Abstract
Background
Some oral probiotics have been shown to prevent necrotizing enterocolitis (NEC) and decrease mortality effectively in preterm very low birth weight (PVLBW) infants. However, it is unclear whether a single probiotic or a mixture of probiotics is most effective for the prevention of NEC.
Objective
A meta-analysis was conducted by reviewing the most up to date literature to investigate whether multiple strains probiotics are more effective than a single strain in reducing NEC and death in PVLBW infants.
Data sources
Relevant studies were identified by searches of the MEDLINE, EMBASE, and Cochrane CENTRAL databases, from 2001 to 2016.
Data extraction and synthesis
The inclusion criteria were randomized controlled trials of any enteral probiotic supplementation that was initiated within the first 7 days and continued for at least 14 days in preterm infants (≤ 34 weeks’ gestation) and/or those of a birth weight ≤1500 g.
Results
A total of 25 trials (n = 7345 infants) were eligible for inclusion in the meta-analysis using a fixed-effects model. Multiple strains probiotics were associated with a marked reduction in the incidence of NEC, with a pooled OR of 0.36 (95% CI, 0.24–0.53; P < .00001). Single strain probiotic using Lactobacillus species had a borderline effect in reducing NEC (OR of 0.60; 95% CI 0.36–1.0; P = .05), but not mortality. Multiple strains probiotics had a greater effectiveness in reducing mortality and were associated with a pooled OR of 0.58 (95% CI, 0.43–0.79; P = .0006). Trials using single strain of Bifidobacterium species and Saccharomyces boulardii did not reveal any beneficial effects in terms of reducing NEC or mortality.
Conclusion
This updated report found that multiple strains probiotics appear to be the most feasible and effective strategy for the prevention of NEC and reduction of mortality in PVLBW neonates. Further clinical trials should focus on which probiotic combinations are most effective.
Introduction
Necrotizing enterocolitis (NEC) remains the most common acquired gastrointestinal and surgical emergency in preterm very low birth weight (PVLBW) infants. The incidence of NEC ≥ stage 2 varies from 2.6% to 28.0% of PVLBW infants, with associated mortality ranging between 16% and 42% [1,2]. Preterm infants with NEC are at risk of long-term complications, including neurodevelopmental impairment, short bowel syndrome, and growth retardation [3,4]. Though there are significant morbidities associated with NEC, few safe and effective therapies are available to prevent this disastrous condition [5]. Available strategies for primary prevention of NEC include antenatal glucocorticoids, breast milk feeding, fluid restriction, and the use of probiotics [4,5]. However, during the past decade, only probiotics have been studied extensively in terms of the prevention of neonatal NEC in prospective randomized control trials (RCTs). Many meta-analyses of RCTs confirmed that oral probiotics effectively prevent NEC and death [6–14]. Although systemic reviews of single strain has been conducted [12,14], clinicians are facing challenges in assessing which probiotics are most effective in PVLBW infants. Unfortunately, published studies have used a variety of different single or multiple strains probiotic with different target populations. Relatively little is known about whether single strain probiotic alone or multiple strains are most effective in the prevention of NEC and death in PVLBW infants.
Recent articles have shown a link between NEC and a lack of microbiotal diversity [15–17]. One review article suggested that mixtures of probiotics were more beneficial than single strains for gut and immune function [18]. Our animal model also revealed that multiple strains probiotics were more effective in the prevention of NEC [19]. Furthermore, NEC does not usually occur after a gestation age of 34 weeks. We thus hypothesize that multiple strains probiotics are more effective in the prevention of NEC and death for preterm infants below a gestation age of 34 weeks. We conducted a meta-analysis by systematically reviewing the most up to date evidence available in the literature to investigate whether multiple strains probiotics are more effective than single-strain probiotics for reducing NEC and death in preterm infants.
Materials and methods
Search strategy and study selection
This meta-analysis was conducted according to PRISMA guidelines (http://www.prisma-statement.org/) (S1 PRISMA Checklist). Any trials following prespecified criteria were enrolled in the analysis: (a) RCTs involving PVLBW infants (≤ 34 weeks' gestation or birth weight ≤ 1500 g by mean or median) and reporting on NEC ≥ stage 2 by the Modified Bell staging criteria [20] and/or death, and (b) enteral administration of any probiotic commenced within the first 7 days of life and continued for at least 28 days. We searched the PubMed, Embase, and CBM databases for studies published from January 2001 to June 2016, with the terms “extremely low birth weight infant” or “very low birth weight infant” or “premature infant” or “preterm infant” and “Lactobacillus” or “probiotics” or “Saccharomyces” or “Bifidobacterium”. There was no language restriction. Similar studies and review articles reference lists in the references were also searched. The primary outcome was the efficacy of probiotic supplementation in preventing NEC ≥ stage 2. The secondary outcome was mortality before the infant was discharged.
Data extraction
Two authors (H.Y.C. and H.C.L.) independently conducted the literature search. Information regarding study inclusion, study design, key characteristics, and outcomes was extracted independently by the 2 reviewers using a standardized data collection form. Inconsistencies were resolved by involving a third author (J.H.C.) or by discussion between all authors.
Statistical analysis
To assess the between-study heterogeneity more precisely, both the χ2-based Q statistic test (Cochran Q statistic) to test for heterogeneity and the I2 statistic to quantify the proportion of the total variation attributable to heterogeneity were used. For each meta-analysis, the Cochran Q statistic was first calculated to assess the heterogeneity of the included trials. For P values less than .10, the assumption of homogeneity was deemed invalid. The outcome for our topic was binary in each trial; for example, whether NEC (or death) or not. The effects of probiotics for NEC (or death) were measured as odds ratios (ORs). For each trial, the OR is shown with a 95% confidence interval (95% CI). For NEC or mortality, a forest plot was used. For the meta-analysis, both the fixed-effects model and the random-effects model were considered. Homogeneity existed between almost all trials in our pooled study. The I2 of all trials or subgrouped trials was approximately zero. The fixed-effects model was considered to pool the estimators. Publication bias was investigated by funnel plot, and an asymmetric funnel plot suggested possible publication bias. Another way to explore the publication bias was the use of Egger’s regression test, which evaluated whether the intercept was significant. The significance level in the association test and the publication bias of our research were 0.05. Statistical analyses were performed using version 2 of the Comprehensive Meta-Analysis program (USA, 2006) and Review Manager (Cochrane Collaboration, Nordic Cochrane Centre) 5.1.
Results
Description of studies
In total, there were 123 studies identified through electronic searches; 39 trials met the inclusion criteria and were selected to be read in full text (Fig 1). Eighteen studies were excluded for the following reasons: two studies had a lack of relevant data [21,22], one study used both lactoferrin and probiotics [23], two study used both prebiotics and probiotics [24,25], five studies did not assess the NEC outcomes [26–30], the full text could not be extracted in four studies [31–34], one study was a report of additional data from a previous paper [35], and three trials were conducted before 2000 [36–38]. After eliminating these trials, this review included data from 25 RCTs [39–63]. Two studies only enrolled infants with a birth weight under 1500 g [51,57]. A total of 7345 infants were included, 3679 in the probiotics group and 3666 in the control group. Each study was evaluated by Jadad score (Table 1). Of the 25 studies included in the analyses, fourteen studies used a single strain of probiotic (5 used a Lactobacillus strain [39,43,49,51,58], 6 used a Bifidobacterium strain [44,48,59,61–63], 3 used Saccharomyces boulardii [40,54,56]). Of the 5 Lactobacillus trials, 2 used Lactobacillus rhamnosus strain [39,43], 2 used Lactobacillus reuteri strain [51,58], and 1 used Lactobacillus sporogenes strain [49]. Of the 6 Bifidobacterium trials, 2 used Bifidobacterium lactis [44,48], 2 used Bifidobacterium breve [61,63], 1 used Bifidobacterium bifidus [59], and 1 used Bifidobacterium clausii [62]. Eleven studies used multiple strains probiotics, including 8 studies that utilized a combination of Bifidobacterium strain and Lactobacillus strain [42,45–47,50,52,57,60], and 3 studies that used a mixture of multiple probiotics strains [41,53,55].
Fig 1. Flowchart showing the selection of studies for inclusion in the meta-analysis.
Table 1. Characteristics of the trials included in the analysis.
| Study | Participants | Gestation or birth weight | Probiotic agents | Outcomes | Jadad score | |
|---|---|---|---|---|---|---|
| Probiotics | Placebo | |||||
| Dani, 2002 [39] | 295 | 290 | < 33 wk, < 1500 g | L. rhamnosus GG | NEC | 4 |
| Costalos, 2003 [40] | 51 | 36 | 28–32 wk | S. boulardii | NEC | 5 |
| Bin-Nun, 2005 [41] | 72 | 73 | < 1500 g | B. infantis, B. bifidus, Strepto. thermophiles | NEC, mortality | 3 |
| Lin, 2005 [42] | 180 | 187 | < 1500 g | L. acidophilus, B. bifidum | NEC, mortality | 5 |
| Manzoni, 2006 [43] | 39 | 41 | < 1500 g | L. rhamnosus GG | NEC, mortality | 4 |
| Stratiki, 2007 [44] | 41 | 36 | 27–37wk | B. lactis | NEC | 3 |
| Lin, 2008 [45] | 217 | 217 | < 34 wk, < 1500 g | L. acidophilus, B. bifidum | NEC, mortality | 5 |
| Rouge, 2009 [46] | 45 | 49 | < 32 wk, < 1500 g | B. longum, L. rhamnosus GG | NEC, mortality | 5 |
| Samanta, 2009 [47] | 91 | 95 | < 32 wk, < 1500 g | B. infantis, B. bifidum, B. longum, L. acidophilus | NEC, mortality | 3 |
| Mihatsch, 2010 [48] | 91 | 89 | < 30 wk, < 1500 g | B. lactis | NEC, mortality | 4 |
| Sari, 2011 [49] | 110 | 111 | < 33 wk, < 1500 g | L. sporogenes | NEC, mortality | 4 |
| Braga, 2011 [50] | 119 | 112 | 750–1499 g | L. casei, B. breve | NEC, mortality | 5 |
| Rojas, 2012 [51] | 176 | 184 | < 1500 g* | L. reuteri | NEC | 5 |
| Al-Hosni, 2012 [52] | 50 | 51 | 501–1000g | L. rhamnosus GG, B. infantis | NEC, mortality | 3 |
| Fernandez-Carrocera, 2013 [53] | 75 | 75 | < 1500 g | L. acidophilus, L. rhamnosus, L. casei, L. plantarum, B. infantis, Strepto. thermophillus | NEC, mortality | 5 |
| Demirel, 2013 [54] | 135 | 136 | ≤ 32 wk, ≤ 1500 g | S. boulardii | NEC, mortality | 4 |
| Jacobs, 2013 [55] | 548 | 551 | < 32 wk, < 1500 g | B. infantis, Strepto. thermophilus, B. lactis | NEC, mortality | 4 |
| Serce, 2013 [56] | 104 | 104 | ≤ 32 wk, ≤ 1500g | S. boulardii | NEC, mortality | 4 |
| Roy, 2014 [57] | 11 | 11 | < 1000 g* | B. infantis, Lactobacillus acidophilus, B. lactis | NEC | 4 |
| Oncel, 2014 [58] | 200 | 200 | ≤ 32 wk, ≤ 1500 g | L. reuteri | NEC, mortality | 4 |
| Totsu, 2014 [59] | 153 | 130 | < 1500 g | B. bifidus | NEC, mortality | 4 |
| Saengtawesin 2014 [60] | 31 | 29 | < 34 wk, < 1500 g | L. acidophilus, B. bifidum | NEC, mortality | 3 |
| Patole 2014 [61] | 74 | 76 | < 33 wk | B. breve M-16V | NEC, mortality | 5 |
| Tewari 2015 [62] | 121 | 123 | < 34 wk | B. clausii | NEC, mortality | 4 |
| Costeloe 2016 [63] | 650 | 660 | < 30 wk | B. breve BBG-001 | NEC, mortality | 5 |
L: Lactobacillus; B: Bifidobacterium; S: Saccharomyces; Strepto: Streptococcus; NEC, necrotizing enterocolitis.
*only patients with a birth weight < 1500 g were included in these studies.
Efficacy of all probiotics on NEC
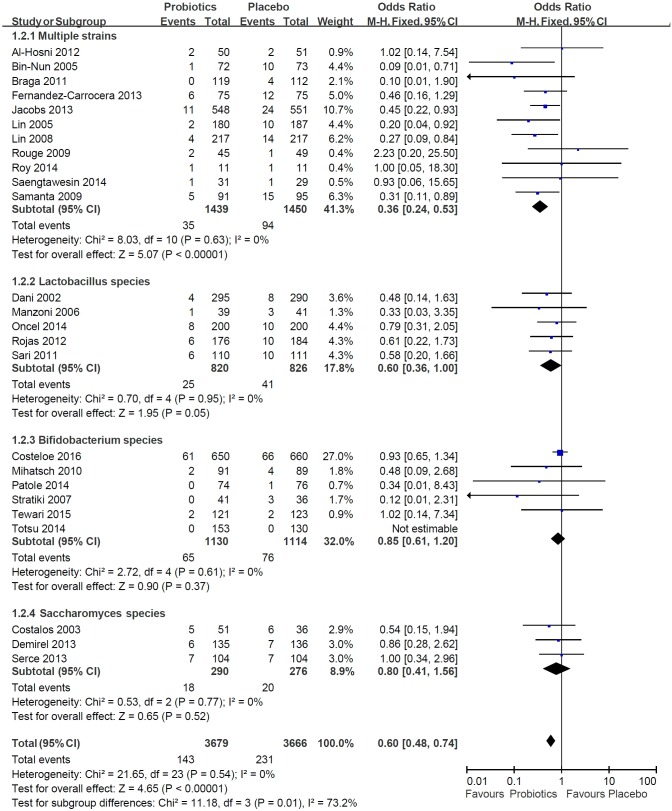
We combined the trials with all probiotics, such as multiple strains probiotics, Lactobacillus, Bifidobacterium, and Saccharomyces. The incidence of NEC stage ≥ 2 was 3.9% in the probiotics group, whereas it was 6.3% in the placebo group. The P-value of heterogeneity was 0.54, and I2 was 0%. The pooled OR was 0.60 with the fixed-effect model and the 95% CI was 0.48–0.74, P < .00001. The probiotics group had a lower risk of developing NEC than the placebo group (Fig 2).
Fig 2. Meta-analysis of the efficacy of probiotics associated with the risk of NEC in preterm infants.
Subgroup analysis: Efficacy of multiple strains probiotics versus single-strain probotics on NEC
In the multiple strains probiotics trials, the incidence of NEC stage ≥ 2 was 2.4% in the probiotics group, whereas in the placebo group it was 6.5%. In the Lactobacillus trials, the developed definite NEC stage ≥ 2 was 3.0% in the Lactobacillus group and 5.0% in the placebo group. In the Bifidobacterium trials, the developed definite NEC stage ≥ 2 was 5.8% in the Bifidobacterium group and 6.8% in the placebo group. In the Saccharomyces trials, the developed definite NEC stage ≥ 2 was 6.2% in the Saccharomyces group and 7.2% in the placebo group.
The meta-analysis showed that the placebo group had a higher risk of developing NEC than the multiple strains probiotics group (pooled OR: 0.36, 95% CI: 0.24–0.53, P < .00001, I2 = 0%). The odds of NEC occurring in the placebo group were higher than those in the single-strain probiotics groups, but these differences did not reach statistical significance (Fig 2). Single strain probiotic using Lactobacillus species had a borderline effect in reducing NEC (OR of 0.60; 95% CI 0.36–1.0; P = .05). Trials using single strain of Bifidobacterium species and Saccharomyces boulardii did not reveal any beneficial effects in terms of reducing NEC.
Efficacy of probiotics on mortality
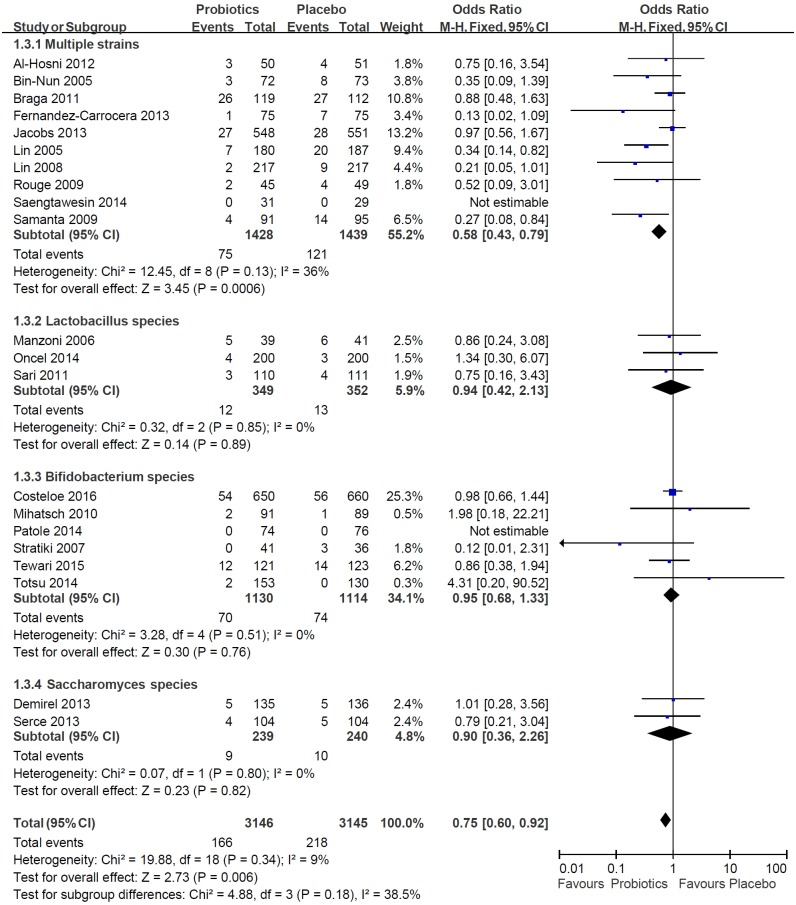
There were 21 trials that discussed the association between mortality and probiotics. The mortality rate in the probiotics group with all the tested probotics, including multiple strains probiotics, Lactobacillus, Bifidobacterium, and Saccharomyces, was 5.3%, whereas in the placebo group it was 6.9%. Probiotics reduced the risk of death by 25% relative to the placebo group (pooled OR: 0.75, 95% CI: 0.60–0.92, P = .006, I2 = 9%) (Fig 3).
Fig 3. Meta-analysis of the efficacy of probiotics associated with the risk of death in preterm infants.
Subgroup: Effect of combined probiotics and single-strain probiotics on mortality
We reported the effects of multiple strains probiotics, Lactobacillus. Bifidobacterium, and Saccharomyces on mortality separately. In the multiple strains probiotics cases, there were 5.3% and 8.4% mortality rates within the treatment and placebo groups, respectively.
Within the multiple strains probiotics group, the P-value of the heterogeneity test was 0.13, and I2 was 36%. The degree of heterogeneity was moderate. The pooled OR between multiple strains probiotics and death was 0.58, and the 95% CI was 0.43 to 0.79 (P-value = .0006). In the single-strain probiotics group, the pooled results in the probiotics group showed no statistical significance in relation to mortality as compared with the placebo group (Fig 3).
Publication bias
A funnel plot was used to show the relationship between effect size and the standard error of estimator for each trial. A symmetric funnel plot would indicate that publication bias did not exist. Egger’s regression test, a weighted linear ordinary least squares regression, was used to explore the publication bias. The funnel plots of NEC and mortality looked generally symmetric separately. Egger’s tests for the intercepts were not statistically significant for NEC (P-value of intercept = .54) or mortality (P-value of intercept = .48). The Egger’s test corresponded with the symmetrical funnel plots. The funnel plots and Egger’s regression indicated no publication bias within our selected trials.
Discussion
Our updated meta-analysis showed that multiple strains probiotics resulted in a marked reduction of the incidence of NEC and of the incidence of mortality in preterm infants ≤ 34 weeks' gestation or of a birth weight ≤ 1500 g.
NEC is a multifactorial disease, and its pathophysiology remains unclear. Several factors appear to contribute to the pathogenesis, including immaturity of multiple intestinal functions, such as gastrointestinal dysmotility, impaired digestive capacity, altered regulation of intestinal blood flow, barrier dysfunction, altered anti-inflammatory control, and impaired host defense. Frequent use of antibiotic therapy and anti-acid medications, followed by enteral feeding, are believed to increase the risk of NEC. Establishing a core microbiota of diverse commensal species is critical of PVLBW infants. Reasons for disruption or delay of this critical process include delivery modes, gestational age, birth weight, infectious diseases, antibiotics therapy, parenteral feeding, feeding type, and hospital period and environment. The dysbiosis of microbial succession [64,65], abnormal bacterial colonization, and lower bacterial diversity [15–17] in PVLBW infants also has been linked to the occurrence of NEC, which is the rationale for the need for probiotic supplements.
Although probiotics are the most promising treatment for reducing NEC, the use of different probiotics in all studies has made the selection of an optimal probiotic regimen difficult. Furthermore, preterm infants range up to ≤ 34 weeks’ gestational age, making it difficult to determine which preterm infants would benefit the most. The 25 trials that were enrolled in our review are summarized in Table 1 [39–53]. Our updated meta-analysis confirmed the results of other systematic reviews, which reported that probiotics prevent NEC and death. However, we focused on preterm infants of ≤ 34 weeks' gestation or of a birth weight ≤ 1500 g, who were a high-risk group for developing NEC or death, who had undergone enteral administration of probiotics commenced within the first 7 days of life and continued for at least 28 days. Our study discovered that multiple strains probiotics resulted in a marked reduction in NEC, which was comparable with results from one recent meta-analysis [12]. The potential mechanisms by which multiple strains probiotics may provide better protection from developing NEC might include increased diversity of the intestinal microbiota and offering healthy bacteria such as Lactobacillus and Bifidobacteria to balance normal microbiota in this vulnerable human population. However, it is unclear whether this is due to synergistic interactions between strains or a consequence of the higher probiotics doses.
Our updated meta-analysis further confirmed the results of other systematic reviews, which reported that probiotics supplements had a significant effect in reducing mortality in preterm infants of ≤ 34 weeks' gestation or of a birth weight ≤ 1500 g. However, after further analysis, 3 trials using a Lactobacillus strain alone, 6 trials using Bifidobacterium alone, and 2 trials using Saccharomyses alone did not reveal a beneficial effect on mortality. On the other hand, analyses from 10 studies showed a greater effectiveness of multiple strains probiotics in reducing mortality in PVLBW infants. It is unclear from this meta-analysis whether this was due to a reduction in NEC-related or sepsis-related deaths or other etiologies. In theory and animal studies, different probiotics may function differently in the modification of the intestinal immune system. Therefore, the effects of multiple strains probiotics probiotics on NEC or death may be acting through the synergetic effect, inhibiting the growth of pathogens, promoting up-regulation of the immune responses, or strengthening the mucosal barrier [17].
Concerns regarding safety issues and complications associated with the use of probiotics in these relatively immunocompromised preterm infants have been debated. However, the reported risk of sepsis due to translocation of the probiotics through the intestinal wall is extremely rare, and no apparent adverse effects were observed in any of the studies. Based on the currently available clinical trial results, probiotics use in preterm infants is generally considered to be safe. We did not analyze the efficacy of probiotics on sepsis, the time to full oral feeding, or the duration of hospitalization, because these items were not the primary or secondary outcomes of the enrolled studies. Only a fraction of the enrolled studies described these outcomes, which could not be analyzed because of publication bias.
There were several possible limitations that warranted careful review in this meta-analysis. First of all, the multiple strains probiotics treatment regimens varied widely. Questions regarding the optimal combination of species and dosing remain unanswered. Further studies directly comparing probiotic mixtures with single strains are warranted. Further research should also identify which multiple strains probiotics might be associated with improved health outcomes or enhance the preparation’s effectiveness. Second, publication bias may have existed for trials using single strain probiotic alone because of the limited study numbers. Probiotic effects are known to be strain specific, statistically and clinically significant benefits could relate to strain-specific differences. Although strainspecific meta-analysis data has been performed [12,14], recommendation cannot be made because of limited RCTs using the same single strain. Furthermore, there is significant heterogeneity among included studies. Some trials with small sample size and inadequate power, NEC or mortality as a secondary outcome are also affected our meta-analysis results. We should also point out that some of the probiotic products or placebos in the control group contained maltodextrin, which according to a recent animal study could increase the incidence of NEC [66]. However, our further analysis showed that probiotics containing maltodextrin had the same effect in terms of the prevention of NEC.
Conclusions
Multiple strains probiotics, a therapeutic modification of the gut microbiota and restoring a healthy complement and diversity of commensal bacteria is the most logical approach to prevent NEC and death in PVLBW infants. The current evidence provided by this meta-analysis supported that multiple strains probiotics seemed to be the most feasible method and the most effective way to prevent NEC and reduce mortality in preterm infants of ≤ 34 weeks' gestation or of a birth weight ≤ 1500 g. Single strain probiotic using Lactobacillus species had a borderline effect in reducing NEC. Single strain of Bifidobacterium species and Saccharomyces boulardii did not reveal any beneficial effects in terms of reducing NEC or mortality. The optimal combination of species and dosing, long-term immune and neurodevelopment outcomes of probiotic supplementation in PVLBW infants still need to be explored in further studies.
Supporting information
(DOC)
Data availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Luig M, Lui K, NSW & ACT NICUS Group. Epidemiology of necrotizing enterocolitis-Part I: Changing regional trends in extremely preterm infants over 14 years. J Paediatr Child Health 2005; 41: 169–173. 10.1111/j.1440-1754.2005.00582.x [DOI] [PubMed] [Google Scholar]
- 2.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009; 44: 1072–1075. 10.1016/j.jpedsurg.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005; 115: 696–703. 10.1542/peds.2004-0569 [DOI] [PubMed] [Google Scholar]
- 4.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med 2007; 161: 583–590. 10.1001/archpedi.161.6.583 [DOI] [PubMed] [Google Scholar]
- 5.Chen AC, Chung MY, Chang JH, Lin HC. Pathogenesis implication for necrotizing enterocolitis prevention in preterm very-low-birth-weight infants. J Pediatr Gastroenterol Nutr 2014; 58: 7–11. [DOI] [PubMed] [Google Scholar]
- 6.Barclay AR, Stenson B, Simpson JH, Weaver LT, Wilson DC. Probiotics for necrotizing enterocolitis: a systematic review. J Pediatr Gastroenterol Nutr 2007; 45: 569–576. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotizing enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007; 369: 1614–1620. 10.1016/S0140-6736(07)60748-X [DOI] [PubMed] [Google Scholar]
- 8.Alfaleh K, Anabrees J, Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology 2010; 97: 93–99. 10.1159/000235684 [DOI] [PubMed] [Google Scholar]
- 9.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010; 125: 921–930. 10.1542/peds.2009-1301 [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 2012; 47: 241–248. 10.1016/j.jpedsurg.2011.09.064 [DOI] [PubMed] [Google Scholar]
- 11.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2014; 4: CD005496. [DOI] [PubMed] [Google Scholar]
- 12.Aceti A, Gori D, Barone G, Callegari ML, Di Mauro A, Fantini MP, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital J Pediatr. 2015; 41: 89 10.1186/s13052-015-0199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau CS, Chamberlain RS. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: A meta-analysis. J Pediatr Surg. 2015; 50: 1405–1412. 10.1016/j.jpedsurg.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 14.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: A strain-specific systematic review. J Parenter Enteral Nutr. 2016; 40: 783–794. [DOI] [PubMed] [Google Scholar]
- 15.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011; 6: e20647 10.1371/journal.pone.0020647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 2009; 3: 944–954. 10.1038/ismej.2009.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiou SR, Yu Y, Guo Y, He SM, Mziray-Andrew CH, Hoenig J, et al. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS One 2013; 8: e65108 10.1371/journal.pone.0065108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 2011; 50: 1–17. 10.1007/s00394-010-0166-z [DOI] [PubMed] [Google Scholar]
- 19.Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC, Caplan M. Efficacy of different probiotic combinations on death and necrotizing enterocolitis in a premature rat model. J Pediatr Gastroenterol Nutr 2013; 57: 23–28. [DOI] [PubMed] [Google Scholar]
- 20.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg 1978; 187: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Indrio F, Riezzo G, Raimondi F, Bisceglia M, Cavallo L, Francavilla R. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr 2008; 152: 801–806. 10.1016/j.jpeds.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Garland SM, Tobin JM, Pirotta M, Tabrizi SN, Opie GF, Donath S, et al. The ProPrems trial: investigating the effects of probiotics on late onset sepsis in very preterm infants. BMC Infect Dis 2011; 11: 210 10.1186/1471-2334-11-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009; 302: 1421–1428. 10.1001/jama.2009.1403 [DOI] [PubMed] [Google Scholar]
- 24.Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr 2009; 48: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilli D, Aydin B, Fettah ND, Özyazıcı E, Beken S, Zenciroğlu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. 2015; 166: 545–551. 10.1016/j.jpeds.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Sharma N, Chaudhry R, Deorari A, Paul VK, Gewolb IH, et al. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. J Pediatr Gastroenterol Nutr 2003; 36: 397–402. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Shimizu T, Hosaka A, Kaneko N, Ohtsuka Y, Yamashiro Y. Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int 2004; 46: 509–515. 10.1111/j.1442-200x.2004.01953.x [DOI] [PubMed] [Google Scholar]
- 28.Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm neonates: a double placebo controlled, randomized study. J Clin Microbiol 2006; 44: 4025–4031. 10.1128/JCM.00767-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umezaki H, Shinohara K, Satoh Y, Shoji H, Satoh H, Ohtsuka Y, et al. Bifidobacteria prevents preterm infants from developing infection and sepsis. Int J Probiotics Prebiotics 2010; 5: 33–36. [Google Scholar]
- 30.Romeo MG, Romeo DM, Trovato L, Oliveri S, Palermo F, Cota F, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 2011; 31: 63–69. 10.1038/jp.2010.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke D, Su Z, Li L. Control study on preventing necrotizing enterocolitis in 438 premature infants by using Bifico. Chin Pediatr Emerg Med 2008; 15: 69–71. [Article in Chinese]. [Google Scholar]
- 32.Huang BZ, Yang HY, Huang XY. Prevention and cure effect of micro ecosystem praeparatum on necrotizing enterocolitis of very low birth weight infant. J Guangdong Med Coll 2009; 27: 37–39. [Article in Chinese]. [Google Scholar]
- 33.Di M, Li X. Effects of Bifidobacterium supplementation for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled trial. Zhong Guo She Qu Yi Shi 2010; 231: 69. [Article in Chinese]. [Google Scholar]
- 34.Ren B. Preventive effect of Bifidobacterium tetravaccine tablets in premature infants with necrotizing enterocolitis. J Pediatr Pharm 2010; 16: 24–25. [Article in Chinese]. [Google Scholar]
- 35.Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 2008; 64: 418–422. 10.1203/PDR.0b013e318181b7fa [DOI] [PubMed] [Google Scholar]
- 36.Reuman PD, Duckworth DH, Smith KL, Kagan R, Bucciarelli RL, Ayoub EM. Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis 1986; 5: 663–668. [DOI] [PubMed] [Google Scholar]
- 37.Millar MR, Bacon C, Smith SL, Walker V, Hall MA. Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child 1993; 69: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997; 76: F101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002; 82: 103–108. [DOI] [PubMed] [Google Scholar]
- 40.Costalos C, Skouteri V, Gounaris A, Sevastiadou S, Triandafilidou A, Ekonomidou C, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003; 74: 89–96. [DOI] [PubMed] [Google Scholar]
- 41.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005; 147: 192–196. 10.1016/j.jpeds.2005.03.054 [DOI] [PubMed] [Google Scholar]
- 42.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115: 1–4. 10.1542/peds.2004-1463 [DOI] [PubMed] [Google Scholar]
- 43.Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006; 42: 1735–1742. 10.1086/504324 [DOI] [PubMed] [Google Scholar]
- 44.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, et al. The effect of a Bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007; 83: 575–579. 10.1016/j.earlhumdev.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 45.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122: 693–700. 10.1542/peds.2007-3007 [DOI] [PubMed] [Google Scholar]
- 46.Rougé C, Piloquet H, Butel MJ, Berger B, Rochat F, Ferraris L,et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009; 89: 1828–1835. 10.3945/ajcn.2008.26919 [DOI] [PubMed] [Google Scholar]
- 47.Samanta M, Sarkar M, Ghosh P, Ghosh J, Sinha M, Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009; 55: 128–131. 10.1093/tropej/fmn091 [DOI] [PubMed] [Google Scholar]
- 48.Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birthweight infants: a randomized controlled trial. Neonatology 2010; 98: 156–163. 10.1159/000280291 [DOI] [PubMed] [Google Scholar]
- 49.Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011; 65: 434–439. 10.1038/ejcn.2010.278 [DOI] [PubMed] [Google Scholar]
- 50.Braga TD, da Silva GA, de Lira PI. de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011; 93: 81–86. 10.3945/ajcn.2010.29799 [DOI] [PubMed] [Google Scholar]
- 51.Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Bastidas JA, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012; 130: e1113–1120. 10.1542/peds.2011-3584 [DOI] [PubMed] [Google Scholar]
- 52.Al-Hosni M, Duenas M, Hawk M, Stewart LA, Borghese RA, Cahoon M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012; 32: 253–259. 10.1038/jp.2011.51 [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, Gallardo-Sarmiento RB, García-Pérez CS, Montaño-Rodríguez R, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013; 98: F5–9. 10.1136/archdischild-2011-300435 [DOI] [PubMed] [Google Scholar]
- 54.Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr 2013; 102: e560–565. 10.1111/apa.12416 [DOI] [PubMed] [Google Scholar]
- 55.Jacobs SE, Tobin JM, Opei GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013; 132: 1055–1062. 10.1542/peds.2013-1339 [DOI] [PubMed] [Google Scholar]
- 56.Serce O, Benzer D, Gursoy T, Karatekin G, Ovali F. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum Dev 2013; 89: 1033–1036. 10.1016/j.earlhumdev.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 57.Roy A, Chaudhuri J, Sarkar D, Ghosh P, Chakraborty S. Role of Enteric Supplementation of Probiotics on Late-onset Sepsis by Candida species in Preterm Low Birth Weight Neonates: A Randomized, Double Blind, Placebo-controlled Trial. N Am J Med Sci 2014; 6: 50–57. 10.4103/1947-2714.125870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014; 99: F110–115. 10.1136/archdischild-2013-304745 [DOI] [PubMed] [Google Scholar]
- 59.Totsu S, Yamasaki C, Terahara M, Uchiyama A, Kusuda S; Probiotics Study Group in Japan. Bifidobacterium and enteral feeding in preterm infants: Cluster-randomized trial. Pediatr Int 2014; 56: 714–719. 10.1111/ped.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014; 97 Suppl 6: S20–5. [PubMed] [Google Scholar]
- 61.Patole S, Keil AD, Chang A, Nathan E, Doherty D, Simmer K, et al. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates -a randomised double blind placebo controlled trial. PLoS One. 2014; 9: e89511 10.1371/journal.pone.0089511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tewari VV, Dubey SK, Gupta G. Bacillus clausii for Prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J Trop Pediatr. 2015; 61: 377–385. 10.1093/tropej/fmv050 [DOI] [PubMed] [Google Scholar]
- 63.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR; Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. 2016; 387: 649–660. 10.1016/S0140-6736(15)01027-2 [DOI] [PubMed] [Google Scholar]
- 64.Cassir N, Simeoni U, La Scola B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol. 2016; 11: 273–292. 10.2217/fmb.15.136 [DOI] [PubMed] [Google Scholar]
- 65.Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016; 387(10031):1928–36. 10.1016/S0140-6736(16)00081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thymann T, Møller HK, Stoll B, Støy AC, Buddington RK, Bering SB, et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol 2009; 297: G1115–1125. 10.1152/ajpgi.00261.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.