Abstract
Objectives
This systematic review and meta-analysis aims to determine the current evidence on risk factors for type II endoleaks after endovascular aneurysm repair (EVAR).
Materials and methods
A systematic literature search was carried out for studies that evaluated the association of demographic, co-morbidity, and other patient-determined factors with the onset of type II endoleaks. Pooled prevalence of type II endoleaks after EVAR was updated.
Results
Among the 504 studies screened, 45 studies with a total of 36,588 participants were included in this review. The pooled prevalence of type II endoleaks after EVAR was 22% [95% confidence interval (CI), 19%–25%]. The main factors consistently associated with type II endoleaks included age [pooled odds ratio (OR), 0.37; 95% CI, 0.31–0.43; P<0.001], smoking (pooled OR, 0.71; 95% CI, 0.55–0.92; P<0.001), patent inferior mesenteric artery (pooled OR, 1.98; 95% CI, 1.06–3.71; P = 0.012), maximum aneurysm diameter (pooled OR, 0.23; 95% CI, 0.17–0.30; P<0.001), and number of patent lumbar arteries (pooled OR, 3.07; 95% CI, 2.81–3.33; P<0.001). Sex, diabetes, hypertension, anticoagulants, antiplatelet, hyperlipidemia, chronic renal insufficiency, types of graft material, and chronic obstructive pulmonary diseases (COPD) did not show any association with the onset of type II endoleaks.
Conclusions
Clinicians can use the identified risk factors to detect and manage patients at risk of developing type II endoleaks after EVAR. However, further studies are needed to analyze a number of potential risk factors.
Introduction
Endovascular aneurysm repair (EVAR) has become the primary choice of treatment for abdominal aortic aneurysms (AAAs) in suitable patients [1]. EVAR always has better short-term outcomes compared with open repair [2,3]. Aortic endograft occlusion, migration, and endoleaks are known complications after EVAR [4], among which endoleaks are the most frequent. Types I and III endoleaks require urgent intervention to relieve aneurysm re-pressurization [5,6]. Type II endoleaks are caused by backflow of collateral arteries into the aneurysm sac, with an occurrence rate of 10.2% after EVAR [7]. Type II endoleaks do not exert any immediate adverse effects. However, persistent type II endoleaks are believed to be associated with increased sac pressure and cause adverse outcomes and even aneurysm rupture [8].
Various studies assessed risk factors for type II endoleaks following EVAR [9–11]. Potential correlations may exist between preoperative characteristics and development of type II endoleaks [9]. A patent inferior mesenteric artery (IMA) and the number of patent lumbar arteries have been investigated as risk factors for type II endoleaks [10,11]. However, no previous systematic review has concentrated on this topic. Hence, the primary objective of the present systematic review and meta-analysis was to determine the current evidence on risk factors for the onset of type II endoleaks. This review concentrated on nonclinical risk factors, such as sex and age, preoperative comorbidities, and aortic anatomy. Moreover, we intended to report the updated pooled prevalence of type II endoleaks after EVAR.
Methods
Search strategy and study selection
Relevant publications were identified by searching the following database: MEDLINE (from Jan 1, 1950, to June 30, 2016) (S1 File), Scopus (from 1966 to June 30, 2016), and EMBASE (from 1966 to June 30, 2016). The terms “type II endoleak” or “type 2 endoleak,” “endovascular,” and “aneurysm” were searched. The references cited in published original and review articles were searched to identify additional studies. The search was limited to human adults and publications in English. Subsequently, two authors (Q.G. and X.D.) independently reviewed the title and abstract of the studies identified in the search to exclude those that did not answer the research question of interest in accordance with prespecified inclusion and exclusion criteria. Records extracted by the initial search were screened, and potentially relevant papers were retrieved and examined in detail. Duplicate publications and case reports were also excluded. Whenever publications of overlapping populations were available, the publication with the most complete and relevant set of data was chosen.
We identified studies in accordance with the following inclusion criteria: (1) participants: human adults (minimum of 18 years of age) with abdominal aortic aneurysm; (2) intervention: EVAR; (3) comparison: patients with potential risk factors versus patients without potential risk factors resulting in type II endoleaks; and (4) sufficient data were available for estimating an odds ratio (OR) with confidence interval (CI).
Data abstraction and quality assessment
Two authors (B.H. and Y.M.) extracted relevant data independently, including the first author’s name, study year, study region, study design, number of type II endoleaks, number of reintervention for type II endoleaks, duration of follow-up, and potential risk factor for type II endoleaks [including age, sex, smoking, diabetes, hypertension, hyperlipidemia, chronic renal insufficiency, chronic obstructive pulmonary diseases (COPD), polytrafluoroethylene-based endografts, anticoagulants, antiplatelet, patent inferior mesenteric artery, maximum aneurysm diameter, and number of patent lumbar arteries]. Conflicts in data abstraction were resolved through consensus by referring back to the original article. The methodological quality of the studies was assessed by two authors (D.Y. and Y.Y.) independently using the Newcastle–Ottawa scale [12]. Quality was calculated on the basis of the following three aspects of study design: patient selection, comparability of study groups, and exposure and outcome of study participants. Studies that scored 5 or greater were considered high quality, whereas those that scored 4 and below were considered low quality.
Statistical analysis
Statistical analyses were performed with Stata 12.0 software (StataCorp LP, College Station, Texas, USA). Dichotomous variables were analyzed by estimating the odds ratio (OR) with a 95% confidence interval (95% CI), whereas continuous variables were analyzed using the weighted mean difference with a 95% CI. Statistical heterogeneity between studies was assessed using the I2 measure. I2 > 50% was considered to indicate high statistical heterogeneity. If no heterogeneity was found, a fixed effect model based on the Mantel and Haenszel estimator was used; otherwise, a random effect model based on the DerSimonian and Laird estimator was used [13]. Several characteristics were identified to analyze their effect on the prevalence of type II endoleaks. Categorical characteristics were treated as moderators, and effectiveness was compared across subgroups formed by year of publication (before 2010 vs. 2011–2016), surveillance test (with categories: CTA vs. others), and study setting (retrospective vs. prospective). Egger’s and Begg’s tests were performed to assess publication bias [14]. For all statistical analyses, except heterogeneity and publication bias, p < 0.05 was regarded to indicate statistical significance, and all tests were two-sided. The present systematic review was conducted and reported in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (S2 File) [15].
Results
Study characteristics and methodological quality
From the 504 studies identified using our search strategy, 73 potentially relevant articles were retrieved and assessed for eligibility. In total, 45 papers were included in this review [8–11,16–56], with 15 included in the meta-analysis [8,9,28,32–34,37,38,45–48,50,55,56]. The flow diagram summarizing the study identification and selection is shown in Fig 1. The characteristics of the included studies are reported in Table 1. The methodological quality of the included studies for meta-analysis is summarized in S3 File. All of the studies enrolled for meta-analysis were of high quality.
Fig 1. PRISMA flow chart for the review.
Table 1. Characteristics of included studies.
| Studies | Country | Study design | No. of patients | Type II endoleaks | types of endoleaks | Reintervention | Follow-up (months) | Surveillance test |
|---|---|---|---|---|---|---|---|---|
| Chuter 2001[16] | USA | retrospective | 114 | 9 | Any | n.s. | n.s. | CTA |
| Haulon 2001[17] | France | retrospective | 60 | 18 | Any | n.s. | 13.3 | CTA/MRI |
| Liewald 2001[18] | Germany | retrospective | 160 | 13 | Any | n.s. | n.s. | CTA |
| Buth 2002[19]* | Netherland | retrospective | 3529 | 240 | Any | 53 | n.s. | CTA |
| Parent 2002[20] | USA | retrospective | 83 | 36 | Any | 3 | 20.7 | CTA |
| Solis 2002[21] | USA | retrospective | 119 | 14 | Any | 10 | 18.2 | CTA |
| Tuerff 2002[22] | USA | prospective | 130 | 22 | Any | n.s. | 15.7 | CTA |
| Faries 2003[23] | USA | retrospective | 597 | 16 | Any | n.s. | 24.5 | CTA |
| Farner 2003[24] | USA | retrospective | 63 | 9 | Any | n.s. | 12 | CTA |
| Kasirajan 2003[25] | USA | retrospective | 104 | 8 | Any | n.s. | n.s. | CTA |
| Carpenter 2004[26] | USA | prospective | 227 | 21 | Any | 8 | n.s. | CTA |
| Steinmetz 2004[27] | USA | retrospective | 486 | 90 | Any | 4 | 21.7 | CTA |
| Van Marrewijk 2004[28]* | Netherland | prospective | 3595 | 320 | Any | n.s. | 15 | CTA |
| Tolia 2005[29] | USA | retrospective | 83 | 16 | Any | 2 | 30 | CTA |
| Sheehan 2006[30] | USA | retrospective | 1909 | 221 | Any | 101 | 36 | CTA |
| Silverberg 2006[31] | USA | retrospective | 965 | 154 | Any | 19 | 22 | CTA |
| Jones 2007[8] | USA | retrospective | 873 | 164 | Any | 16 | 38 | CTA |
| Abularrage 2010[9] | USA | retrospective | 832 | 136 | Persistent | 39 | 34.8 | CTA |
| Brountzos 2012[10] | Greece | retrospective | 136 | 31 | Persistent | n.s. | 13.5 | CTA |
| Koole 2012[32]* | Netherland | prospective | 8638 | 4518 | Any | 661 | 26 | CTA |
| Nolz 2012[33] | Austria | retrospective | 407 | 49 | Any | 20 | n.s. | CTA |
| Batti 2013[34] | France | prospective | 700 | 201 | Any | 30 | 31.3 | CTA |
| Chang 2013[35] | USA | retrospective | 1736 | 474 | Any | 251 | 32.4 | CTA |
| Jouhannet 2013[36] | France | retrospective | 232 | 94 | Any | 14 | 23 | CTA |
| Cieri 2014[37] | Italy | prospective | 1412 | 218 | Any | 52 | 45 | CTA |
| Maeda 2014[11] | Japan | retrospective | 469 | 111 | Any | n.s. | 21.0 | CTA |
| Sidloff 2014[38] | UK | prospective | 904 | 175 | Any | 9 | 43.2 | DUS |
| Ward 2014[39] | USA | retrospective | 326 | 99 | Any | n.s. | n.s. | CTA |
| Dudeck 2015[40] | Germany | retrospective | 188 | 62 | Any | n.s. | 39 | CTA |
| Hiraoka 2015[41] | Japan | retrospective | 148 | 36 | Any | n.s. | 28.8 | CTA |
| Löwenthal 2015[42] | Germany | retrospective | 130 | 62 | Any | n.s. | 22 | CTA |
| Müller-Wille 2015[43] | Germany | retrospective | 384 | 56 | Any | 20 | 36 | CTA |
| Gallitto 2015[44] | Italy | prospective | 200 | 47 | Any | 6 | 22 | CTA |
| Kray 2015[45] | USA | retrospective | 191 | 18 | Any | n.s. | n.s. | CTA |
| Nolz 2015[46] | Austria | retrospective | 143 | 69 | Any | n.s. | n.s. | CTA |
| Pini 2015[47] | Italy | retrospective | 753 | 85 | Any | 2 | 19 | CTA/DUS |
| Walker 2015[48] | USA | retrospective | 1736 | 474 | Any | 111 | 32.2 | CTA |
| Rousié 2015[49] | Belgium | retrospective | 77 | 17 | Any | 0 | 79 | CTA |
| Phan 2015[50] | Germany | retrospective | 82 | 51 | Any | 13 | 29.5 | CTA |
| Piazza 2016[51] | USA | prospective | 126 | 34 | Any | 7 | 16 | CTA |
| Lo 2016[52] | USA | retrospective | 2367 | 807 | Persisent | n.s. | 15.4 | n.s. |
| Mursalin 2016[53] | Japan | retrospective | 145 | 47 | Any | n.s. | n.s. | CTA |
| Ikoma 2016[54] | Japan | prospective | 34 | 12 | Any | n.s. | n.s. | CTA |
| Pippin 2016[55] | USA | retrospective | 163 | 66 | Any | 4 | 24.7 | CTA |
| Fujimura 2016[56] | Japan | retrospective | 832 | 234 | Any | 11 | 35.6 | CTA/MRI |
*Three papers were published from the EUROSTAR registry and may present duplicated data.
n.s., Not stated.
Prevalence of type II endoleaks after EVAR
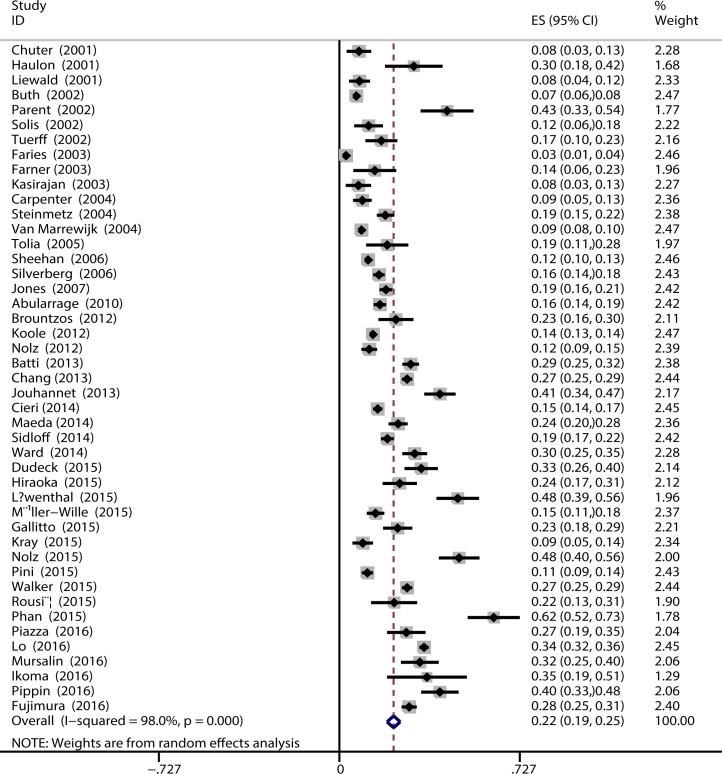
A total of 9,654 type II endoleaks were reported to have occurred over a range of 12–79 follow-up months in 36,588 patients. The pooled prevalence of type II endoleaks after EVAR was 22% (95%CI, 19%–25%) (Fig 2). Reintervention for type II endoleaks was reported in 1466 (19%) of 7885 patients. The proportion of type II endoleaks was significantly greater in subgroups of studies published after 2010 [13%, 95% CI (11%–16%) vs. 27% 95% CI (24%–31%)] (Table 2). The proportion of type II endoleaks was similar in subgroups of studies using CTA as surveillance test with those using others [21%, 95% CI (14%–23%) vs. 24%, 95% CI (14%–34%)]. The proportion of type II endoleaks was similar in subgroups of prospective studies with retrospective studies [18%, 95% CI (15–21%) vs. 23%, 95% CI (19%–27%)].
Fig 2. Pooled prevalence of type II endoleaks after EVAR.
Table 2. Subgroup analysis for prevalence of Type II endoleak.
| Study subgroup | No. of studies | Total no. of participants | Pooled prevalence | 95% CI | I2 |
|---|---|---|---|---|---|
| Total | 45 | 36588 | 0.22 | 0.19–0.25 | 98.0 |
| Year of the study | |||||
| 2001–2010 | 18 | 13929 | 0.13 | 0.11–0.16 | 95.1 |
| 2011–2016 | 27 | 22659 | 0.27 | 0.24–0.31 | 97.5 |
| Study setting | |||||
| Prospective | 10 | 15966 | 0.18 | 0.15–0.21 | 96.1 |
| Retrospective | 35 | 20622 | 0.23 | 0.19–0.27 | 98.3 |
| Surveillance test | |||||
| CTA | 40 | 31672 | 0.21 | 0.19–0.24 | 97.6 |
| Others | 5 | 4916 | 0.24 | 0.14–0.34 | 98.4 |
Meta-analysis of possible factors associated with type II endoleak
In total, eight studies reported on the effect of age on type II endoleaks (Table 3). These studies consistently demonstrated that age was a risk factor for the onset of type II endoleaks, although considerable heterogeneity was present among the results reported (I2 = 99.0%). The pooled OR of the eight studies was 0.37 (95% CI, 0.31–0.43; P<0.001). Twelve studies investigated smoking as a risk factor for the onset of type II endoleaks. Among these 14 studies, a considerable heterogeneity was observed between the study findings (I2 = 86.4%); however, all studies were generally consistent in reporting smoking as a protective factor for the onset of type II endoleaks. The pooled OR of the 12 studies was 0.71 (95% CI, 0.55–0.92; P<0.001).
Table 3. Pooled ORs for association of commonly studied risk factors with Type II endoleak.
| Potential risk factors | No. of studies | Total no. of participants | Pooled OR | 95% CI | P value | I2 |
|---|---|---|---|---|---|---|
| Age | 8 | 6278 | 0.37 | 0.31–0.43 | <0.001 | 99.0 |
| Male | 12 | 11775 | 0.83 | 0.67–1.02 | 0.059 | 46.4 |
| Smoking | 14 | 20477 | 0.71 | 0.55–0.92 | <0.001 | 86.4 |
| Diabetes | 10 | 7303 | 0.91 | 0.76–1.09 | 0.251 | 20.9 |
| Hypertension | 10 | 7281 | 0.98 | 0.85–1.12 | 0.484 | 0 |
| Hyperlipidemia | 7 | 5522 | 1.12 | 0.83–1.49 | 0.814 | 74.7 |
| Chronic renal insufficiency | 10 | 9201 | 0.85 | 0.53–1.36 | 0.600 | 85.3 |
| COPD | 10 | 5745 | 0.84 | 0.69–1.03 | 0.135 | 34.1 |
| Polytrafluoroethylene-based endografts | 7 | 8396 | 0.88 | 0.65–1.18 | 0.390 | 70.8 |
| Anticoagulants | 5 | 3758 | 1.27 | 0.97–1.67 | 0.537 | 0 |
| Antiplatelet | 5 | 3758 | 1.09 | 0.79–1.51 | 0.220 | 65.6 |
| Patent IMA | 3 | 4353 | 1.98 | 1.06–3.71 | 0.012 | 77.6 |
| Number of patent lumbar arteries | 2 | 758 | 3.07 | 2.81–3.33 | <0.001 | 99.8 |
| Maximum aneurysm diameter | 7 | 4858 | 0.23 | 0.17–0.30 | <0.001 | 98.0 |
Seven cohort studies assessed the effect of maximum aneurysm diameter on the onset of type II endoleaks. The I2 among the seven studies was 98.0%, and the resulting pooled OR was 0.23 (CI, 0.17–0.30; P = 0.012), showing an increased risk of type II endoleaks in patients with a greater diameter of AAA. Three studies were included in the meta-analysis of patent IMA as a risk factor for the onset of type II endoleaks. The extent of heterogeneity present between the findings reported was considerable (I2, 77.6%), and the pooled OR was 1.98 (95% CI, 1.06–3.71; P<0.001). Two studies reported the effect of number of patent lumbar arteries on type II endoleaks, and the pooled OR was 3.07 (95% CI, 2.81–3.33; P<0.001).
Twelve studies assessed male sex as a potential risk factor. The pooled OR of 0.83 (95% CI, 0.67–1.02; I2, 46.4%) suggests that male sex is not associated with type II endoleaks. Diabetes (OR 0.91; 95% CI, 0.76–1.09), hypertension (OR, 0.98; 95% CI, 0.85–1.12), Polytrafluoroethylene-based endografts (OR, 0.88; 95% CI, 0.65–1.18), anticoagulants (OR, 1.27; 95% CI, 0.97–1.67), antiplatelet (OR, 1.09; 95% CI, 0.79–1.51), hyperlipidemia (OR, 1.12; 95% CI, 0.83–1.49), chronic renal insufficiency (OR, 0.85; 95% CI, 0.53–1.36), and COPD (OR, 0.84; 95% CI, 0.63–1.03) are not risk factors of type II endoleaks (Table 3).
Sensitivity analyses by quality (methodological quality >6), and sample size (n>100) were done for the enrolled studies. All meta-analytic conclusions remained robust to this testing. The results of Begg’s test (p = 0.432) and Egger’s test (p = 0.338) revealed no significant publication bias in this study.
Discussion
Endoleaks are specific complications of EVAR. Type II endoleaks are the most common type of endoleaks, occurring in approximately 10% of patients after EVAR [7]. Although Type II endoleaks are less likely to require secondary reintervention than type I or III endoleaks because of the possibility of arterial rupture [9], type II endoleaks are a risk factor for aneurysm sac growth, and ruptures occur in approximately 1% of patients with type II endoleaks after EVAR [7]. Thus, patients need continuous surveillance after EVAR to detect aneurysm growth and endoleaks. Previous studies explored risk factors for type II endoleaks after EVAR [9–11]. However, controversies have been found between these studies. These discrepancies may be attributed to the insufficient statistical power of individual studies and the inability to perform separate analyses. Therefore, we conducted a systematic review and meta-analysis to identify the risk factors for type II endoleaks after EVAR. Furthermore, the pooled prevalence of type II endoleaks must be updated on the basis of the latest evidence.
Among the potential risk factors for type II endoleaks, older age, patent inferior mesenteric artery, number of patent lumbar arteries, and maximum aneurysm diameter significantly increase the risk for type II endoleaks after EVAR. By contrast, smoking is a protective factor for type II endoleaks. Sex, diabetes, hypertension, anticoagulants, hyperlipidemia, chronic renal insufficiency, COPD, and polytrafluoroethylene-based endografts are not associated with the onset of type II endoleaks. To the best of our knowledge, this systematic review and meta-analysis is the first to identify the risk factors of type II endoleaks after EVAR. This paper also revealed a higher prevalence of type II endoleaks after EVAR compared with an earlier review [7]. Most of the included studies exhibited high methodological quality on the basis of the Newcastle–Ottawa scale. Statistical tests were performed to examine the issue of publication bias, and results revealed no statistically significant publication bias.
Sidloff et al. [7] performed a systematic review that included 22 studies to evaluate the prevalence of type II endoleaks. A total of 1,515 type II endoleaks were reported to have occurred in 14,794 patients (10.2%). In this study, 9,654 of 36,588 patients developed type II endoleaks after 12–45 months of follow-up (26.4%). The proportion of type II endoleaks was significantly greater in the subgroup analysis of studies published after 2010 compared with those published before 2010 (27% vs. 13%). These results may be attributed to the improved accuracy of imaging tools for the surveillance after EVAR, more endoleaks might be detected by the upgraded imaging tools.
Reintervention for type II endoleaks has been reported in 1,466 (19%) of 7,885 patients. Management of type II endoleaks is still debated because clinical outcomes vary in published literature [34,36,37,44]. Some studies have indicated that persistence of type II endoleaks is not a negligible complication that could require re-interventions and decrease the long-term EVAR success [44]. Moreover, the endovascular reinterventions for type II endoleaks associated with an enlargement of the aneurysmal sac after EVAR have a poor effectiveness on the stabilization of the diameter of the AAA [36]. Other studies stated that recurrent and persistent type II endoleaks are prone to life-threatening complications, and type II endoleak appears to be a marker of EVAR failure that is difficult to predict and treat effectively [34,37]. The debate about the indication of intervention for type II endoleaks seems to last.
From the anatomic aspect, we identified patent IMA, number of patent lumbar arteries, and maximum aneurysm diameter as risk factors for the onset of type II endoleaks. Preoperative embolization of the IMA has been proved to be associated with decreased risk of type-2 endoleak and aneurysm sac enlargement after EVAR [57]. Considering aneurysm diameter, it is likely that the increased flow channel within the aneurysm sac coupled with a larger number of patent aortic branch vessels leads to increased flow velocities within the sac, thus decreasing the likelihood of type II endoleak resolution [58]. It is reported that the number of patent lumbar arteries was also correlated with onset of type II endoleak, however, this study did not include the analysis because no other studies reported the similar issue [9].
In total, 12 studies investigated smoking as a risk factor for the onset of type II endoleaks. All studies were generally consistent in reporting smoking as a protective factor for the onset of type II endoleaks. Koole et al. [32] reported a study that analyzed the data of 8638 patients who were enrolled prospectively in the European Collaborators on Stent-Graft Techniques for Aortic Aneurysm Repair database. The study observed decreased intraoperative perfusion from the mesenteric inferior artery or lumbar arteries in smokers. The explanation of these findings remains unclear; however, we hypothesized that tobacco use can induce the atherosclerotic injury of medium- and small-sized vessels, which may narrow or occlude these arteries and thereby impair the blood flow [32]. There is also an increased tendency of coagulation in smokers, which also might narrow or occlude the arteries. We also found that older age is a risk factor for the onset of type II endoleaks. Long history and large diameter of AAA in aging patients, favoring high-pressure aneurysm sac, may be a possible explanation of this fact. Besides, aging patients might also have more severe atherosclerotic injury of vessels which induced impair of the retrograde flow into abdominal aorta.
Our review also has some limitations. First, only the main predictors drawn from the articles were analyzed because of data deficiencies. Second, our study was subjected to heterogeneity in some of the inclusion criteria, as well as relatively small sample sizes and numbers of articles, which may have caused potential publication bias. Only three studies were included in the meta-analysis of patent IMA as a risk factor for the onset of type II endoleaks, whereas only four studies provided data that compared the effects of anticoagulants used on the onset of type II endoleaks. Furthermore, most of the articles included in our review are retrospective observational studies. A long-term prospective study to specifically define risk factors of endoleaks would be instructive.
In conclusion, age, patent inferior mesenteric artery, number of patent lumbar arteries, maximum aneurysm diameter, and non-tobacco use are significant factors in predicting type II endoleaks. Meanwhile, sex, diabetes, hypertension, anticoagulants, antiplatelet, hyperlipidemia, chronic renal insufficiency, types of graft material, and COPD show no statistical significance. Thus, clinicians should pay attention to these factors in AAA patients after EVAR.
Supporting information
(DOC)
(DOC)
(DOC)
Acknowledgments
All of the authors contributed to the collection and analysis of the data and to the preparation of the report. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors have no conflicts of interest or financial ties to disclose.
Data availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomized controlled trial. Lancet. 2005;365(9478):2179–86. 10.1016/S0140-6736(05)66627-5 [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–42. 10.1001/jama.2009.1426 [DOI] [PubMed] [Google Scholar]
- 3.United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1863–71. 10.1056/NEJMoa0909305 [DOI] [PubMed] [Google Scholar]
- 4.Ilyas S, Shaida N, Thakor AS, Winterbottom A, Cousins C. Endovascular aneurysm repair (EVAR) follow-up imaging: the assessment and treatment of common postoperative complications. Clin Radiol. 2010;362(20):1863–71. [DOI] [PubMed] [Google Scholar]
- 5.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4 Suppl):S2–49. 10.1016/j.jvs.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- 7.Sidloff DA, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak after endovascular aneurysm repair. Br J Surg. 2013;100(10):1262–70. 10.1002/bjs.9181 [DOI] [PubMed] [Google Scholar]
- 8.Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg. 2007;46(1):1–8. 10.1016/j.jvs.2007.02.073 [DOI] [PubMed] [Google Scholar]
- 9.Abularrage CJ, Crawford RS, Conrad MF, Lee H, Kwolek CJ, Brewster DC, et al. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg. 2010;52(1):19–24. 10.1016/j.jvs.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 10.Brountzos E, Karagiannis G, Panagiotou I, Tzavara C, Efstathopoulos E, Kelekis N. Risk factors for the development of persistent type II endoleaks after endovascular repair of infrarenal abdominal aortic aneurysms. Diagn Interv Radiol. 2012;18(3):307–13. 10.4261/1305-3825.DIR.4646-11.1 [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Ito T, Kurimoto Y, Watanabe T, Kuroda Y, Kawaharada N, Higami T. Risk factors for a persistent type 2 endoleak after endovascular aneurysm repair. Surg Today. 2015;45(11):1373–7. 10.1007/s00595-014-1070-6 [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 11 Jan 2015).
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 In: The Cochrane Library, Issue 3. Chichester, UK: Wiley; 2006. [Google Scholar]
- 14.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med.2006;25(20):3443–57. 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 16.Chuter TA, Faruqi RM, Sawhney R, Reilly LM, Kerlan RB, Canto CJ, et al. Endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2001;34(1):98–105. 10.1067/mva.2001.111487 [DOI] [PubMed] [Google Scholar]
- 17.Haulon S, Tyazi A, Willoteaux S, Koussa M, Lions C, Beregi JP. Embolization of type II endoleaks after aortic stent-graft implantation: technique and immediate results. J Vasc Surg. 2001;34(4):600–5. 10.1067/mva.2001.117888 [DOI] [PubMed] [Google Scholar]
- 18.Liewald F, Ermis C, Görich J, Halter G, Scharrer-Pamler R, Sunder-Plassmann L. Influence of treatment of type II leaks on the aneurysm surface area. Eur J Vasc Endovasc Surg. 2001;21(4):339–43. 10.1053/ejvs.2001.1333 [DOI] [PubMed] [Google Scholar]
- 19.Buth J, Harris PL, van Marrewijk C. Causes and outcomes of open conversion and aneurysm rupture after endovascular abdominal aortic aneurysm repair: can type II endoleaks be dangerous? J Am Coll Surg. 2002;194(1 Suppl):S98–S102. [DOI] [PubMed] [Google Scholar]
- 20.Parent FN, Meier GH, Godziachvili V, LeSar CJ, Parker FM, Carter KA, et al. The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan. J Vasc Surg. 2002;35(3):474–81. [DOI] [PubMed] [Google Scholar]
- 21.Solis MM, Ayerdi J, Babcock GA, Parra JR, McLafferty RB, Gruneiro LA, et al. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg. 2002;36(3):485–91. [DOI] [PubMed] [Google Scholar]
- 22.Tuerff SN, Rockman CB, Lamparello PJ, Adelman MA, Jacobowitz GR, Gagne PJ, et al. Are type II (branch vessel) endoleaks really benign? Ann Vasc Surg. 2002;16(1):50–4. 10.1007/s10016-001-0126-4 [DOI] [PubMed] [Google Scholar]
- 23.Faries PL, Cadot H, Agarwal G, Kent KC, Hollier LH, Marin ML. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg. 2003;37(6):1155–61. [DOI] [PubMed] [Google Scholar]
- 24.Farner MC, Carpenter JP, Baum RA, Fairman RM. Early changes in abdominal aortic aneurysm diameter after endovascular repair. J Vasc Interv Radiol. 2003;14(1):205–10. [DOI] [PubMed] [Google Scholar]
- 25.Kasirajan K, Matteson B, Marek JM, Langsfeld M. Technique and results of transfemoralsuperselective coil embolization of type II lumbar endoleak. J Vasc Surg. 2003;38(1): 61–6. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter JP, Anderson WN, Brewster DC, Kwolek C, Makaroun M, Martin J, et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg. 2004;39(1):34–43. 10.1016/j.jvs.2003.10.036 [DOI] [PubMed] [Google Scholar]
- 27.Steinmetz E, Rubin BG, Sanchez LA, Choi ET, Geraghty PJ, Baty J, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg. 2004;39(2):306–13. 10.1016/j.jvs.2003.10.026 [DOI] [PubMed] [Google Scholar]
- 28.van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J; EUROSTAR Collaborators. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004;27(2):128–37. 10.1016/j.ejvs.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 29.Tolia AJ, Landis R, Lamparello P, Rosen R, Macari M. Type II endoleaks after endovascular repair of abdominal aortic aneurysms: natural history. Radiology. 2005;235(2):683–6. 10.1148/radiol.2352040649 [DOI] [PubMed] [Google Scholar]
- 30.Sheehan MK, Ouriel K, Greenberg R, McCann R, Murphy M, Fillinger M, et al. Are type II endoleaks after endovascular aneurysm repair endograft dependent? J Vasc Surg. 2006;43(4): 657–61. 10.1016/j.jvs.2005.12.044 [DOI] [PubMed] [Google Scholar]
- 31.Silverberg D, Baril DT, Ellozy SH, Carroccio A, Greyrose SE, Lookstein RA, et al. An 8-year experience with type II endoleaks: natural history suggests selective intervention is a safe approach. J Vasc Surg. 2006;44(3):453–9. 10.1016/j.jvs.2006.04.058 [DOI] [PubMed] [Google Scholar]
- 32.Koole D, Moll FL, Buth J, Hobo R, Zandvoort H, Pasterkamp G, et al. The influence of smoking on endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2012;55(6):1581–6. 10.1016/j.jvs.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 33.Nolz R, Teufelsbauer H, Asenbaum U, Beitzke D, Funovics M, Wibmer A, et al. Type II Endoleaks After Endovascular Repair of Abdominal Aortic Aneurysms: Fate of the Aneurysm Sac and Neck Changes During Long-term Follow-up. J Endovasc Ther. 2012;19(2):193–9. 10.1583/11-3803.1 [DOI] [PubMed] [Google Scholar]
- 34.Batti S, Cochennec F, Roudot-Thoraval F, Becquemin JP. Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg. 2013;57(5):1291–8. 10.1016/j.jvs.2012.10.118 [DOI] [PubMed] [Google Scholar]
- 35.Chang RW, Goodney P, Tucker LY, Okuhn S, Hua H, Rhoades A, et al. Ten-year results of endovascular abdominal aortic aneurysm repair from a large multicenter registry. J Vasc Surg. 2013;58(2): 324–32. 10.1016/j.jvs.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouhannet C, Alsac JM, Julia P, Sapoval M, El Batti S, Di Primio M, et al. Reinterventions for type 2 endoleaks with enlargement of the aneurismal sac after endovascular treatment of abdominal aortic aneurysms. Ann Vasc Surg. 2014;28(1):192–200. 10.1016/j.avsg.2012.10.038 [DOI] [PubMed] [Google Scholar]
- 37.Cieri E, De Rango P, Isernia G, Simonte G, Ciucci A, Parlani G, et al. Type II endoleak is an enigmatic and unpredictable marker of worse outcome after endovascular aneurysm repair. J Vasc Surg. 2014;59(4):930–7. 10.1016/j.jvs.2013.10.092 [DOI] [PubMed] [Google Scholar]
- 38.Sidloff DA, Gokani V, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak: conservative management is a safe strategy. Eur J Vasc Endovasc Surg. 2014;48(4):391–9. 10.1016/j.ejvs.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 39.Ward TJ, Cohen S, Patel RS, Kim E, Fischman AM, Nowakowski FS, et al. Anatomic Risk Factors for Type-2 Endoleak Following EVAR: A Retrospective Review of Preoperative CT Angiography in 326 patients. Cardiovasc Intervent Radiol. 2014;37(2):324–8. 10.1007/s00270-013-0646-7 [DOI] [PubMed] [Google Scholar]
- 40.Dudeck O, Schnapauff D, Herzog L, Löwenthal D, Bulla K, Bulla B, et al. Can Early Computed Tomography Angiography after Endovascular Aortic Aneurysm Repair Predict the Need for Reintervention in Patients with Type II Endoleak? Cardiovasc Intervent Radiol. 2015;38(1)45–52. 10.1007/s00270-014-0901-6 [DOI] [PubMed] [Google Scholar]
- 41.Hiraoka A, Chikazawa G, Ishida A, Miyake K, Totsugawa T, Tamura K, et al. Impact of Age and Intraluminal Thrombus Volume on Abdominal Aortic Aneurysm Sac Enlargement after Endovascular Repair. Ann Vasc Surg. 2015;29(7):1440–6. 10.1016/j.avsg.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 42.Löwenthal D, Herzog L, Rogits B, Bulla K, Weston S, Meyer F, et al. Identification of predictive CT angiographic factors in the development of high-risk type 2 endoleaks after endovascular aneurysm repair in patients with infrarenal aortic aneurysms. Rofo. 2015;187(1):49–55. 10.1055/s-0034-1385123 [DOI] [PubMed] [Google Scholar]
- 43.Müller-Wille R, Schötz S, Zeman F, Uller W, Güntner O, Pfister K, et al. CT features of early type II endoleaks after endovascular repair of abdominal aortic aneurysms help predict aneurysm sac enlargement. Radiology. 2015;274(3):906–16. 10.1148/radiol.14140284 [DOI] [PubMed] [Google Scholar]
- 44.Gallitto E, Gargiulo M, Mascoli C, Freyrie A, De Matteis M, Serra C, et al. Persistent type II endoleak after EVAR: the predictive value of the AAA thrombus volume. J Cardiovasc Surg (Torino). 2015. July 29. [DOI] [PubMed] [Google Scholar]
- 45.Kray J, Kirk S, Franko J, Chew DK. Role of type II endoleak in sac regression after endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg. 2015;61(4):869–74. 10.1016/j.jvs.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 46.Nolz R, Schwartz E, Langs G, Loewe C, Wibmer AG, Prusa AM, et al. Stent graft Surface Movement after Infrarenal Abdominal Aortic Aneurysm Repair: Comparison of Patients with and without a Type 2 Endoleak. Eur J Vasc Endovasc Surg. 2015;50(2):181–8. 10.1016/j.ejvs.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 47.Pini R, Faggioli G, Mascoli C, Gallitto E, Freyrie A, Gargiulo M, et al. Influence of Statin Therapy on Type 2 Endoleak Evolution. Ann Vasc Surg. 2015;29(6):1167–73. 10.1016/j.avsg.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 48.Walker J, Tucker LY, Goodney P, Candell L, Hua H, Okuhn S, et al. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J Vasc Surg. 2015;62(3):551–61. 10.1016/j.jvs.2015.04.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousié M, Douillez V, Arend S, Arend P. Endovascular Aneurysm Repair (EVAR): A Ten-Year Retrospective Study in a Low-Volume Center. Acta Chir Belg. 2016;115:404–7. [DOI] [PubMed] [Google Scholar]
- 50.Phan DD, Meyer F, Pech M, Halloul Z. Length of abdominal aortic aneurysm and incidence of endoleaks type II after endovascular repair. Wien Klin Wochenschr. 2015;127(21–22):851–7. 10.1007/s00508-015-0871-y [DOI] [PubMed] [Google Scholar]
- 51.Piazza M, Squizzato F, Zavatta M, Menegolo M, Ricotta JJ 2nd, Lepidi S,et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. J Vasc Surg. 2016; 63(1):32–8. 10.1016/j.jvs.2015.08.049 [DOI] [PubMed] [Google Scholar]
- 52.Lo RC, Buck DB, Herrmann J, Hamdan AD, Wyers M, Patel VI, et al. Risk factors and consequences of persistent type II endoleaks. J Vasc Surg. 2016;63(4):895–901. 10.1016/j.jvs.2015.10.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mursalin R, Sakamoto I, Nagayama H, Sueyoshi E, Tanigawa K, Miura T, et al. Imaging-Based Predictors of Persistent Type II Endoleak After Endovascular Abdominal Aortic Aneurysm Repair. AJR Am J Roentgenol. 2016;206(6):1335–40. 10.2214/AJR.15.15254 [DOI] [PubMed] [Google Scholar]
- 54.Ikoma A, Nakai M, Sato M, Sato H, Minamiguchi H, Sonomura T, et al. Systolic Sac Pressure Index for the Prediction of Persistent Type II Endoleak for 12 Months After Endovascular Abdominal Aortic Aneurysm Repair. Cardiovasc Intervent Radiol. 2016;39(4):522–9. 10.1007/s00270-015-1191-3 [DOI] [PubMed] [Google Scholar]
- 55.Pippin K, Hill J, He J, Johnson P. Outcomes of type II endoleaks after endovascular abdominal aortic aneurysm (AAA) repair: a single-center, retrospective study. Clin Imaging. 2016;40(5):875–9. 10.1016/j.clinimag.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Fujimura N, Obara H, Matsubara K, Watada S, Shibutani S, Akiyoshi T, et al. Characteristics and Risk Factors for Type 2 Endoleak in an East Asian Population From a Japanese Multicenter Database. Circ J. 2016;80(1):118–23. 10.1253/circj.CJ-15-0850 [DOI] [PubMed] [Google Scholar]
- 57.Ward TJ, Cohen S, Fischman AM, Kim E, Nowakowski FS, Ellozy SH, et al. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol. 2013;24(1):49–55. 10.1016/j.jvir.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 58.Arko FR, Filis KA, Siedel SA, et al. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003;37(1):8–15. 10.1067/mva.2003.55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.