Abstract
High acrylamide (ACR) content in heat-processed carbohydrate-rich foods, as well as roasted products such as coffee, almonds etc., has been found to be as a risk factor for carcinogenicity and genotoxicity by The World Health Organization. Glycidamide (GLY), the epoxide metabolite of ACR, is processed by the cytochrome P-450 enzyme system and has also been found to be a genotoxic agent. The aim of this study was to determine whether ACR and/or GLY have any detrimental effect on the meiotic cell division of oocytes. For this purpose, germinal vesicle-stage mouse oocytes were treated with 0, 100, 500, or 1000 μM ACR or 0, 25, or 250 μM GLY in vitro. In vivo experiments were performed after an intraperitoneal injection of 25 mg/kg/day ACR of female BALB/c mice for 7 days. The majority of in vitro ACR-treated oocytes reached the metaphase-II stage following 18 hours of incubation, which was not significantly different from the control group. Maturation of the oocytes derived from in vivo ACR-treated mice was impaired significantly. Oocytes, reaching the M-II stage in the in vivo ACR-treated group, were characterized by a decrease in meiotic spindle mass and an increase in chromosomal disruption. In vitro GLY treatment resulted in the degeneration of all oocytes, indicating that ACR toxicity on female germ cells may occur through its metabolite, GLY. Thus, ACR exposure must be considered, together with its metabolite GLY, when female fertility is concerned.
Introduction
Acrylamide (ACR) is a colorless and odorless industrial compound formed by the hydration of acrylonitrile. It is widely used in the treatment of wastewater, paper/pulp manufacturing, mining, scientific research, surface coatings, textile and cosmetics [1, 2]. Environmental exposure to ACR may happen either by polymer construction or polymer use [3]. Once taken into the body, approximately 50% of ACR is metabolized to its epoxide metabolite glycidamide (GLY) by the cytochrome P-450 enzyme CYP2E1 [4]. Following ACR exposure, ACR is metabolized to GLY, and both molecules can be found in several tissues in the body [5].
ACR was first defined as a neurotoxic agent in 1989, after a detailed study on workers who were exposed to ACR and acrylonitrile for two or more years [6]. It was reported that the levels of hemoglobin adducts, which are biomarkers for ACR exposure [7], were correlated with the degree of peripheral neuropathy [6]. In 2001, Hagmar et al. [7] discovered that the reaction products of ACR may also be found in human blood without a known exposure history. Several hypotheses were proposed considering the unknown source of ACR exposure [8–10]. In April 2002, Tareke et al. [11] published a comparative analysis that documented the levels of ACR in heat-processed protein- and carbohydrate-rich foods, at 120°C or above, for the first time. This study showed that heated protein-rich foods possess 5–50 μg/kg ACR, whereas ACR content in heated carbohydrate-rich foods such as in potato, beetroot and crisp bread is significantly higher (150–4000 μg/kg). It was also reported that processed foods, such as roasted almonds, coated peanuts, fried potato, biscuits, and coffee, contain high levels of ACR [10]. In June 2002, The United Nations Food and Agricultural Organization (FAO) and The World Health Organization (WHO) held a consultation on “Health Implications of Acrylamide in Food” [12]. The report from the joint FAO/WHO consultation emphasized that ACR consuming diets may lead to severe carcinogenic implications and genotoxicity. Moreover, Hsu et al. [13] documented a heat-dependent increase of ACR levels in French fries, fried oil and vapor and noted that “fast-food restaurant workers are potentially subject to occupational hazards from acrylamide inhalation” in their very recently published article. Mojska et al. [14] also determined the ACR level in cigarette smoke with liquid chromatography–mass spectrometry in a Polish population and noted that tobacco smoke includes significant amounts of ACR with a total ACR exposure rate for smokers over 50% greater than for non-smokers.
The primary pathway responsible for the ACR genotoxicity is the production of GLY through CYP2E1 [15]. As reviewed by Favor and Shelby in 2005 [16], exposure of spermatozoa or spermatids to ACR or GLY increases the frequency of mutational events. In another study, a stronger effect was observed for GLY than ACR on the male reproductive system, especially on sperm cell viability [17]. In 1986, Sakamoto and Hashimoto [18] reported a decrease in fertility rates and litter size in mice exposed to 1.25 to 24 mg/kg/day ACR for 4 weeks. Sperm counts decreased and an increase in abnormal sperm was observed [18]. Male rats exposed to 4.2 to 7.9 mg/kg/day ACR for 10 weeks were characterized with reduced mounting activities and decreased numbers of sperm deposited in the uterus [19, 20]. It was also shown that 10 weeks exposure of ACR causes testicular atrophy, a decreased testes weight and the degeneration of seminiferous epithelium [21].
One possible mechanism of ACR-related neurotoxicity is the interference of GLY with kinesin-related motor proteins, which also play a crucial role in meiotic division [22]. Both meiotic division and chromosome integrity are fundamental to oocyte maturation, which occurs through a series of events including germinal vesicle (GV) breakdown and extrusion of the polar body. Mature oocytes, undergoing nuclear and cytoplasmic changes from pro-metaphase-I to metaphase-II (M-II), are key to fertilization and embryo development [23]. However, the number of ACR studies focusing on the female reproductive system has been limited. To our knowledge, this study is the first to evaluate the effects of GLY treatment on mouse oocytes. Therefore, the aim of this study was to examine the effect of ACR and its metabolite GLY on mouse oocytes in vitro and in vivo, focusing on meiotic maturation.
Materials and methods
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Republic of Turkey Ministry of Food, Agriculture and Livestock. Ethical approval of the study was obtained from Ankara University Local Ethical Board of Animal Experiments (approval no. 2012-17-109). All animals were housed in an appropriate temperature and dark-light cycles controlled room in the Laboratory Animal Unit of Ankara University. Mice were sacrificed by cervical dislocation.
All statistical analyses were performed through an SPSS software package (version 15.0; SPSS Inc., Chicago, IL, USA). A chi-square test was utilized to compare groups. The significance level was set at p < 0.05.
Isolation of mouse oocytes
The ovarian follicular development of 21-day-old female BALB/c mice (n = 37) was stimulated by the intraperitoneal injection of 0.5 IU pregnant mare’s serum gonadotropin. Animals were sacrificed 48 hours following injection, and oocytes were collected from the ovaries by follicular puncture. Denuded GV-stage oocytes (n = 1084) were transferred into gamete maturation medium (G-IVF Plus, Vitrolife, Sweden) and incubated in a humidified atmosphere of 5% CO2 at 37°C for 18 hours.
ACR and GLY experiments
ACR (purity 99.9%, MW 71.08 g/mol) was dissolved in ultrapure water to prepare a 10 mM stock solution. Working solutions were freshly prepared and dissolved either in a culture medium or 0.9% physiological saline depending on the experimental procedure. The in vitro and in vivo effects of ACR on oocyte maturation were tested by two different sets of experiments. To test the in vitro effects of ACR on oocyte meiotic maturation, GV-stage oocytes (n = 452) collected from female BALB/c mice (n = 15) were cultured in gamete maturation medium containing progressive doses of ACR (0, 100, 500 and 1000 μM) for 18 hours. M-II stage oocytes were then fixed with a microtubule-stabilizing buffer containing 2% formaldehyde and 0.1% Triton-X for 30 minutes at 37°C to assess the meiotic spindle and chromosome morphology.
The in vivo effects of ACR were tested by the intraperitoneal injection of ACR to female BALB/c mice (n = 12) for 7 days (25 mg/kg/day). Oocytes were then collected from the ovaries as described above. The maturation stage of the oocytes (n = 342) was determined with an inverted microscope (Olympus, Melville, NY, USA) equipped with a heating stage. M-II stage oocytes were fixed as described above.
To determine whether the in vivo effect of ACR was related to GLY metabolism or not, GV-stage oocytes from female BALB/c mice (n = 10) were exposed to 0, 25 or 250 μM GLY in vitro. One millimolar stock GLY (purity ≥96.0%, MW 87.08 g/mol) solution was prepared by dissolving 87.08 mg GLY in 1 mL ultrapure water. Working solutions were freshly prepared and dissolved in a culture medium. The maturation of oocytes (n = 290) was monitored for 18 hours and the maturation stage of each oocyte was determined.
Labeling of oocytes and image acquisition
Fixed oocytes were incubated in a 1:100 dilution of anti-α-tubulin/anti-β-tubulin (1:1 mixture) mouse monoclonal antibodies for 90 minutes at 37°C for the evaluation of meiotic spindle integrity. Oocytes were then washed in phosphate-buffered saline (PBS) and incubated in a 1:100 dilution of an affinity-purified fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, PA, USA) for 60 minutes at 37°C. Oocytes were stained with 10 μM 7-aminoactinomycine-D (7-AAD) as a nuclear stain. Finally, oocytes were mounted between glass slides and coverslips with a glycerol/PBS solution (1:1 mixture) containing 25 mg/mL sodium azide as an anti-fading reagent.
Slides were assessed with a laser scanning confocal microscope (Zeiss LSM-510, Jena, Germany) equipped with 488 nm Argon, 543 nm He-Ne and 633 nm He-Ne lasers and a 63× Zeiss Plan-Apo objective. Single and z-axis optical sections were collected, and 3D images were constructed using LSM-510 software.
Results
In vitro effect of ACR on mouse oocytes
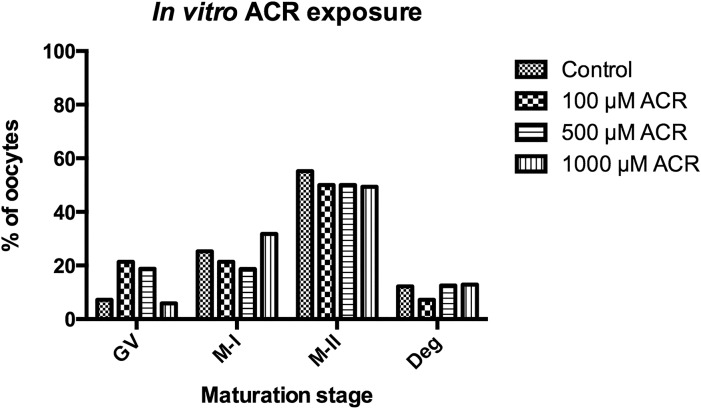
Meiotic spindle formation during in vitro maturation was monitored following treatment to GV-stage oocytes with 100, 500 and 1000 μM ACR. Following 18 hours of incubation, 55.2% of the oocytes reached the M-II stage in the control group. There was no significant difference between the control and ACR-treated groups in terms of meiotic resumption of the oocytes (50.0% for 100 μM, p = 0.613; 50.0% for 500 μM, p = 0.705; and 49.4% for 1000 μM, p = 0.373) (Fig 1). The meiotic spindle was observed in its typical barrel-shape and chromosomes were aligned properly along the metaphase plate in both the control (Fig 2A, 2B and 2C) and ACR-treated (Fig 2D, 2E and 2F) groups. The number of degenerated oocytes was also comparable between the control and ACR-treated groups following 18 hours of incubation (Fig 1).
Fig 1. In vitro Effect of ACR on Oocyte Maturation.
In vitro maturation stages of 0, 100, 500 and 1000 μM ACR-treated oocytes following 18 hours of incubation. There was no significant difference between control and ACR-treated groups in terms of oocytes reaching the M-II stage or degenerated oocytes.
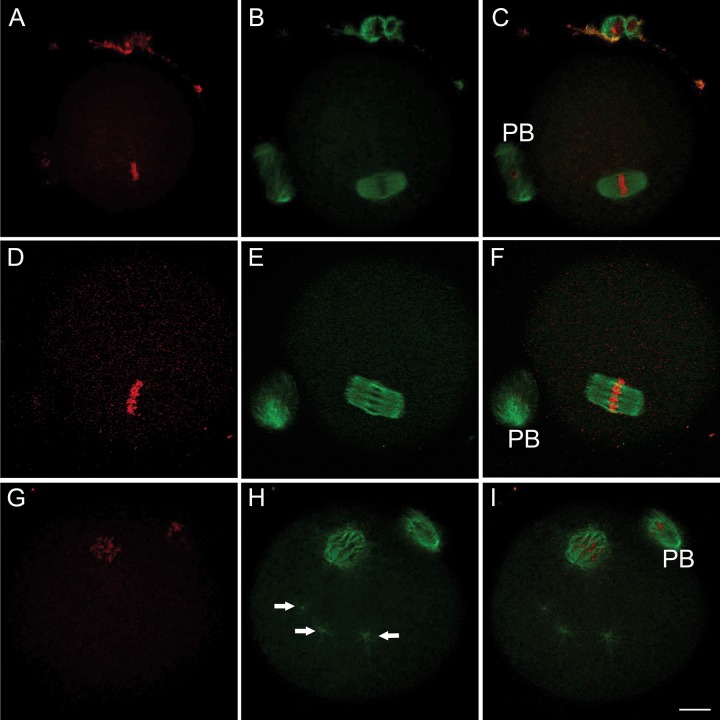
Fig 2. In vitro and In vivo Effects of ACR on Meiotic Spindle.
In vitro-matured M-II stage oocytes in control (A-C), in vitro ACR-treated (D-F) and in vivo ACR-treated (25 mg/kg) groups (G-I). Representative images in D-F were taken from a 1000 μM ACR-treated group. In vitro ACR-treated M-II stage oocytes showed no disruption in the meiotic spindle (green signal) and chromosome organization (red signal) appeared to be intact. In vivo ACR-treatment caused a decrease in spindle microtubule mass and disruption in the chromosome alignment. Multiple microtubule-organizing centers (MTOCs) were detected (arrows). PB: polar body. Scale bar: 10 μm.
In vivo effect of ACR on mouse oocytes
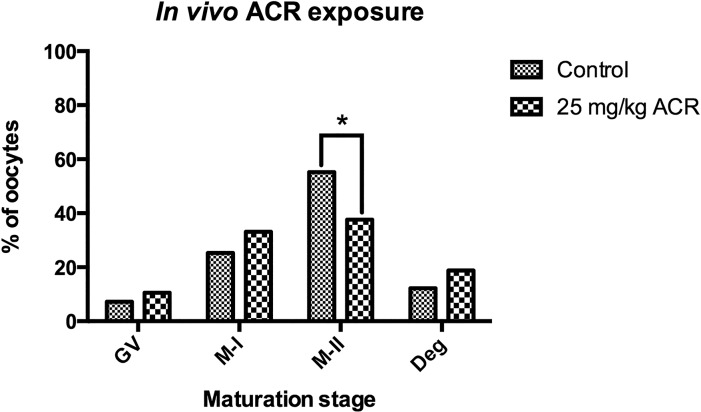
Isolated GV-stage oocytes from the ovaries of control or ACR-treated mice were incubated in a maturation medium for 18 hours. Oocytes were then subjected to meiotic resumption analyses. The percentage of M-II stage oocytes in the control group (55.2%) was significantly higher than in the ACR-treated group (37.6%, p = 0.001) (Fig 3). Although the degeneration rate was higher in the ACR-treated group (18.8%) compared to the controls (12.2%), it did not reach statistically significant levels (Fig 3). The majority of the M-II stage oocytes showed a distinct pattern in which the meiotic spindle mass was reduced and the chromosome alignment was disrupted in the ACR-treated group (Fig 2G, 2H and 2I). Moreover, multiple microtubule-organizing centers (MTOCs) were detected in the ACR-treated group (Fig 2H, arrows).
Fig 3. In vivo Effect of ACR on Oocyte Maturation.
Maturation stages of oocytes isolated from control and ACR-treated mice were presented. *The percentage of M-II stage oocytes was significantly higher in the control group compared to the ACR-treated group (p = 0.001).
In vitro effect of GLY on mouse oocytes
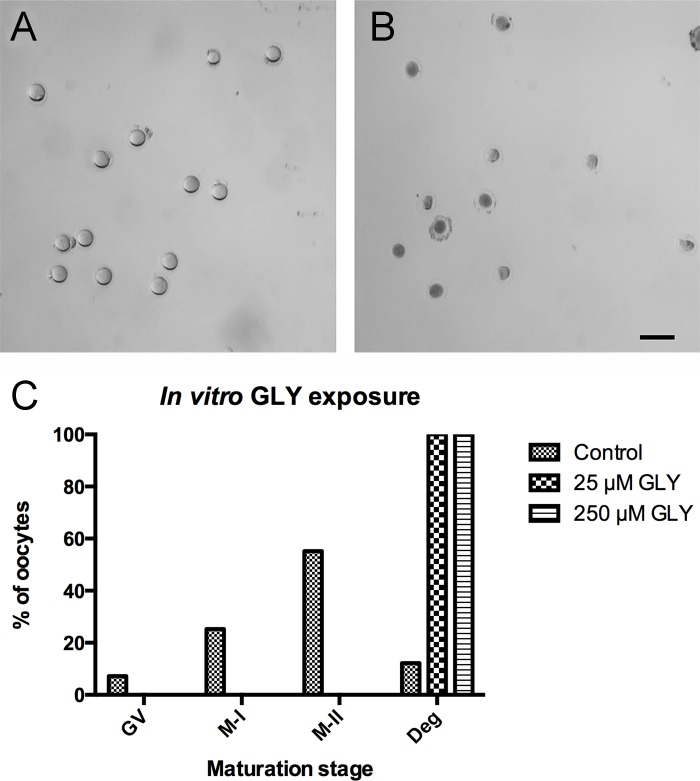
The discrepancy between the results of in vivo and in vitro ACR experiments led us to focus on the effects of GLY, the epoxide metabolite of ACR. To this end, isolated GV-stage oocytes were treated with 25 or 250 μM GLY for 18 hours in vitro. All oocytes were degenerated in both GLY treated groups, whereas 55.2% of the oocytes reached the M-II stage in the control group (Fig 4).
Fig 4. In vitro Effect of GLY on Oocyte Maturation.
In vitro-maturated oocytes were presented in control (A) and in vitro GLY-treated (B) groups. Bar graphs show the in vitro maturation stages of 0, 25 and 250 μM ACR-treated oocytes following 18 hours of incubation (C). The representative image in B was taken from a 25 μM GLY-treated group. The majority of the GV-stage oocytes reached the M-II stage in the control group, whereas all GLY-treated oocytes were degenerated following 18 hours of incubation (A-C). Scale bar: 200 μm.
Discussion
ACR is an industrial chemical produced in large quantities. Although exposure to industrial forms of ACR is limited to particular occupational groups, the discovery of the high ACR content in heat-processed and/or carbohydrate-rich foods has drawn worldwide attention. ACR has been declared as a priority existing chemical by numerous governments and organizations such as the United Kingdom, Australia, the WHO/FAO Joint Commission, the European Union and the Organization of Economic Cooperation and Development (OECD) [3].
According to our previous experience, particular environmental agents such as dicoumarol [24, 25] and bisphenol-A [24, 25] may affect germline and somatic cells in the female reproductive system. Therefore, we took the potential detrimental effects of ACR and GLY on reproductive health into consideration. To this end, firstly, the in vitro maturation of GV-stage oocytes was monitored following in vitro ACR treatment. Our findings showed that 100, 500 or 1000 μM ACR exposure did not block the in vitro maturation of mouse oocytes. In contrast, a recent study revealed that even low doses (10 and 20 μM) of in vitro ACR exposure to cumulus–oocyte complexes may cause a significant decrease in the number of mature oocytes. In the same study, chromosome misalignment and abnormal spindle configurations were distinguished following in vitro ACR exposure [26]. On the contrary, we have shown that in vitro ACR treatment had no adverse effects on meiotic spindle or chromosomes. Since Liu et al tested the in vitro effects of ACR on cumulus-enclosed oocytes, we wanted to examine the effects of ACR on denuded oocytes. As discussed later in this section, we hypothesize that the incubation of oocytes with the granulosa cells may have caused this effect.
Secondly, oocytes derived from in vivo ACR-treated (25 mg/kg/day) BALB/c mice were subjected to meiotic resumption analysis. Our findings demonstrated that in vivo ACR treatment impaired oocyte maturation. The number of M-II stage oocytes was significantly higher in the control group compared to the ACR-treated groups. Moreover, a diminished meiotic spindle mass and disrupted chromosome alignment were observed in M-II stage oocytes following in vivo ACR exposure. Likewise, Duan et al. [27] noted that oral ACR administration (10 mg/kg/day and 50 mg/kg/day for 6 weeks) reduced the GV-breakdown and polar body extrusion rates and induced abnormal meiotic spindle rates, as well as reactive oxygen species (ROS) levels and Annexin-V immunopositivity [27]. In the same study, it was reported that litter sizes were significantly smaller following ACR-feeding compared to control mice. In a study by Hulas-Stasiak et al. [28], the ovaries of 3 mg/kg/day ACR-treated pregnant guinea pig pups were analyzed. It was demonstrated that in vivo ACR exposure caused a decrease in the number of healthy follicles and an increase in the apoptotic cells. Hulas-Stasiak et al. also argued that ACR exposure breaks oocyte–cumulus cell connections by disrupting vimentin filaments resulting in apoptosis [28]. Similarly, Wei et al. [29] reported that oral ACR exposure causes a significant reduction in body weights, organ weights and the number of corpora lutea in mice, possibly acting through the nitric oxide synthase (NOS)-signaling pathway. In a very recently published study, Dobrovolsky et al. [30] evaluated the genotoxicity of ACR by treating rats with similar doses (20 mg/kg/day) of ACR and noted that ACR exposure resulted in DNA damage in the liver but not in the bone marrow, indicating a tissue-specific genotoxic effect of ACR.
Inconsistency between the results of in vivo and in vitro ACR experiments in our study led us to focus on the effects of GLY, the epoxide metabolite of ACR, which was previously suspected to cause genotoxicity [15, 31]. Sickles et al. [22] suggested that ACR displays its toxic effects on peripheral neuropathies through GLY, causing a concentration-dependent reduction in the binding of motor-protein kinesin to microtubules [22, 32]. Consistent with this hypothesis, there are studies indicating that a stable metabolite may be involved in ACR toxicity on germ cells, rather than ACR itself. For example, the long-term administration of ACR induces chromatid exchanges and breaks in spermatogonia of mice, whereas adverse effects are not remarkable during short-term administration [33]. It has also been reported that reproductive toxicity is less severe when a single high dose of ACR is administered compared to recurrent low doses [34]. In addition, ALKarim et al. [35] determined that long-term exposure to a 60 μg/kg/day dose of ACR was associated with cystic ovarian changes and degenerative changes of the zona pellucida, granulosa cells and oocytes in post-weaning Sprague Dawley rats. In light of these data, we conclude that short-term or single-dose exposures to ACR are not solely responsible for toxicity. Rather, one of the major metabolites of ACR causes this effect.
To test whether GLY is involved in ACR toxicity on female germ cells, isolated GV-stage oocytes were treated directly with 25 or 250 μM GLY for 18 hours in vitro. GLY exposure resulted in the degeneration of all oocytes, indicating that ACR toxicity may occur through GLY metabolism. It is known that the conversion of ACR to GLY is catalyzed by the CYP2E1 enzyme, a member of cytochrome P-450 family [34]. One of the essential questions of this study is where this conversion occurs. As indicated above, ACR blocks the in vitro oocyte maturation process in cumulus-enclosed oocytes but not in denuded oocytes. Therefore, the existence of the CYP2E1 enzyme in either granulosa cells or oocytes appears to be important. According to a study by Sobinoff et al. [36], granulosa cells and oocytes from control C57BL/6 mice do not have CYP2E1 immunopositivity, whereas oocyte nuclei show a strong CYP2E1 positivity following smoke exposure. Therefore, we hypothesize that CYP2E1 enzyme-mediated ACR-to-GLY conversion may be an inducible situation and that cumulus cells may induce this conversion. In a study by Ghanayem et al. [34], CYP2E1-null and wild-type male mice were treated with 0, 12.5, 25 and 50 mg/kg/day ACR for 5 days and then mated to untreated females. ACR administration to CYP2E1-null mice resulted in healthy offspring [34], indicating that ACR itself is not toxic to the male reproductive system in tested doses, but rather its metabolite GLY is. Ghanayem et al. [34] also reported that the number of pregnant females and live fetuses decreased in females mated to wild-type mice that can metabolize ACR to GLY. The results, indicating ACR-GLY conversion, of our in vivo and in vitro ACR experiments are consistent with the findings of Ghanayem et al. [34] Although the results of a study by Liu. et al. [26] conflict with this hypothesis, the presence of granulosa cells in culture conditions and the CYP2E1 enzyme activity of granulosa cells should be considered when in vitro ACR results are compared to our study, in which only denuded oocytes were studied.
In conclusion, since the direct exposure to ACR does not have toxic effects on mouse oocytes whereas systemic exposure does, we hypothesize that ACR toxicity is caused by its metabolite GLY and we believe that future studies focusing on ACR metabolites, particularly focusing on microtubule polymerization dynamics and microtubule related proteins, should be performed. In one study, internal doses of ACR and GLY were evaluated in humans. Tolerable daily intake (TDI) for neurotoxicity for ACR was estimated to be 40 μg/kg-day whereas the TDI for cancer was estimated to be 2.6 and 16 μg/kg/day for ACR and GLY levels, respectively [37]. A dose–response curve has not been assessed for ACR or GLY in humans, in terms of female fertility. In view of our results, we strongly believe that chronic exposure to ACR must be taken into consideration and a TDI for reproductive toxicity should be estimated for both ACR and GLY in future studies. We also believe that since ACR has tissue-specific genotoxic effects, these effects should be investigated in the ovary, particularly in folliculogenesis. We have initiated a prospective study aiming to measure acrylamide and/or glycidamide levels in the follicular fluid from couples undergoing in vitro fertilization treatment in terms of the effects of these chemicals on female infertility.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by Ankara University Scientific Research Council BAP13B3330012 to SO, AC, and OC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith E, Prues S, Oehme F. Environmental degradation of polyacrylamides. 1. Effects of artificial environmental conditions: Temperature, light, and ph. Ecotoxicol Environ Saf. 1996;35: 121–135. 10.1006/eesa.1996.0091 [DOI] [PubMed] [Google Scholar]
- 2.Smith E, Prues S, Oehme F. Environmental degradation of acrylamides 2. Effects of environmental (outdoor) exposure. Ecotoxicol Environ Saf. 1997;37: 76–91. [DOI] [PubMed] [Google Scholar]
- 3.NICNAS. Acrylamide—priority existing chemical assessment report. Australia: National Industrial Chemicals Notification and Assessment Scheme (NICNAS); 2002. [Google Scholar]
- 4.Sumner SC, MacNeela JP, Fennell TR. Characterization and quantitation of urinary metabolites of [1, 2, 3-13c] acrylamide in rats and mice using carbon-13 nuclear magnetic resonance spectroscopy. Chem Res Toxicol. 1992;5: 81–89. [DOI] [PubMed] [Google Scholar]
- 5.Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in b6c3f 1 mice. Toxicol Applied Pharm. 2005;202: 258–267. [DOI] [PubMed] [Google Scholar]
- 6.He F, Zhang S, Wang H, Li G, Zhang Z, Li F, et al. Neurological and electroneuromyographic assessment of the adverse effects of acrylamide on occupationally exposed workers. Scand J Work Env Hea. 1989;15: 125–129. [DOI] [PubMed] [Google Scholar]
- 7.Hagmar L, Törnqvist M, Nordander C, Rosén I, Bruze M, Kautiainen A, et al. Health effects of occupational exposure to acrylamide using hemoglobin adducts as biomarkers of internal dose. Scand J Work Env Hea. 2001;27: 219–226. [DOI] [PubMed] [Google Scholar]
- 8.Dybing E, Sanner T. Risk assessment of acrylamide in foods. Toxicol Sci. 2003;75: 7–15. 10.1093/toxsci/kfg165 [DOI] [PubMed] [Google Scholar]
- 9.Exon JH. A review of the toxicology of acrylamide. J Toxicol Environ Health B Crit Rev. 2006;9: 397–412. 10.1080/10937400600681430 [DOI] [PubMed] [Google Scholar]
- 10.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51: 4504–4526. 10.1021/jf030204 [DOI] [PubMed] [Google Scholar]
- 11.Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50: 4998–5006. [DOI] [PubMed] [Google Scholar]
- 12.FAO, WHO. Health implications of acrylamide in food. Switzerland: FAO/WHO Joint Commission; 2002. [Google Scholar]
- 13.Hsu H-T, Chen M-J, Tseng T-P, Cheng L-H, Huang L-J, Yeh T-S. Kinetics for the distribution of acrylamide in french fries, fried oil and vapour during frying of potatoes. Food Chemistry. 2016;211: 669–678. 10.1016/j.foodchem.2016.05.125 [DOI] [PubMed] [Google Scholar]
- 14.Mojska H, Gielecińska I, Cendrowski A. Acrylamide content in cigarette mainstream smoke and estimation of exposure to acrylamide from tobacco smoke in poland. Ann Agric Environ Med. 2016;23: 456 10.5604/12321966.1219187 [DOI] [PubMed] [Google Scholar]
- 15.Manjanatha MG, Aidoo A, Shelton SD, Bishop ME, McDaniel LP, Lyn-Cook LE, et al. Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female big blue mice. Environ Mol Mutagen. 2006;47: 6–17. 10.1002/em.20157 [DOI] [PubMed] [Google Scholar]
- 16.Favor J, Shelby MD. Transmitted mutational events induced in mouse germ cells following acrylamide or glycidamide exposure. Mutat Res Genet Toxicol Environ Mutagen. 2005;580: 21–30. [DOI] [PubMed] [Google Scholar]
- 17.Costa LG, Deng H, Gregotti C, Manzo L, Faustman EM, Bergmark E, et al. Comparative studies on the neuro- and reproductive toxicity of acrylamide and its epoxide metabolite glycidamide in the rat. Neurotoxicology. 1992;13: 219–224. [PubMed] [Google Scholar]
- 18.Sakamoto J, Hashimoto K. Reproductive toxicity of acrylamide and related compounds in mice—effects on fertility and sperm morphology. Arch Toxicol. 1986;59: 201–205. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Tanii H. Mutagenicity of acrylamide and its analogues in salmonella typhimurium. Mutat Res Genet Tox. 1985;158: 129–133. [DOI] [PubMed] [Google Scholar]
- 20.Zenick H, Hope E, Smith MK. Reproductive toxicity associated with acrylamide treatment in male and female rats. J Toxicol Environ Health. 1986;17: 457–472. 10.1080/15287398609530840 [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Sakamoto J, Tanii H. Neurotoxicity of acrylamide and related compounds and their effects on male gonads in mice. Arch Toxicol. 1981;47: 179–189. [DOI] [PubMed] [Google Scholar]
- 22.Sickles DW, Sperry AO, Testino A, Friedman M. Acrylamide effects on kinesin-related proteins of the mitotic/meiotic spindle. Toxicol Appl Pharmacol. 2007;222: 111–121. 10.1016/j.taap.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 23.Can A, Semiz O, Cinar O. Centrosome and microtubule dynamics during early stages of meiosis in mouse oocytes. Mol Hum Reprod. 2003;9: 749–756. [DOI] [PubMed] [Google Scholar]
- 24.Aras D, Cinar O, Cakar Z, Ozkavukcu S, Can A. Can dicoumarol be used as a gonad-safe anticancer agent: An in vitro and in vivo experimental study. Mol Hum Reprod. 2016;22: 57–67. 10.1093/molehr/gav065 [DOI] [PubMed] [Google Scholar]
- 25.Cinar O, Semiz O, Can A. Carbofuran alters centrosome and spindle organization and delays cell division in oocytes and mitotic cells. Toxicol Sci. 2015;144: 298–306. 10.1093/toxsci/kfu317 [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Jiang L, Zhong T, Kong S, Zheng R, Kong F, et al. Effect of acrylamide on oocyte nuclear maturation and cumulus cells apoptosis in mouse in vitro. PloS one. 2015;10: e0135818 10.1371/journal.pone.0135818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan X, Wang Q-C, Chen K-L, Zhu C-C, Liu J, Sun S-C. Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Sci Rep. 2015;5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulas-Stasiak M, Dobrowolski P, Tomaszewska E, Kostro K. Maternal acrylamide treatment reduces ovarian follicle number in newborn guinea pig offspring. Reproductive Toxicology. 2013;42: 125–131. 10.1016/j.reprotox.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 29.Wei Q, Li J, Li X, Zhang L, Shi F. Reproductive toxicity in acrylamide-treated female mice. Reproductive Toxicology. 2014;46: 121–128. 10.1016/j.reprotox.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 30.Dobrovolsky VN, Pacheco-Martinez MM, McDaniel LP, Pearce MG, Ding W. In vivo genotoxicity assessment of acrylamide and glycidyl methacrylate. Food Chem Toxicol. 2016;87: 120–127. 10.1016/j.fct.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 31.Nixon B, Stanger S, Nixon B, Roman S. Chronic exposure to acrylamide induces DNA damage in male germ cells of mice. Toxicol Sci. 2012;129: 135–145. 10.1093/toxsci/kfs178 [DOI] [PubMed] [Google Scholar]
- 32.Sickles DW, Brady ST, Testino A, Friedman MA, Wrenn RW. Direct effect of the neurotoxicant acrylamide on kinesin-based microtubule motility. J Neurosci Res. 1996;46: 7–17. [DOI] [PubMed] [Google Scholar]
- 33.Shiraishi Y. Chromosome aberrations induced by monomeric acrylamide in bone marrow and germ cells of mice. Mutat Res. 1978;57: 313–324. [DOI] [PubMed] [Google Scholar]
- 34.Ghanayem BI, Witt K, El-Hadri L, Hoffler U, Kissling G, Shelby M, et al. Comparison of germ cell mutagenicity in male cyp2e1-null and wild-type mice treated with acrylamide: Evidence supporting a glycidamide-mediated effect. Biol Reprod. 2005;72: 157–163. 10.1095/biolreprod.104.033308 [DOI] [PubMed] [Google Scholar]
- 35.ALKarim S, ElAssouli S, Ali S, Ayuob N, ElAssouli Z. Effects of low dose acrylamide on the rat reproductive organs structure, fertility and gene integrity. Asian Pac J Reprod. 2015;4: 179–187. [Google Scholar]
- 36.Sobinoff A, Beckett E, Jarnicki A, Sutherland J, McCluskey A, Hansbro P, et al. Scrambled and fried: Cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol Appl Pharm. 2013;271: 156–167. [DOI] [PubMed] [Google Scholar]
- 37.Tardiff RG, Gargas ML, Kirman CR, Carson ML, Sweeney LM. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem Toxicol. 2010;48: 658–667. 10.1016/j.fct.2009.11.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.