 | 21 |
|
| Georgoulilas [40] | III | NSCLC | 1100 mg/m2 | 75 mg/m2 | 21 | |
| Ebeling [41] | Retrosp | STS | 900 mg/m2 | 100 mg/m2 | 21 |
|
| Boukovinas [42] | II | elderly NSCLC | 1100 mg/m2 | 100 mg/m2 | 21 |
|
| Labourey [43] | II | HNC | 1000 mg/m2 | 75 mg/m2 | 21 |
|
| Maki [44] | II | STS | 900 mg/m2 FDR | 100 mg/m2 | 21 |
|
The addition of imatinib may have amplified the toxicities of gemcitabine in our patients. Though we did not see severe hematological toxicities in our previous study of gemcitabine and imatinib, neutropenia was noted on day 10 in the current study, even before docetaxel administration. The combination of chemotherapy with imatinib may be problematic, and may be worse in patients with prior radiotherapy. In other Phase I studies of imatinib with gemcitabine or doxorubicin, the initial dose levels produced DLT when imatinib was given daily with chemotherapy [32].
It has been hypothesized that inhibition of PDGFR may decrease tumor interstitial fluid pressure, allowing better penetration of chemotherapeutic drugs. A mouse xenograft study showed increased gemcitabine efficacy when administered with imatinib, an outcome postulated as due to increased gemcitabine delivery [33]. Perfusion MRI to evaluate for possible increased tumoral uptake of chemotherapy after imatinib administration was designed as part of the present study. Unfortunately, due to the limited number of patients evaluated with functional MRI, no conclusions could be drawn from this analysis. One might hypothesize that increased uptake of gemcitabine was seen less in tumor cells and more in normal hematopoietic cells as was reflected in the increased hematologic toxicity without any change in efficacy.
There is no noted pK interaction between imatinib and docetaxel [34]. Our previous study did not show any effect of imatinib on gemcitabine pK [21]. Because of this, no formal pK evaluations were performed as part of this trial, a limitation of the study.
Pleural and pericardial effusions, among the dose limiting toxicities observed, are generally rare with gemcitabine and docetaxel, but can be associated with imatinib [35–37]. One patient each had pericardial effusion and pleural effusion during cycle #1, which is likely related to imatinib. The role of docetaxel and gemcitabine in those cases was unclear.
Conclusion
In this combination the maximally tolerated dose was: imatinib 400 mg orally per day on days 1–5 and 8–12, gemcitabine 600 mg/m2 in FDR infusion over 60 min on days 3 and 10, and docetaxel 40 mg/m2 on day 10. The combination was unexpectedly toxic without any apparent increase in efficacy in this limited patient population. Further studies involving imatinib in combination with cytotoxic chemotherapy should be performed with caution and close monitoring of toxicities. Perfusion and diffusion MRI is feasible in this population of patients and further studies should be performed to determine the utility of this technique.
Acknowledgments
This study was supported in part by NIH grants P30 CA046592 and NIH P01 CA085878 (Thomas Chenevert).
Contributor Information
Biren Saraiya, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA.
Rashmi Chugh, Hematology/Oncology, University of Michigan, 1500 E. Medical Center Drive, Ann Arbor, MI 48109, USA.
Vassiliki Karantza, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA.
Janice Mehnert, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA.
Rebecca A. Moss, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA
Nelli Savkina, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA.
Mark N. Stein, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA
Laurence H. Baker, Hematology/Oncology, University of Michigan, 1500 E. Medical Center Drive, Ann Arbor, MI 48109, USA
Thomas Chenevert, Radiology, University of Michigan, 1500 E. Medical Center Drive, Ann Arbor, MI 48109, USA.
Elizabeth A. Poplin, The Cancer Institute of New Jersey, UMDNJ-Robert Wood Johnson Medical School, 195 Little Albany St., New Brunswick, NJ 08901, USA
References
- 1.Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, Rahman A, Chen G, Staten A, Griebel D, Pazdur R. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8(10):3034–3038. [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457348/11/994. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, O’Brien S, Cortes JE, Smith TL, Rios MB, Shan J, Yang Y, Giles FJ, Thomas DA, Faderl S, Garcia-Manero G, Jeha S, Wierda W, Issa JP, Kornblau SM, Keating M, Resta D, Capdeville R, Talpaz M. Treatment of philadelphia chromosome-positive, accelerated-phase chronic myelogenous leukemia with imatinib mesylate. Clin Cancer Res. 2002;8(7):2167–2176. [PubMed] [Google Scholar]
- 4.Kantarjian HM, Cortes J, O’Brien S, Giles FJ, Albitar M, Rios MB, Shan J, Faderl S, Garcia-Manero G, Thomas DA, Resta D, Talpaz M. Imatinib mesylate (sti571) therapy for philadelphia chromosome-positive chronic myelogenous leukemia in blast phase. Blood. 2002;99(10):3547–3553. doi: 10.1182/blood.v99.10.3547. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Javier Rodriguez JC, Calvo E, Azinovic I, Fernandez-Hildago O, Martinez-Monge R, Garzon C, de Irala J, Martinez-Aguillo M, Ramon y Cajal T, Brugarolas A. Paclitaxel, cisplatin, and gemcitabine combination chemotherapy within a multidisciplinary therapeutic approach in metastatic nonsmall cell lung carcinoma. Cancer. 2000;89(12):2622–2629. doi: 10.1002/1097-0142(20001215)89:12<2622::aid-cncr15>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Lerouge D, Monnier A, Bennouna J, Haller AM, Sun XS, Assouline D, Grau B, Riviere A. Combined paclitaxel and gemcitabine as first-line treatment in metastatic non-small cell lung cancer: a multicentre phase ii study. Br J Cancer. 2001;84(9):1179–1184. doi: 10.1054/bjoc.2001.1784S0007092001917847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, Murphy WK, Lippman S, Benner S, Glisson B, et al. Phase ii study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995;13(3):645–651. doi: 10.1200/JCO.1995.13.3.645. [DOI] [PubMed] [Google Scholar]
- 9.Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna A, Fidias P, Millward M, Belani CP. Randomized, multinational, phase iii study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the tax 326 study group. J Clin Oncol. 2003;21(16):3016–3024. doi: 10.1200/JCO.2003.12.046JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans SS. Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol. 2000;18(11):2245–2249. doi: 10.1200/JCO.2000.18.11.2245. [DOI] [PubMed] [Google Scholar]
- 11.Murad AM, Guimaraes RC, Aragao BC, Scalabrini-Neto AO, Rodrigues VH, Garcia R. Phase ii trial of the use of paclitaxel and gemcitabine as a salvage treatment in metastatic breast cancer. Am J Clin Oncol. 2001;24(3):264–268. doi: 10.1097/00000421-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kornek GV, Haider K, Kwasny W, Raderer M, Schull B, Payrits T, Depisch D, Kovats E, Lang F, Scheithauer W. Treatment of advanced breast cancer with docetaxel and gemcitabine with and without human granulocyte colony-stimulating factor. Clin Cancer Res. 2002;8(5):1051–1056. [PubMed] [Google Scholar]
- 13.Nabholtz JM, Crown J. Phase iii studies of single-agent docetaxel in patients with metastatic breast cancer who have progressed despite previous chemotherapy regimens: preliminary results. Semin Oncol. 1998;25(6 Suppl 13):4–9. [PubMed] [Google Scholar]
- 14.Lund B, Hansen OP, Theilade K, Hansen M, Neijt JP. Phase ii study of gemcitabine (2′, 2′-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst. 1994;86(20):1530–1533. doi: 10.1093/jnci/86.20.1530. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, Kuzel T, Nicol S, Oh W, Stadler W. Phase ii trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000;18(9):1921–1927. doi: 10.1200/JCO.2000.18.9.1921. [DOI] [PubMed] [Google Scholar]
- 16.Palmeri S, Vaglica M, Spada S, Filippelli G, Farris A, Palmeri L, Massidda B, Misino A, Ferrau F, Comella G, Leonardi V, Condemi G, Mangiameli A, De Cataldis G, Macaluso MC, Cajozzo M, Iannitto E, Danova M. Weekly docetaxel and gemcitabine as first-line treatment for metastatic breast cancer: results of a multicenter phase ii study. Oncology. 2005;68(4–6):438–445. doi: 10.1159/000086986. [DOI] [PubMed] [Google Scholar]
- 17.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D, Thall PF, Benjamin RS, Baker LH, Hensley ML. Randomized phase ii study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007;25(19):2755–2763. doi: 10.1200/jco.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 18.Georgoulias V, Androulakis N, Kotsakis A, Hatzidaki D, Syrigos K, Polyzos A, Agelidou A, Varthalitis I, Ziras N, Agelidou M, Chandrinos V, Boukovinas I, Geroyianni A, Vamvakas L, Mavroudis D. Docetaxel versus docetaxel plus gemcitabine as front-line treatment of patients with advanced non-small cell lung cancer: a randomized, multicenter phase iii trial. Lung Cancer. 2008;59(1):57–63. doi: 10.1016/j.lungcan.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a gynecologic oncology group phase ii study. Gynecol Oncol. 2008;109(3):323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of pdgf receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–5484. [PubMed] [Google Scholar]
- 21.Ali Y, Lin Y, Gharibo MM, Gounder MK, Stein MN, Lagattuta TF, Egorin MJ, Rubin EH, Poplin EA. Phase i and pharmacokinetic study of imatinib mesylate (gleevec) and gemcitabine in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5876–5882. doi: 10.1158/1078-0432.CCR-07-0883. [DOI] [PubMed] [Google Scholar]
- 22.Mathew P, Thall PF, Jones D, Perez C, Bucana C, Troncoso P, Kim S-J, Fidler IJ, Logothetis C. Platelet-derived growth factor receptor inhibitor imatinib mesylate and docetaxel: a modular phase i trial in androgen-independent prostate cancer. J Clin Oncol. 2004;22(16):3323–3329. doi: 10.1200/jco.2004.10.116. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Bhargava P, Marshall JL, Fried K, Williams M, Lefebvre P, Dahut W, Hanfelt J, Gehan E, Figuera M, Hawkins MJ, Rizvi NA. Phase i and pharmacokinetic study of two sequences of gemcitabine and docetaxel administered weekly to patients with advanced cancer. Cancer Chemother Pharmacol. 2001;48(2):95–103. doi: 10.1007/s002800100317. [DOI] [PubMed] [Google Scholar]
- 25.Dumez H, Louwerens M, Pawinsky A, Planting AS, de Jonge MJ, Van Oosterom AT, Highley M, Guetens G, Mantel M, de Boeck G, de Bruijn E, Verweij J. The impact of drug administration sequence and pharmacokinetic interaction in a phase i study of the combination of docetaxel and gemcitabine in patients with advanced solid tumors. Anticancer Drugs. 2002;13(6):583–593. doi: 10.1097/00001813-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Spiridonidis CH, Laufman LR, Jones J, Rhodes VA, Wallace K, Nicol S. Phase i study of docetaxel dose escalation in combination with fixed weekly gemcitabine in patients with advanced malignancies. J Clin Oncol. 1998;16(12):3866–3873. doi: 10.1200/JCO.1998.16.12.3866. [DOI] [PubMed] [Google Scholar]
- 27.Brandi M, Vici P, Lopez M, Valerio MR, Giotta F, Gebbia N, Schittulli F, Colucci G. Novel association with gemcitabine and docetaxel as salvage chemotherapy in metastatic breast cancer previously treated with anthracyclines: Results of a multicenter phase ii study. Semin Oncol. 2004;31(2 Suppl 5):13–19. doi: 10.1053/j.seminoncol.2004.03.022. S0093775404001265. [DOI] [PubMed] [Google Scholar]
- 28.Casal J, Amenedo M, Mel JR, Anton LM, Rodriguez-Lopez R, Lopez-Lopez R, Gonzalez-Ageitos A, Castellanos J, Constenla M, Tisaire JL. Gemcitabine plus docetaxel as first-line chemotherapy in patients with advanced non-small cell lung cancer: a lung cancer galician group phase ii study. Cancer Chemother Pharmacol. 2007;60(5):725–732. doi: 10.1007/s00280-007-0418-7. [DOI] [PubMed] [Google Scholar]
- 29.Cascinu S, Gasparini G, Catalano V, Silva RR, Pancera G, Morabito A, Giordani P, Gattuso D, Catalano G. A phase i–ii study of gemcitabine and docetaxel in advanced pancreatic cancer: a report from the italian group for the study of digestive tract cancer (giscad) Ann Oncol. 1999;10(11):1377–1379. doi: 10.1023/a:1008394111533. [DOI] [PubMed] [Google Scholar]
- 30.Ganjoo KN, Gordon MS, Sandler AB, Warner RE, Fife K, Poirier S, Seshadri R, Loehrer PJ. A phase i study of weekly gemcitabine and docetaxel in patients with advanced cancer: a hoosier oncology group study. Oncology. 2002;62(4):299–304. doi: 10.1159/000065060. ocl62299. [DOI] [PubMed] [Google Scholar]
- 31.Ricotti L, Tesei A, De Paola F, Ulivi P, Frassineti GL, Milandri C, Amadori D, Zoli W. In vitro schedule-dependent interaction between docetaxel and gemcitabine in human gastric cancer cell lines. Clin Cancer Res. 2003;9(2):900–905. [PubMed] [Google Scholar]
- 32.George S, Desai J, Paul Eder J, Manola J, Ryan DP, Appleman LJ, Demetri GD. Selective kinase inhibition with daily imatinib intensifies toxicity of chemotherapy in patients with solid tumours. Eur J Cancer. 2006;42(7):864–870. doi: 10.1016/j.ejca.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Bertino P, Piccardi F, Porta C, Favoni R, Cilli M, Mutti L, Gaudino G. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res. 2008;14(2):541–548. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 34.Chatta GS, Fakih M, Ramalingam S, Belani CP, Ramanathan RK, Zamboni W, Friedland D, Lis D, Tutchko S, Egorin M. Phase i pharmacokinetic (pk) study of daily imatinib in combination with docetaxel for patients with advanced solid tumors. J Clin Oncol (Meeting Abstracts) 2004;22(14_suppl):2047. [Google Scholar]
- 35.Goldsby R, Pulsipher M, Adams R, Coffin C, Albritton K, Wagner L. Unexpected pleural effusions in 3 pediatric patients treated with sti-571. J Pediatr Hematol/Oncol. 2002;24(8):694–695. doi: 10.1097/00043426-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Breccia M, D’Elia G, D’Andrea M, Latagliata R, Alimena G. Pleural-pericardic effusion as uncommon complication in cml patients treated with imatinib. Eur J Haematol. 2005;74(1):89–90. doi: 10.1111/j.1600-0609.2004.00347.x. [DOI] [PubMed] [Google Scholar]
- 37.Ishii Y, Shoji N, Kimura Y, Ohyashiki K. Prominent pleural effusion possibly due to imatinib mesylate in adult philadelphia chromosome-positive acute lymphoblastic leukemia. Intern Med. 2006;45(5):339–340. doi: 10.2169/internalmedicine.45.1602. [DOI] [PubMed] [Google Scholar]
- 38.Matsui K, Hirashima T, Nitta T, Kobayashi M, Ogata Y, Furukawa M, Kudoh S, Yoshimura N, Mukohara T, Yamauchi S, Shiraishi S, Kamoi H, Negoro S, Takeda K, Nakagawa K, Takada M, Yana T, Fukuoka M. A phase i/ii study comparing regimen schedules of gemcitabine and docetaxel in japanese patients with stage iiib/iv non-small cell lung cancer. Jpn J Clin Oncol. 2005;35(4):181–187. doi: 10.1093/jjco/hyi057. [DOI] [PubMed] [Google Scholar]
- 39.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a gynecologic oncology group phase ii trial. Gynecol Oncol. 2008;109(3):329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgoulias V, Samonis G, Papadakis E, Alexopoulos A, Tsiafaki X, Rapti A, Veslemes M, Grigoratou T, Palamidas P, Kouroussis C, Mavroudis D, Kakolyris S, Giannakakis T, Vlachonikolis J. Comparison of docetaxel/cisplatin to docetaxel/gemcitabine as first-line treatment of advanced non-small cell lung cancer: early results of a randomized trial. Lung Cancer. 2001;34(Suppl 4):S47–51. doi: 10.1016/s0169-5002(01)00417-2. S0169500201004172. [DOI] [PubMed] [Google Scholar]
- 41.Ebeling P, Eisele L, Schuett P, Bauer S, Schuette J, Moritz T, Seeber S, Flasshove M. Docetaxel and gemcitabine in the treatment of soft tissue sarcoma—a single-center experience. Onkologie. 2008;31(1–2):11–16. doi: 10.1159/0000111756. [DOI] [PubMed] [Google Scholar]
- 42.Boukovinas I, Souglakos J, Hatzidaki D, Kakolyris S, Ziras N, Vamvakas L, Polyzos A, Geroyianni A, Agelidou A, Agelaki S, Kalbakis K, Kotsakis A, Mavroudis D, Georgoulias V. Docetaxel plus gemcitabine as front-line chemotherapy in elderly patients with lung adenocarcinomas: a multicenter phase ii study. Lung Cancer. 2008 doi: 10.1016/j.lungcan.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Labourey JL, Cupissol D, Calais G, Tourani JM, Kohser F, Borel C, Eymard JC, Germann N, Tubiana-Mathieu N. Docetaxel plus gemcitabine in recurrent and/or metastatic squamous cell carcinoma of the head and neck: a phase ii multicenter study. Am J Clin Oncol. 2007;30(3):278–282. doi: 10.1097/01.coc.0000258117.75487.2b00000421-200706000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D, Thall PF, Benjamin RS, Baker LH, Hensley ML. Randomized phase ii study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25(19):2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]