Abstract
Introduction
Understanding of swallowing pressures after total laryngectomy (TL) and what constitutes a “functional” swallow are limited. Mobile structures are altered or removed after TL, with consequent effects on pressure profiles. High-resolution manometry (HRM) can characterize these pressures.
Methods
Six TL subjects without dysphagia and six controls underwent pharyngeal HRM. Timing and pressure variables for the velopharynx, mesopharynx, and upper esophageal sphincter (UES) were compared. Changes in variables due to bolus volume were evaluated in TL subjects.
Results
TL subjects had increased duration of velopharyngeal pressure (p=0.012). Maximum mesopharyngeal pressure was lower versus controls (p=0.003). Maximal and total pre-opening (p=0.002; p=0.002) and post-closure (p=0.001; p=0.002) UES pressures were lower. Maximum mesopharyngeal pressure (p=0.032) decreased with increasing bolus volume.
Conclusions
Increased velopharyngeal pressure duration and total swallow duration reflect separation of the pharynx into distinct conduits for air and food, thus ensuring successful bolus passage without the need for respiration. Decreased UES pressure highlights the effects of disrupting the cricopharyngeal and rostral esophageal muscle fibers from their attachments to the larynx and performing a cricopharyngeal myotomy. Additional studies including subjects with dysphagia could further characterize the functional TL swallow and identify aspects susceptible to dysfunction.
Keywords: laryngectomy, high-resolution manometry, pharyngeal swallow
INTRODUCTION
Dysphagia is a common problem after total laryngectomy (TL), with an incidence up to 70% (1), and contributes to poor health outcomes such as compromised nutrition and weight loss (2-4), diminished quality of life (QOL), and decreased psychological well-being (1-7). Severe distress due to dysphagia symptoms has been reported in up to 40% of TL patients (1). Diet and social interactions can also be severely limited (8). Despite the prevalence and significance of dysphagia after TL, we still have a limited understanding of swallowing physiology following TL and better understanding of the mechanism would diminish the significant burden of dysphagia on patients and caregivers.
Oropharyngeal swallowing is inherently complex, involving precise timing and movement of structures in the oral and pharyngeal cavities for bolus transport while protecting the airway (7,9). With a normal, intact swallow, anterosuperior movement of the hyolaryngeal complex during swallowing enlarges the pharyngeal space, creates negative pressure, and contributes to the mechanical opening of the upper esophageal sphincter (9). After a TL, although airway protection is not a concern, anatomic alterations affect the oropharyngeal swallow (7,9-10). Removing the larynx and hyoid bone eliminates hyolaryngeal elevation, increasing pharyngeal bolus transit time and the presence of residue in the neopharynx (11-12). The pharyngeal constrictors, laryngopharyngeal elevators, and cricopharyngeus are altered as the muscle insertion points have been removed. Further, the cricopharyngeus is dilated by performing a myotomy. TL also compromises sensory and motor function of the laryngeal nerves that innervate the pharynx (9,10).
Characterization of the oropharyngeal swallow requires consideration of pharyngeal pressure. Pharyngeal pressure changes during swallowing are more rapid than those within the esophagus, as muscles in this region are striated. Accordingly, quantification of swallowing events requires equipment with superior spatial and temporal resolution. As such, we applied high-resolution manometry (HRM), as this method can directly measure pressure patterns along the length of the pharynx with adequate spatial and temporal resolution (13) and provide a quantifiable, comprehensive manometric dataset directly characterizing each swallow. We hypothesized that swallowing physiology changes after total laryngectomy, and to maintain successful swallowing, pressure patterns will emerge with alterations at key anatomic regions, including the velopharynx, mesopharynx, and upper esophageal sphincter (UES). Specifically, greater pressure must be generated at the velopharynx and/or mesopharynx to facilitate forward bolus propulsion in the absence of the negative pressure created by hyolaryngeal excursion. Further, pressure at the UES will be lower at baseline as well as immediately before and after opening, but will remain positive during bolus passage due to lack of hyolaryngeal elevation. With increasing bolus volumes, there will be increased pressure at the velopharynx, decreased pressure at the mesopharynx, and increased total swallow duration. In this study, we used HRM to collect pressure and timing data from the pharynx and upper esophagus in individuals after TL and compared them to a group of age- and sex-matched controls.
Our goal was to determine the physiologic differences in swallowing pressure patterns after TL in individuals who are not symptomatic for dysphagia and compare them to control participants using HRM. This is a crucial first step toward understanding adaptations to normal physiology after TL and will provide a basis to compare individuals after TL who exhibit dysphagia. This is important to define targets for behavioral and surgical therapy for patients with a TL-related dysphagia. This study required the use of a select set of subjects to avoid confounds, such as prior radiation therapy or pharyngeal reconstruction with regional or free flaps.
MATERIALS AND METHODS
Participants
This study was conducted under the approval of the University of Wisconsin-Madison Health Sciences Institutional Review Board. Six participants post-TL (age: 61.2±11.1 years; 5 males, 1 female) and six age (±6 years; age: 61.8±13.3 years) and sex-matched controls participated in this study. The surgeries were performed by one of two surgeons, the senior author or his partner. Control subjects were previously enrolled for a larger study to collect normative data and had no history of dysphagia or head and neck surgery. Inclusion criteria for the TL subjects were: primary closure of the neopharynx, all nutrition taken orally, and no symptoms of dysphagia as reported on the validated Sydney Swallowing Questionnaire (14). All post-TL subjects in this study had a single layer closure of the pharyngeal mucosa in a T-pattern without reapproximation of the pharyngeal constrictor muscles. Exclusion criteria were: flap reconstruction of the neopharynx, prior radiation or chemotherapy, surgical manipulation of the tongue or base of tongue at the time of TL, dilation or other procedures in the head and neck prior to evaluation, and having a feeding tube in place at the time of participation in the study. Summary subject characteristics are presented in table 1.
Table 1.
Summary characteristics of subjects who underwent total laryngectomy.
| Subject No. | Age | Sex | Indication for total laryngectomy |
|---|---|---|---|
| 1 | 53 | M | T4N0M0 squamous cell carcinoma of the transglottic larynx |
| 2 | 51 | M | T3N1M0 squamous cell carcinoma of the transglottic larynx |
| 3 | 62 | M | T4N0M0 squamous cell carcinoma of the transglottic larynx |
| 4 | 55 | M | T3N0M0 squamous cell carcinoma of the glottic larynx |
| 5 | 81 | F | T3N0M0 squamous cell carcinoma of the glottic larynx |
| 6 | 65 | M | T3N2aM0 squamous cell carcinoma of the transglottic larynx |
Data collection
A solid-state high-resolution manometer with 36 sensors and outer diameter of 4.2 mm was used (ManoScan360 High Resolution Manometry System, Sierra Scientific Instruments, Los Angeles, CA). The catheter was calibrated before each use according to manufacturer specifications. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Sierra Scientific Instruments). A small amount of topical 2% viscous lidocaine hydrochloride was applied to the catheter to ease catheter insertion. Once the catheter was in position, participants rested for approximately five minutes prior to performing experimental swallows.
All participants swallowed three to five 10 cc water boluses delivered to the oral cavity by syringe. TL subjects also swallowed 5 and 20 cc liquid boluses. Data from control subjects were not collected specifically for this study and thus multiple trials at each bolus volume were not available for analysis; only 10 cc water swallows were analyzed. Swallows were performed with the head in a neutral position. Participants held the bolus in the oral cavity until cued to swallow by the experimenter.
Experimental variables
Data were extracted using a customized MATLAB program (The MathWorks, Inc., Natick, MA) described previously (15). Using the customized data extraction program, five regions/events of interest were identified for each swallow: velopharynx, mesopharynx, upper esophageal sphincter (UES), pre-UES opening pressure peak, and post-closure UES pressure peak. A user selects regions corresponding to the velopharynx, inferior boundary of UES pressure, pre-UES opening pressure peak, and post-closure pressure peak. The mesopharynx (which includes the area at the levels of the tongue base to the superior border of the UES) and UES are then identified automatically.
Maximum pressure, rise time, fall time, duration of pressure above baseline, and rise rate are calculated for the velopharynx and mesopharynx. The UES zone contains three events of interest: the pre-swallow baseline pressure and pressure declination, the duration of low pressure during UES muscular relaxation, and the time of repressurization as the UES restores muscle tone post-swallow. For the pre-opening and post-closure UES pressure peak regions, maximum pressure, rise time, and rise rate are calculated; for the period of UES relaxation, minimum pressure, duration from pre-opening pressure peak to post-closure pressure peak (also termed UES activity time), and nadir pressure duration (bolus passage time) are calculated. In the calculation of nadir pressure duration, all sensors in the UES are first combined and then the second-order derivative is used to find the onset and offset times. Total swallow duration is defined as the time between onset of velopharyngeal pressure and the post-closure UES pressure peak. For all five regions, a three-dimensional integral, defined as the total pressure generated across all sensors in the area of interest, was calculated (15).
Statistical analysis
Timing and pressure variables for the velopharynx, mesopharynx, and upper esophageal sphincter (UES) were extracted and compared between TL and control subjects using independent samples t-tests. If data did not meet assumptions of parametric testing, a Wilcoxon-Mann-Whitney rank sum test was performed. Although multiple t-tests were performed, corrections to the significance level were not made in order to decrease the likelihood of type II error. One-way repeated measures analysis of variance (ANOVA) was performed to determine if pressure and timing variables differed across bolus volume within TL subjects. If data did not meet assumptions of parametric testing, repeated measures ANOVA on ranks was performed. All tests were two-tailed with a significance level of α=0.05. The Holm-Sidak correction was used to determine the significance level for pairwise comparisons within the repeated measures ANOVA.
RESULTS
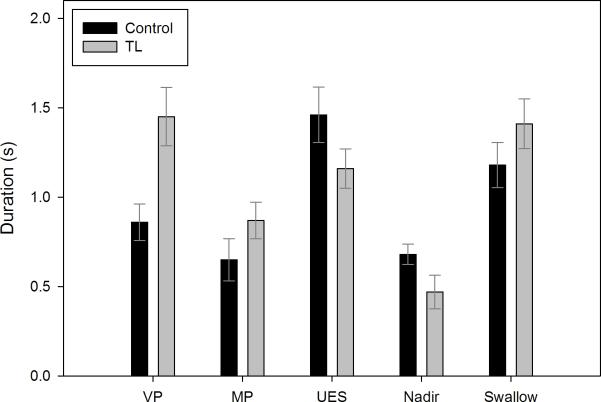
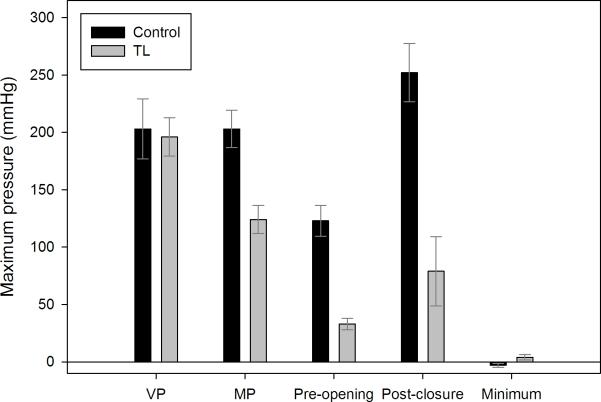
Summary data comparing TL and control subjects are presented in table 2. Compared to controls, TL subjects exhibited increased duration of velopharyngeal pressure (p=0.012) (figure 1) and slower velopharyngeal pressure rise rate (p=0.005); there were no differences in maximum velopharyngeal pressure (p=0.821) (figure 2) or total pressure generated at the velopharynx (p=0.163).
Table 2.
Summary data comparing total laryngectomy and control subjects.
| Parameter | TL | Control | p-value |
|---|---|---|---|
| Velopharynx | |||
| Duration (s) | 1.45 ± 0.40 | 0.86 ± 0.25 | 0.012 |
| Rise rate (mmHg/s) | 306 ± 127 | 1023 ± 479 | 0.005 |
| Maximum pressure (mmHg) | 196 ± 41 | 203 ± 64 | 0.821 |
| 3D integral (mmHg*s) | 272 ± 85 | 203 ± 73 | 0.163 |
| Mesopharynx | |||
| Duration (s) | 0.87 ± 0.25 | 0.65 ± 0.29 | 0.093 |
| Rise rate (mmHg/s) | 352 ± 158 | 600 ± 343 | 0.093 |
| Maximum pressure (mmHg) | 124 ± 30 | 203 ± 40 | 0.003 |
| 3D integral (mmHg*s) | 125 ± 57 | 138 ± 33 | 0.485 |
| Pre-opening UES pressure peak | |||
| Maximum pressure (mmHg) | 33 ± 12 | 123 ± 33 | 0.002 |
| 3D integral (mmHg*s) | 63 ± 26 | 269 ± 82 | 0.002 |
| Post-closure UES pressure peak | |||
| Maximum pressure (mmHg) | 79 ± 74 | 252 ± 62 | 0.001 |
| 3D integral (mmHg*s) | 122 ± 93 | 400 ± 137 | 0.002 |
| Upper esophageal sphincter | |||
| Minimum pressure (mmHg) | 4 ± 6 | −3 ± 4 | 0.054 |
| Activity time (s) | 1.16 ± 0.27 | 1.46 ± 0.38 | 0.147 |
| Nadir duration (s) | 0.47 ± 0.23 | 0.68 ± 0.14 | 0.086 |
| 3D integral (mmHg*s) | 66 ± 18 | 231 ± 162 | 0.015 |
| Total swallow duration (s) | 1.41 ± 0.34 | 1.18 ± 0.31 | 0.244 |
Values are presented as mean ± standard deviation. UES = upper esophageal sphincter; 3D = three-dimensional.
Figure 1.
Comparison of durations for each manometric event of interest between control and total laryngectomy (TL) subjects. Bars represent mean duration and error bars represent standard error of the mean. VP = velopharynx; MP = mesopharynx; UES = upper esophageal sphincter.
Figure 2.
Comparison of maximum pressure for each region of interest between control and total laryngectomy (TL) subjects. Bars represent mean duration and error bars represent standard error of the mean. VP = velopharynx; MP = mesopharynx.
TL subjects exhibited lower maximum mesopharyngeal pressure (p=0.003) (figures 1, 2). There were also trends towards slower mesopharyngeal pressure rise rate (p=0.093) and longer duration of pressure above baseline (p=0.093). There was no difference in total pressure generated (p=0.886).
Maximum (p=0.002) and total pressure generated at the UES (p=0.002) prior to UES opening was higher in controls (figure 2). Similarly, maximum (p=0.001) and total pressure generated (p=0.002) at the UES after UES closure were higher in control subjects. Average nadir pressure during UES opening remained positive in most TL subjects while reaching subatmospheric levels in controls (p=0.054).
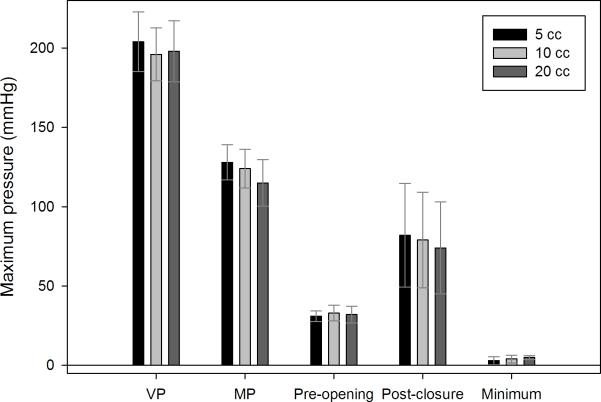
Summary data evaluating changes across bolus volumes within TL subjects are presented in table 3 and figure 3. Few differences were observed. Mesopharyngeal pressure rise rate (p=0.011) decreased with increasing bolus volume. Maximum mesopharyngeal pressure (p=0.032) decreased with increasing bolus volume. A similar trend was observed for the post-closure UES pressure peak, though this was not statistically significant (p=0.125). Changes across bolus volume were not observed at the velopharynx.
Table 3.
Summary data evaluating changes in manometric parameters due to bolus characteristics within total laryngectomy subjects.
| Parameter | 5 cc liquid | 10 cc liquid | 20 cc liquid | p-value |
|---|---|---|---|---|
| Velopharynx | ||||
| Duration (s) | 1.39 ± 0.49 | 1.45 ± 0.40 | 1.51 ± 0.41 | 0.319 |
| Rise rate (mmHg/s) | 448 ± 349 | 306 ± 127 | 278 ± 99 | 0.270 |
| Maximum pressure (mmHg) | 204 ± 46 | 196 ± 41 | 198 ± 47 | 0.142 |
| 3D integral (mmHg*s) | 270 ± 79 | 272 ± 85 | 281 ± 105 | 0.652 |
| Mesopharynx | ||||
| Duration (s) | 0.89 ± 0.31 | 0.88 ± 0.25 | 0.88 ± 0.35 | 0.946 |
| Rise rate (mmHg/s) | 387 ± 165 | 352 ± 158 | 285 ± 161 | 0.011 |
| Maximum pressure (mmHg) | 128 ± 27 | 124 ± 30 | 115 ± 36 | 0.032 |
| 3D integral (mmHg*s) | 132 ± 58 | 125 ± 57 | 106 ± 41 | 0.328 |
| Pre-opening UES pressure peak | ||||
| Maximum pressure (mmHg) | 31 ± 8 | 33 ± 12 | 32 ± 13 | 0.892 |
| 3D integral (mmHg*s) | 58 ± 30 | 63 ± 26 | 62 ± 20 | 0.831 |
| Post-closure UES pressure peak | ||||
| Maximum pressure (mmHg) | 82 ± 80 | 79 ± 74 | 74 ± 71 | 0.125 |
| 3D integral (mmHg*s) | 125 ± 103 | 122 ± 93 | 124 ± 100 | 0.697 |
| Upper esophageal sphincter | ||||
| Minimum pressure (mmHg) | 3 ± 6 | 4 ± 6 | 5 ± 3 | 0.500 |
| Activity time (s) | 1.02 ± 0.35 | 1.16 ± 0.27 | 1.16 ± 0.35 | 0.695 |
| Nadir duration (s) | 0.49 ± 0.23 | 0.47 ± 0.23 | 0.36 ± 0.20 | 0.335 |
| 3D integral (mmHg*s) | 56 ± 22 | 66 ± 18 | 68 ± 26 | 0.355 |
| Total swallow duration (s) | 1.35 ± 0.33 | 1.41 ± 0.34 | 1.41 ± 0.29 | 0.580 |
Values are presented as mean ± standard deviation. UES = upper esophageal sphincter.
Figure 3.
Comparison of maximum pressure for each region of interest across bolus volume within the total laryngectomy subjects. Bars represent mean duration and error bars represent standard error of the mean. VP = velopharynx; MP = mesopharynx.
DISCUSSION
Using HRM, we found changes in pressure and timing variables between TL and control subjects at all levels of the pharynx. Increased duration of velopharyngeal pressure above baseline may serve to increase the propulsive force mediating bolus movement towards the esophagus.
Overall, group differences were most evident at the level of the UES and included lower pre-opening and post-closure pressure peaks as well as the aforementioned preserved positive pressure during opening in subjects with TL. The difference in pressure patterns can be further elucidated by evaluating the 3D integral, which in the case of the UES, is reflective of the drop in pressure during bolus passage. For TL subjects, the decrease is less prominent and thus the 3D integral is lower.
Prolongation of the total swallow is consistent with the absence of aspiration risk. By separating the pharynx into different conduits for air and food, pharyngeal contractions can continue as long as is necessary to ensure bolus clearance, without concern for respiration. Lack of hyolaryngeal excursion likely also contributes to prolonged swallow duration. Simultaneous evaluation of deglutition and respiration may provide clarification.
There was also notable variability in the appearance of the spatiotemporal plots across TL subjects (figure 4). Decreased pressure at the UES was a common feature, occurring at baseline, the pre-opening pressure peak, and the post-closure pressure peak. This can be attributed to alteration of the muscular connections at the level of the UES, particularly disruption of the cricopharyngeus. Interestingly, some TL subjects displayed what could be termed a “common cavity pressure” between the velopharynx and UES (figures 4, 5), likely attributable to the absent hyolaryngeal excursion and decreased peristaltic contractions of the pharyngeal constrictors. Certainly larynx removal and mesopharyngeal closure can change the compliance of the pharyngeal outlet to create a zone of high resistance. The high velopharyngeal pressure and common cavity pressure effect will manifest as the patient swallows larger boluses. This can be contrasted to a swallow from a control subject where a clear peak corresponding to the mesopharynx is observed (figure 5). Decreased hyolaryngeal excursion is also compensated for in part by increasing UES compliance, as each TL includes a cricopharyngeal myotomy.
Figure 4.
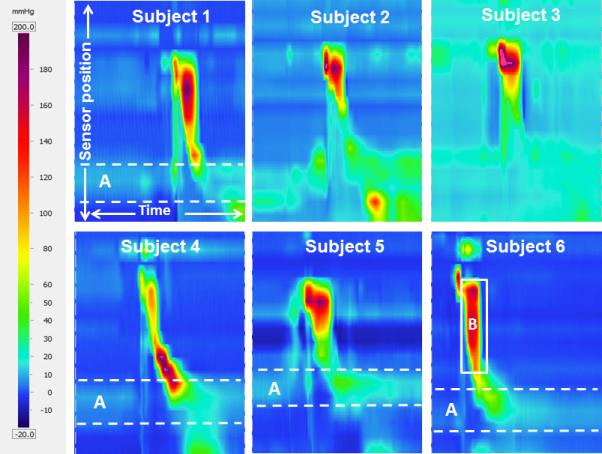
Sample high-resolution manometry spatiotemporal plots representing one 10 cc swallow from each of the total laryngectomy subjects, demonstrating variability in the appearance of functional swallows. Sensor position is on the y- (vertical) axis, with sensors at the velopharynx at the top of the image and sensors in the esophagus at the bottom. Time is on the x- (horizontal) axis; each image represents 5 seconds of data collection. Pressure is represented by color as depicted on the left of the figure. A) Dashed white lines outline the zone which typically exhibits high tonic resting pressure at the upper esophageal sphincter; the high pressure zone is absent here. B) The solid white box outlines a region of common cavity pressure, where pressure is elevated and approximately uniform throughout the neopharynx.
Figure 5.
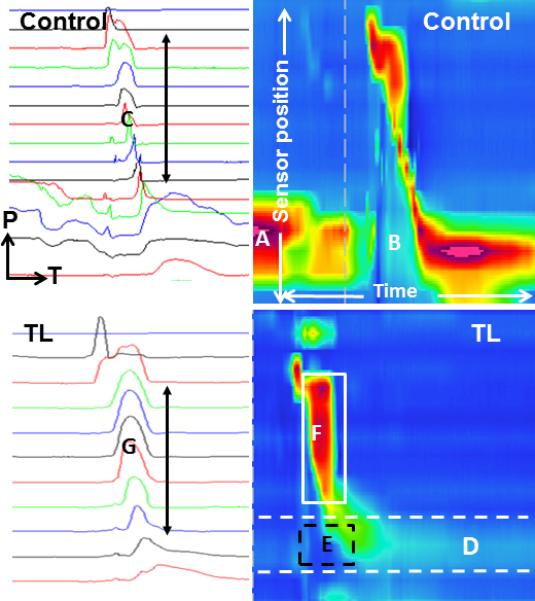
Comparison of two-dimensional and spatiotemporal plots obtained from a control subject (above) and total laryngectomy subject (below). Two-dimensional plots are on the left, with each line representing one pressure tracing from one sensor; pressure (P) is on the y-axis, time (T) is on the x-axis, and more caudal sensors are at the bottom of the image. Spatiotemporal plots are on the right, with sensor position on the y-axis, time on the x-axis, and pressure represented by color. Each image represents 5 seconds of data collection. The vertical dashed line in the upper spatiotemporal plot is an artifact marking a time window from the data collection program. The control subject demonstrates a high resting upper esophageal sphincter (UES) pressure (A), low nadir pressure during bolus passage (B), and variation in shape of the pressure curves generated along the pharynx (C). The total laryngectomy subject demonstrates low resting UES tone (D, dashed white lines), maintenance of positive UES pressure during bolus passage (E, dashed black box), and a common cavity pressure (F, solid white box) which manifests as uniform pressure peaks along the neopharynx (G).
Three prior studies have used HRM to evaluate changes in pharyngeal pressure profiles due to bolus volume in healthy young adults (16-18). Hoffman et al. evaluated 12 healthy adults aged 20.9±1.8 years swallowing 1, 5, 10, and 20 cc water boluses and observed increasing maximum velopharyngeal pressure and duration of velopharyngeal pressure above baseline with increasing bolus volume (16). At the mesopharynx, there was a trend towards decreasing rise rate and maximum pressure with increasing volume. Increasing duration of UES opening as well as increased total swallow duration also occurred with increasing bolus volume. In a study of 34 healthy adults (24.3±5.9 years) swallowing 3, 5, and 10 ml water boluses, Lin et al. reported increased duration of UES relaxation duration and decreased maximum mesopharyngeal pressure with increasing bolus volume (17). No changes were observed for maximum pre-opening or post-closure UES pressure, nor for mesopharyngeal pressure rise rate or duration. Lastly, Matsubara et al. evaluated 30 healthy adults (25.3±3.6 years) swallowing 2, 5, and 10 cc hot and cold liquid boluses (18). Bolus volume did not affect maximum swallowing pressure at the velopharynx or meso-mesopharynx. Duration of UES relaxation was longer with 10 versus 2 ml cold water swallows.
It is important to note that the subjects were older in our study than in previous studies using HRM. Additionally, differences in the specific bolus volumes evaluated are likely also relevant. Despite these differences, a few comparisons can be made. First, similar decreases in mesopharyngeal pressure rise rate and maximum mesopharyngeal pressure as bolus volumes increased were observed in our sample and two of the prior studies (16-17). Second and most interesting, an increase in UES nadir duration with increasing bolus volume was not observed in our TL subjects, perhaps a direct consequence of eliminating hyolaryngeal excursion. Our subjects, though, did not experience any change in dysphagia symptoms with the increasing bolus volume, indicating that successful swallow is possible even with the altered physiology after TL. Further investigation in additional subjects, likely incorporating simultaneous videofluoroscopy, may be beneficial in evaluating this phenomenon.
It is currently thought that the major change occurring in the pharynx after a TL is an increase in resistance (9). McConnel et al. stated that to overcome this resistance, higher propulsive forces are needed, and that TL patients produce higher pressures at the tongue to achieve this (9). Notably, these observations are based on traditional manometry which can be limited in its ability to capture all salient pressure changes during the pharyngeal swallow. While traditional manometry uses 1 to 3 pressure sensors, HRM uses 36 pressure sensors placed 1 cm apart to capture key pressure and timing events along the length of the entire pharynx, including areas such as the velopharynx and base of tongue. Due to these advantages, we see changes in pressure dynamic in the velopharynx, mesopharynx, and UES after laryngectomy. HRM provides the needed precision to study the entire pharynx and has been used to provide previously unexamined anatomic and physiologic information about normal swallowing along the velopharynx and upper esophagus (13). It has also been used to successfully reveal subtle differences during deglutition due to head position and bolus size, not previously detected by traditional manometry (16, 19-20). These characteristics make HRM the ideal tool to study the anatomy and physiology of the pharyngeal swallow, the phase of most interest in TL patients.
Invasive, costly, and potentially morbid interventions are often required to treat dysphagia in post-TL patients. Non-surgical treatments such as diet modification and swallowing therapy are often used, but are not always successful (7). When more conservative management fails, invasive treatments such as botulinum toxin injection, dilation, or surgical treatment must be performed (7, 21). In some patients, extensive surgery with flap reconstruction is necessary (4, 22-23). These invasive procedures subject patients to further major surgery that has significant morbidity associated with the procedure itself and the donor site, and necessitate long hospital stays with the potential for complications. To date, these interventions are based on diagnostic techniques that fail to accurately depict swallowing physiology in TL patients. Accordingly, an approach that provides a more thorough understanding of this physiology may enhance our ability to diagnose and characterize the swallowing deficits. Quantification of pharyngeal pressures using HRM could fill this role and, as a result, provide better targets for therapeutic and surgical intervention. This will potentially limit unnecessary and morbid procedures in some patients while guiding the application of interventions in other patients in a more informed fashion.
This study has several limitations which will be the focus of future investigations. Most notably, a small sample size was included. This is in part attributable to the strict inclusion and exclusion criteria employed, as our objective was to describe the pharyngeal pressure profiles in a homogeneous group of post-TL subjects without any evidence of dysphagia. As dysphagia is common after TL, recruiting subjects who met this criterion was a challenge. Excluding patients who had undergone flap reconstruction or chemoradiation further limited our sample size, but did promote sample homogeneity. Second, the control subjects only completed 10 cc liquid swallows, thus precluding a direct comparison of the effects of bolus volume between our two subject groups. While comparisons can be made to prior studies using HRM evaluating changes in pharyngeal pressures due to bolus volume, a direct comparison to the age- and sex-matched controls in this study would have been preferable. Slight changes in physiology may have also occurred due to the use of experimenter-cued swallows, but all of the subjects in this study swallowed in the same manner. Lastly, it is important to evaluate how pressure profiles are altered in TL patients with dysphagia as well as patients who have undergone flap reconstruction or chemoradiotherapy. This is beyond the scope of this preliminary study, but will be the focus of future research.
The implications of better understanding swallowing physiology in TL patients are vast. Manometric data could be used to diagnose the underlying cause of dysphagia with specific information on anatomic site and failure of appropriate pressure gradients to safely and effectively clear the bolus. Such information could also be used to guide swallowing therapy by determining which anatomic locations are of greatest importance for a functional post-TL swallow and tailoring the therapy to those sites. In the future, enhanced understanding of this altered physiology could impact procedural interventions, surgical approach, and reconstruction.
ACKNOWLEDGEMENTS
This study was funded by National Institutes of Health grant number R33 DC011130 from the National Institute on Deafness and other Communicative Disorders.
Footnotes
Financial disclosures: None.
Conflict of interest: None.
REFERENCES
- 1.Maclean J, Cotton S, Perry A. Post-laryngectomy: It's hard to swallow: an Australian study of prevalence and self-reports of swallowing function after total laryngectomy. Dysphagia. 2009 Jun;24(2):172–9. doi: 10.1007/s00455-008-9189-5. [DOI] [PubMed] [Google Scholar]
- 2.Ackerstaff AH, Hilgers FJM, Aaronson NK, Balm AJM. Communication, functional disorders and lifestyle changes after total laryngectomy. Clin Otolaryngol. 1994;19:295–300. doi: 10.1111/j.1365-2273.1994.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 3.Maclean J, Cotton S, Perry A. Dysphagia following a total laryngectomy: the effect on quality of life, functioning, and psychological well-being. Dysphagia. 2009 Sep;24(3):314–21. doi: 10.1007/s00455-009-9209-0. [DOI] [PubMed] [Google Scholar]
- 4.Sweeny L, Golden JB, White HN, Magnuson JS, Carroll WR, Rosenthal EL. Incidence and outcomes of stricture formation post-laryngectomy. Otolaryngol Head Neck Surg. 2012 Mar;146(3):395–402. doi: 10.1177/0194599811430911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong E, Isman K, Dooley P, Brine D, Riley N, Dentice R, King S, Khanbhai F. An investigation into the quality of life of individuals after laryngectomy. Head Neck. 2001 Jan;23(1):16–24. [PubMed] [Google Scholar]
- 6.Ward EC, Bishop B, Frisby J, Stevens M. Swallowing outcomes following laryngectomy and pharyngolaryngectomy. Arch Otolaryngol Head Neck Surg. 2002;128:181–6. doi: 10.1001/archotol.128.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Landera M, Lundy D, Sullivan P. Dysphagia after total laryngectomy. Perspect Swallowing Swallowing Disorders. 2010 Jun;19:39–44. [Google Scholar]
- 8.Chone CT, Spina AL, Barcellos IH, Servin HH, Crespo AN. A prospective study of long-term dysphagia following total laryngectomy. B-ENT. 2011;7(2):103–9. [PubMed] [Google Scholar]
- 9.McConnel FM, Mendelsohn MS, Logemann JA. Examination of swallowing after total laryngectomy using manofluorography. Head Neck Surg. 1986 Sep-Oct;9(1):3–12. doi: 10.1002/hed.2890090103. [DOI] [PubMed] [Google Scholar]
- 10.Balfe DM. Dysphagia after laryngeal surgery: radiologic assessment. Dysphagia. 1990;5(1):20–34. doi: 10.1007/BF02407390. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus C, Logemann JA, Shi G, Kahrilas P, Pelzer H, Kleinjan K. Does laryngectomy improve swallowing after chemoradiotherapy? A case study. Arch Otolaryngol Head Neck Surg. 2002 Jan;128(1):54–7. doi: 10.1001/archotol.128.1.54. [DOI] [PubMed] [Google Scholar]
- 12.Hutcheson KA, Alvarez CP, Barringer DA, Kupferman ME, Lapine PR, Lewin JS. Outcomes of elective total laryngectomy for laryngopharyngeal dysfunction in disease-free head and neck cancer survivors. Otolaryngol Head Neck Surg. 2012 Apr;146(4):585–90. doi: 10.1177/0194599811432264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takasaki K, Umeki H, Enatsu K, Tanaka F, Sakihama N, Kumagami H, Takahashi H. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008 Oct;118(10):1729–32. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi RC, St Rose S, Roe JW, Khan AS, Pepper C, Nutting CM, Clarke PM, Kerawala CJ, Rhys-Evans PH, Harrington KJ, Kazi R. Validation of the Sydney Swallow Questionnaire (SSQ) in a cohort of head and neck cancer patients. Oral Oncol. 2010 Apr;46(4):e10–4. doi: 10.1016/j.oraloncology.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-resolution manometry data. Laryngoscope. 2013 Jul;123(7):1746–53. doi: 10.1002/lary.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. Laryngoscope. 2010;120(12):2367–73. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin T, Xu G, Dou Z, Lan Y, Yu F, Jiang L. Effect of bolus volume on pharyngeal swallowing assessed by high-resolution manometry. Physiol Behav. 2014 Apr 10;128:46–51. doi: 10.1016/j.physbeh.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara K, Kumai Y, Samejima Y, Yumoto E. Swallowing pressure and pressure profiles in young healthy adults. Laryngoscope. 2014 Mar;124(3):711–7. doi: 10.1002/lary.24311. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012 Sep;27(3):418–26. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCulloch T, Hoffman MR, Ciucci MR. High resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119(6):369–76. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crary MA, Glowasky AL. Using botulinum toxin A to improve speech and swallowing function following total laryngectomy. Arch Otolaryngol Head Neck Surg. 1996 Jul;122(7):760–3. doi: 10.1001/archotol.1996.01890190056013. [DOI] [PubMed] [Google Scholar]
- 22.Iteld L, Yu P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J Reconstr Microsurg. 2007 Aug;23(6):339–45. doi: 10.1055/s-2007-992343. [DOI] [PubMed] [Google Scholar]
- 23.Withrow KP, Rosenthal EL, Gourin CG, Peters GE, Magnuson JS, Terris DJ, et al. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. Laryngoscope. 2007 May;117(5):781–4. doi: 10.1097/MLG.0b013e3180332e39. [DOI] [PubMed] [Google Scholar]