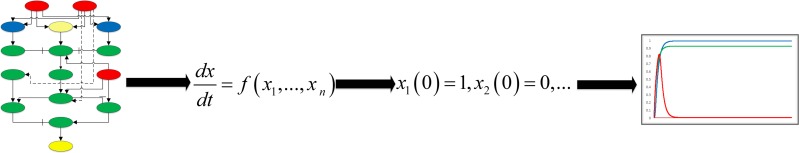Abstract
Stomatal closure is affected by various stimuli such as light, atmospheric carbon dioxide concentration, humidity and phytohormones. Our research focuses on phytohormones, specifically: abscisic acid (ABA), ethylene (ET) and methyl jasmonate (MeJA) that are responsible for the regulation of several plant processes, especially in guard cell signalling. While several studies show that these three phytohormones cause stomatal closure in plants, only two studies are notable for establishing a mathematical model of guard cell signalling involving phytohormones. Those two studies employed Boolean modelling and mechanistic ordinary differential equations modelling. In this study, we propose a new mathematical model of guard cell transduction network for stomatal closure using continuous logical modelling framework. Results showed how the different components of the network function. Furthermore, the model verified the role of antioxidants in the closure mechanism, and the diminished closure level of stomata with combined ABA-ET stimulus. The analysis was extended to ABA-ET-MeJA crosstalk.
Introduction
Stomata are microscopic pores commonly found in the lower epidermis of plant leaves that are very important in the growth and survival of plants [1]. Each pore is formed by two guard cells that regulate the stomatal closure mechanism by controlling turgor pressure on them. When the guard cells are swollen, the pore opens. In contrast, when the guard cells are flaccid, the pore closes [2]. The loss of turgor pressure is a consequence of the efflux of ions out of the guard cells which results to stomatal closure [3].
The opening and closing of the stomata is caused by a variety of stimuli such as light, atmospheric carbon dioxide concentration, humidity and plant hormones [2,4]. For instance, during hot or dry days, plants close their stomata as a natural response to conserve water [3]. It is important for the plants to regulate stomatal closure to adapt to these external challenges.
Drought stress is a major abiotic condition that has adverse effects on plant growth and yield. Water deficiency may result to cellular dehydration leading to damage and may eventually be fatal to plants [5]. As defense response to water stress, certain plant hormones trigger stomatal closure. One of these hormones is abscisic acid (ABA) which is synthesized during drought stress. As the soil dries, ABA builds up in leaves, thus promoting closure [2,6]. Ethylene (ET) is another effector of stomatal closure. It is involved in the regulation of various plant processes [7]. Although both ABA and ET are known to cause stomatal closure, they fail to achieve full closure when applied simultaneously [2]. Likewise, jasmonates are phytohormones that trigger closure. They regulate plant processes such as pollen maturation and tendril coiling. Methyl jasmonate (MeJA) is a volatile methyl ester of jasmonic acid which has been used in studying jasmonic signaling pathway [8–10].
Various studies have described crosstalk in guard cell signalling [1,2,8,9,11,12]. Some of these studies include the interaction between hormones such as ABA, ET and MeJA [1,2,8,9]. One notable work about guard cell signalling was the study of Li et al. [13] which adapted Boolean modelling in predicting essential components of the ABA guard cell signalling transduction network. Beguerisse-Diaz et al. [2], on the other hand, have developed an ordinary differential equation (ODE) model of stomatal closure based on biochemical pathway information. They were able to describe the role of antioxidant mechanisms in the lack of stomatal closure when guard cells are subjected to the combined stimulus of ABA and ET.
However, using ODEs to describe the biochemical processes of a system requires adequate information about biological mechanisms and kinetic parameters—which implies that mechanistic ODE modelling may not be possible. In this study, we explored on analyzing the guard cell signalling network using continuous logical modelling. This modelling formalism was developed by Mendoza and Xenarios [14] and was described as a semi-quantitative technique dealing with logic-based ordinary differential equations. Although it cannot provide comprehensive quantitative information about signalling network, it can confer substantial qualitative information such as trends in various biological networks. Moreover, analysis using semi-quantitative modelling can be useful in determining essential properties of the network through its topology despite the unavailability of experimental data [15]. Sankar et al. [16] have successfully used the continuous logical framework to analyze cellular auxin and brassinosteriod signalling and their interaction.
The interaction between the three phytohormones ABA, ET and MeJA in guard cell signalling was the focus of this study. To our knowledge, no mathematical models on stomatal closure have considered the interaction among these hormones.
This paper proceeds with a further discussion of signal transduction network of stomatal closure. We then describe the methodology used to construct the mathematical model of guard cell signalling pathway. Results and discussions highlight the role of antioxidants, the effect of the combined ABA-ET stimulus and the inclusion of MeJA in the network in the closure mechanism. Based on these, we draw conclusions on the guard cell signalling network.
Signal transduction for stomatal closure
The phytohormones ABA, ET and MeJA are known to be effectors of stomatal closure in plants. An integrated ABA and ethylene signalling network in guard cells was shown in the study of [2]. Additionally, [9] proposed signalling pathway and signal crosstalk between MeJA and ABA in guard cells. Based on these two studies and supported by other relevant literature, we constructed a signal transduction network. It should be noted, however, that the model established in [2] was considered in this study with additional connections to incorporate MeJA in the network.
There are several identified components in the integrated ABA and ethylene signalling network in guard cells. Reactive oxygen species (ROS) and nitric oxide (NO) are central components of the signalling network that regulate stomatal movement in response to hormones such as ABA [7]. ABA sequesters the protein phosphatase 2C ABA-insensitive 1 (ABI1) which lead to phosphorylation of NADPH-oxidase Arabidopsis thaliana respiratory burst oxidase homolog F (AtrbohF) by kinase open stomata 1 that produces ROS such as superoxide and hydrogen peroxide [17]. During the ethylene-induced stomatal closure, AtrbohF upregulates the production of ROS [18]. Consequently, the increase on the level of ROS results to the increased production of NO through the nitrate reductase 1 (NIA1) [19–21].
Other ABA-induced cellular responses include the activation of vacuolar proton pumps that promote the cytosolic pH (pHcyt) [22]. The positive regulation of NO and pHcyt reduces the concentration of K+ ions by increasing its efflux and decreasing the influx. An increased efflux means an escalation of the number of available outwards-rectifying K+ channels (IK,out), while reduced influx means a down-regulation of inwards-rectifying K+ channels(IK,in) [12,23,24]. The resulting lower concentration of K+ ions leads to the loss of turgor and eventually to the closure of the stomatal pore [4,23]. We denote as active IK,out and as active IK,in.
Antioxidants are also essential components of the transduction network. Based on experimental data, the network in [2] suggests two antioxidant mechanisms which are active in guard cells. Two antioxidants included were described by AXO1 and AXO2 lying at the end of two linear activation cascades due to doses of ABA and ET. One is a generic antioxidant in response to an individual stimulus, ABA or ET. This mechanism allows ROS to signal downstream to control oxidative stress of around two hours. The other is an antioxidant response which is active only when both hormones, ABA and ET, are present simultaneously. This response happens for about 10 minutes, and disrupts the closure process. Additional hypothesized connections are then established, which include the activation of both pHcyt and NO by ET.
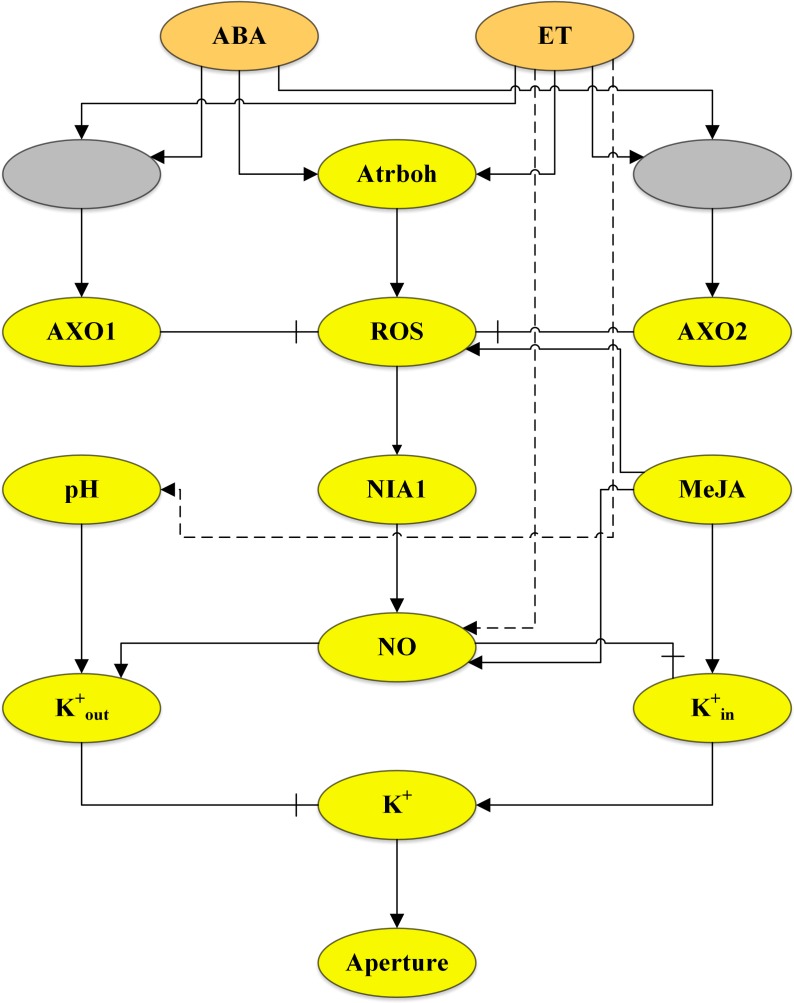
ROS and NO play a significant role in MeJA-induced stomatal closure. It is shown that MeJA induces ROS in guard cells. Similarly, MeJA facilitates the production of NO to induce stomatal closure [9]. Fig 1 shows the complete transduction network in guard cells involving the three phytohormones.
Fig 1. Integrated ABA, ethylene and MeJA signalling network in guard cells.
The hormones ABA, ET, and MeJA are the input nodes shown as orange ellipses. The two grey ellipses represent components of the network that are not yet known and regarded as linear activation cascades. Yellow ellipses are the variable nodes. Interactions between the components are shown by the green lines connecting them. Activation or production is represented as solid lines ending in an arrowhead while inactivation, repression, or scavenging is represented by solid lines ending in a hammerhead. Broken lines represent hypothesized connections that require experimental validation.
Methodology
Construction of the different models of the guard cell
Signalling pathway
Sixteen models have been considered in the analysis of the guard cell signalling network. These models include all 16 components of the guard cell transduction system shown in Fig 1. The variations were made by removing connections from one component to the other and incorporating logical connections, delay, and other assumptions. Table 1 provides a summary of the models.
Table 1. Sixteen models of the guard cell signalling.
| Model | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| M1 | Yes | No | No | No | No | No | No |
| M2 | Yes | No | Yes | No | No | No | No |
| M3 | Yes | Yes | No | No | No | No | No |
| M4 | Yes | Yes | Yes | No | No | No | No |
| M5 | Yes | Yes | No | Yes | No | No | No |
| M6 | Yes | Yes | Yes | Yes | No | No | No |
| M7 | Yes | Yes | No | No | Yes | No | No |
| M8 | Yes | Yes | Yes | No | Yes | No | No |
| M9 | Yes | Yes | No | No | Yes | Yes | No |
| M10 | Yes | Yes | Yes | No | Yes | Yes | No |
| M11 | Yes | Yes | No | No | Yes | No | Yes |
| M12 | Yes | Yes | Yes | No | Yes | No | Yes |
| M13 | Yes | Yes | No | No | Yes | Yes | Yes |
| M14 | Yes | Yes | Yes | No | Yes | Yes | Yes |
| M15 | Yes | Yes | No | No | No | No | Yes |
| M16 | Yes | Yes | Yes | No | No | No | Yes |
where
C1: Inclusion of the 16 components
C2: Logical and operation on ABA and ET signals to activate AXO2 without cascading
C3: Incorporation of cascading to activate AXO1 and AXO2
C4: Activation of AXO1 is only affected by ABA
C5: Activation of AXO1 is only affected by ET
C6: Elimination of the connection of ET to pH
C7: Elimination of the connection of ET to NO
Implementation of continuous logical modelling framework
A continuous logical modelling was used in analyzing the guard cells transduction network. Mendoza and Xenarios [14] have proposed a standard technique of transforming a signalling network into a continuous dynamical system model. The first step in continuous logical modelling formalism is to convert the network into a continuous dynamical system. This is followed by setting the initial state of the variables and specifying all the parameter values. The system is then run until it converges to a steady state. The complete process is illustrated in Fig 2.
Fig 2. Schematic diagram of constructing a continuous dynamical system.
To describe the network as a continuous dynamical system, the following set of ordinary differential equations was used:
Let be the set of activators and be the set of inhibitors of xi, for every species xi. Then
| (1) |
| (2) |
where * indicates use if xi has both activators and inhibitors, ** indicates use if xi has activators only, and, *** indicates use if xi has inhibitors only.
The differential equation’s right-hand side incorporates an activation function and a term for decay. The activation is a sigmoid function of ω which corresponds to the total input to the node. The values of the following variables and parameters were given as follows: 0 ≤ xi ≤ 1, 0 ≤ ωi ≤ 1, h, αn, βm and γi > 0. Since the value of node x is bounded to be within the interval [0,1], it suggests that the level of activation is normalized and not an absolute value. The decay rate γi is directly proportional to the level of activation of the node [14]. The total input to the node is an incorporation of several inhibition and activation mechanisms acting on a node. The function ω is defined in three forms to account for the effect of both activatory and inhibitory information. The weight of the activators and inhibitors are represented by the parameters α and β, respectively. Moreover, ω gives a bounded sigmoid form, regardless of the values of α and β. The parameter h is called the gain of the sigmoid function and controls the steepness of the curve.
Each model constructed has a total of 13 differential equations associated with the mechanism of the signalling network. These equations are as follows:
| (3) |
The values of the parameters α, β and γ are all set to 1, while parameter h = 10. These are default values adapted from the work of Mendoza and Xenarios [14]. A software package called Berkeley Madonna, created by Robert Macey and George Oster of the University of California at Berkeley [25], was used to solve the system of ODEs. The fixed delay mechanism and the logical and operation were incorporated using default functions in the software. The delay was added to incorporate the activation mechanism of the two antioxidants. The initial activation levels of the involved components were all set to zero, except for x1, x2 and x16 which are the input variables. The models were run using the different values of the input variables. The effect of the hormones on the various components of the network, upon introduction to the system, was observed. The simulation started with all input variables set to 0. The level was increased by increments of 0.1 until the maximum level of 1.0 was achieved. The response of the different components was then observed. Several combinations of these values for ABA, ET and MeJA levels were used for the simulation process. The list of notations used in the implementation is given in Table 2.
Table 2. Notations used in the continuous logical model.
| Component | Notation |
|---|---|
| ABA | x1 |
| ET | x2 |
| Unknown component 1 | x3 |
| Atrboh | x4 |
| Unknown component 2 | x5 |
| AXO1 | x6 |
| ROS | x7 |
| AXO2 | x8 |
| pH | x9 |
| NIA1 | x10 |
| NO | x11 |
| x12 | |
| x13 | |
| K+ | x14 |
| Aperture | x15 |
| MeJA | x16 |
Results and discussion
Upon analyzing all the models, M16 showed the most consistent result. Unlike M16, the other 15 models did not show the diminished stomatal closure level when guard cells were presented with combined ABA and ET stimulus. The model considered the logical operator on AXO2 and delay mechanism on the antioxidants. Moreover, the model incorporated the hypothesized connection between pH and ET.
By examining M16 closely, we deduced that in the absence of both ET and ABA, guard cells cannot sustain stomatal closure. This result was expected since both ET and ABA are effectors of stomatal closure response [2,26]. Moreover, the results confirmed the role of ABA and ET in inducing closure of the stomata. The increase in the level of ABA was important in maintaining the closure. Closure was sustained when an ABA level of 0.35 was introduced. Similar results were observed when ET was introduced in the system.
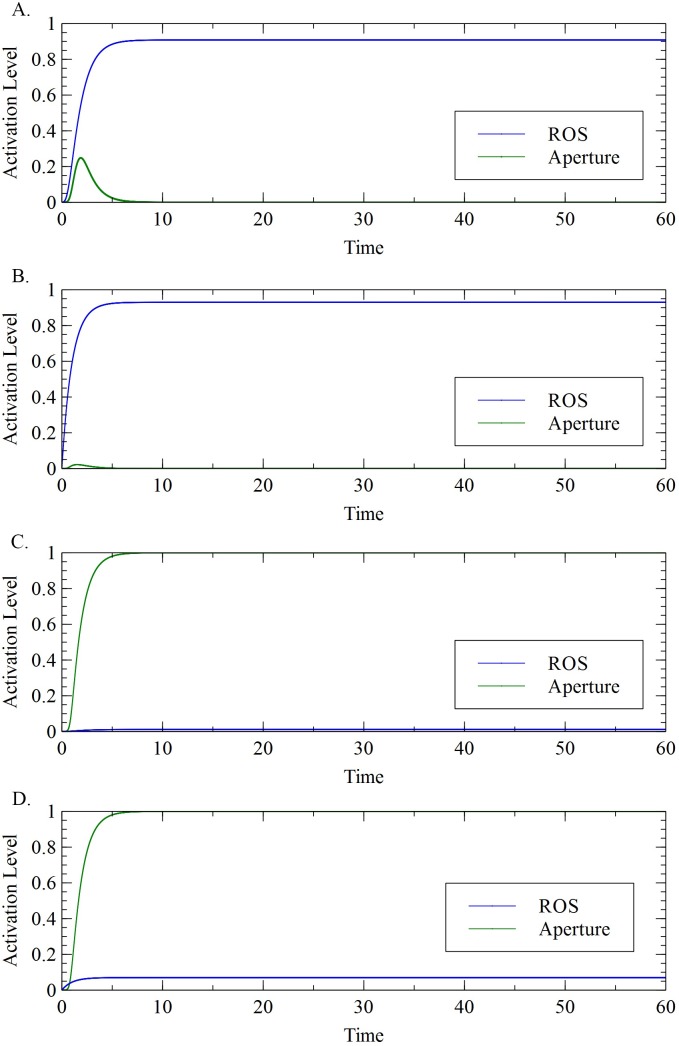
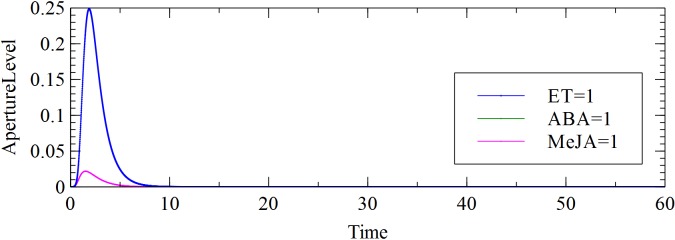
Results also showed that a sufficient level of ROS production was needed to maintain the closed state of stomata (Fig 3). This confirmed the significance of ROS in ABA and ET-induced stomatal closure [2,18,24,27,28]. When the level of ROS is close to zero, the stomata were open.
Fig 3. Activation level of ROS and aperture.
(A) ABA or ET level is equal to 1; (B) MeJA level equal to 1; (C) ABA or ET level is equal to 0.2; (D) MeJA level is equal to 0.2.
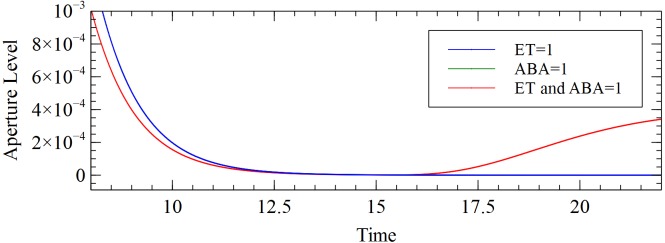
Under the combined ABA and ET stimulus, stomatal closure is diminished as compared to the effect of either individual ABA or ET (Fig 4). This is consistent with the reports in [2,18,29]. The low level of ROS, due to the antioxidant response that activates when both ABA and ET are present, corresponds to the diminished closure. The model also confirmed the important role of the two antioxidant mechanisms in the stomatal closure. These mechanisms were the delayed response activated by a single stimulus and the more rapid antioxidant activity that is only activated when both ABA and ET stimuli are present.
Fig 4. Aperture response to combined ABA and ET stimulus.
The model was extended by establishing a crosstalk with another phytohormone, MeJA. Two connections were added: activation of ROS by MeJA and activation of NO by MeJA. Observation and analysis show that MeJA had a positive effect on stomatal closure, as shown in Fig 5. The MeJA-induced stomatal closure was caused by the increased level of both NO and ROS, both of which play a significant role in the guard cell MeJA signalling [9]. When compared to the individual effect of ABA or ET, MeJA was slightly inducing more closure than ABA or ET.
Fig 5. Aperture response to ABA, ET and MeJA.
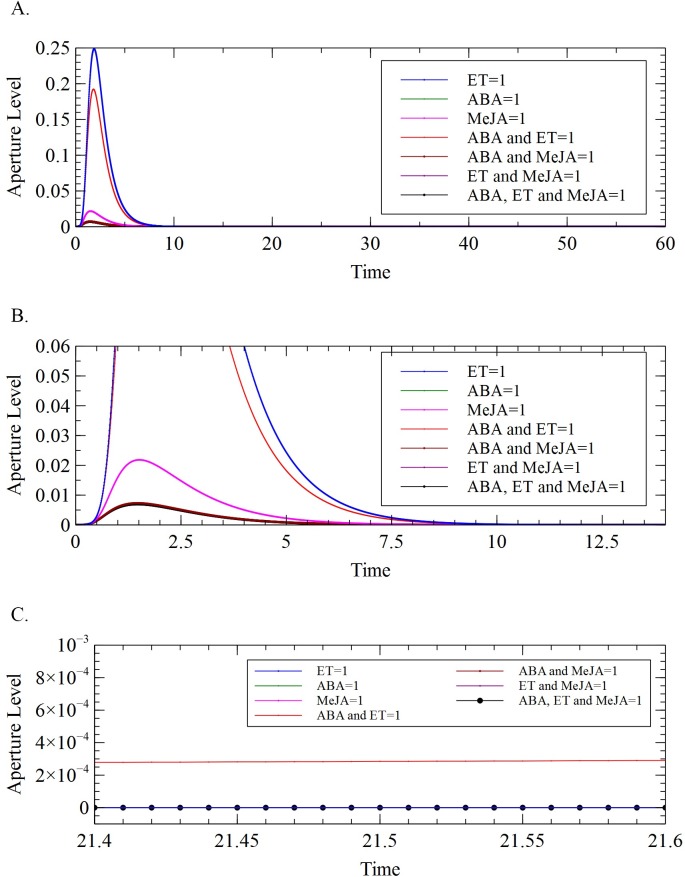
The interactions of ET with MeJA and of ABA with MeJA were also investigated using the model. Results showed that MeJA enhanced the effect of both ABA and ET in the closing of the stomata. The enhanced effect is apparent since MeJA directly evokes ROS and NO productions that promote closure. However, a significant decrease on the level of stomatal closure was observed when the system was subjected to the combined stimulus of the three hormones (Fig 6). This may be caused by the activation of the antioxidant AXO2 that reverses stomatal closure when both ABA and ET are present.
Fig 6.
(A) Aperture levels of stomata at different levels of ABA, ET, MeJA and their combinations (B)-(C) magnified versions of (A) at different time intervals.
Summary, conclusion and recommendation
In this study, we constructed a mathematical model of guard cell transduction network for stomatal closure involving the phytohormones ABA, ET and MeJA using continuous logical modelling framework. Based on this, we verified the existing findings about the role of antioxidants in the closure mechanism, as well as the diminished closure level of stomata with combined ABA-ET stimulus. A significant part of our research was the analysis of the ABA-ET-MeJA crosstalk.
Sixteen models were constructed to analyze the signalling network. Results showed that M16 exhibited the most consistent outcome. It considered the logical operator on AXO2 and the hypothesized connection between pH and ET. The model showed that guard cells cannot sustain stomatal closure in the absence of both ET and ABA.
Stomatal closure is diminished under the combined ABA and ET stimulus as compared to the effect of either ABA or ET individually. The low level of ROS due to the response of antioxidant that activates when both ABA and ET are present resulted to diminishing closure.
An extension of the model was established through crosstalk with another phytohormone, MeJA. The activation of ROS by MeJA and activation of NO by MeJA were additional connections in the model. It was shown that MeJA induces stomatal closure. Results showed that MeJA improved the effect of both ABA and ET in stomatal closure. However, when the system was subjected to the combined stimulus of the three hormones, a significant decrease on the level of the closure was observed.
The use of standard methodology proved to be helpful in describing the behavior of components of the guard cell signalling network. Despite its simpler approach, the model was able to present the important characteristics of the system’s dynamics. This is particularly important for when networks become large, it becomes more difficult to build predictive quantitative models. Additionally, mechanistic information and kinetic parameter data are often not fully available for complex networks.
Our analysis only included established interactions of ABA, ET, and MeJA on stomatal closure in plants. Further analysis can be done when newly found pathways are incorporated in the transduction network. Furthermore, the effect of other phytohormones can be examined using the similar modelling framework.
Supporting information
(DOCX)
Abbreviations
- ABA
abscisic acid
- AOX1
antioxidant 1
- AOX2
antioxidant 2
- AtrbohF
Arabidopsis thaliana respiratory burst oxidase homolog F
- ABI1
ABA-insensitive 1
- ET
ethylene
- IK,in
inwards-rectifying K+ channels
- IK,out
outwards-rectifying K+ channels
active IK,in
active IK,out
- MeJA
methyl jasmonate
- NIA1
nitrate reductase 1
- NO
nitric oxide
- ODEs
ordinary differential equations
- pHcyt
cystolic pH
- ROS
reactive oxygen species
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Daszkowska-Golec A, Szarejko I. Open or close the gate—stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci. 2013;4: 138 10.3389/fpls.2013.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beguerisse-Dıaz M, Hernández-Gómez MC, Lizzul AM, Barahona M, Desikan R. Compound stress response in stomatal closure: a mathematical model of ABA and ethylene interaction in guard cells. BMC Syst Biol. 2012;6: 146 10.1186/1752-0509-6-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reece J, Urry L, Cain M, Wasserman S, Minorsky P, Jackson R. Cambell Biology. 9th ed. Pearson; 2010.
- 4.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu Rev Plant Phisiology Plant Mol Biol. 2001;52: 627–658. [DOI] [PubMed] [Google Scholar]
- 5.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot. 2008;59: 165–176. 10.1093/jxb/erm293 [DOI] [PubMed] [Google Scholar]
- 6.Hetherington AM. Guard cell signaling. Cell. 2001;107: 711–714. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell Environ. 2010;33: 510–525. [DOI] [PubMed] [Google Scholar]
- 8.Harrison M. Cross-talk between phytohormone signaling pathways under both optimal and stressful environmental conditions In: Nafees K, Nazar R, Iqbal N, Naser A, editors. Phytohormones and abiotic stress tolerance in plants. Springer Berlin; Heidelberg; 2012. pp. 49–76. [Google Scholar]
- 9.Munemasa S, Mori IC, Murata Y. Methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid in guard cells. Plant Signal Behav. 2011;6: 939–941. 10.4161/psb.6.7.15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito N, Munemasa S, Nakamura Y, Shimoishi Y, Mori IC, Murata Y. Roles of RCN1, regulatory a subunit of protein phosphatase 2a, in methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid. Plant Cell Physiol. 2008;49: 1396–1401. 10.1093/pcp/pcn106 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Kinoshita T. Crosstalk between blue-light- and ABA-signaling pathways in stomatal guard cells. Plant Signal Behav. 2011;6: 1662–1664. 10.4161/psb.6.11.17800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Mata C, Lamattina L. Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol. 2002;128: 790–792. 10.1104/pp.011020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: A dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006;4: 1732–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza L, Xenarios I. A method for the generation of standardized qualitative dynamical systems of regulatory networks. Theor Biol Med Model. 2006;3: 13 10.1186/1742-4682-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaga R, Klamt S. Modeling approaches for qualitative and semi-quantitative analysis of cellular signaling networks. Cell Commun Signal. Cell Communication and Signaling; 2013;11: 43 10.1186/1478-811X-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankar M, Osmont KS, Rolcik J, Gujas B, Tarkowska D, Strnad M, et al. A qualitative continuous model of cellular auxin and brassinosteroid signaling and their crosstalk. Bioinformatics. 2011;27: 1404–1412. 10.1093/bioinformatics/btr158 [DOI] [PubMed] [Google Scholar]
- 17.Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14: 3089–99. 10.1105/tpc.007906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, et al. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006;47: 907–916. 10.1111/j.1365-313X.2006.02842.x [DOI] [PubMed] [Google Scholar]
- 19.Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant, Cell Environ. 2008;31: 622–631. [DOI] [PubMed] [Google Scholar]
- 20.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45: 113–122. 10.1111/j.1365-313X.2005.02615.x [DOI] [PubMed] [Google Scholar]
- 21.Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot. 2004;55: 205–212. 10.1093/jxb/erh033 [DOI] [PubMed] [Google Scholar]
- 22.Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid- induced stomatal closure. Plant Physiol. 2004;134: 1536–1545. 10.1104/pp.103.032250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak JM, Mäser P, Schroeder JI. The clickable guard cell, version II: interactive model of guard cell signal transduction mechanisms and pathways. Arabidopsis Book. 2008;6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen G, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406: 731–734. 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- 25.Macey R, Oster G. Berkeley Madonna. Version 8.3.23.0 [software]. 2001 [cited 10 Dec 2016]. Available from: http://www.berkeleymadonna.com
- 26.Pallas J, Kays S. Inhibition of photosynthesis by ethylene-a stomatal effect. Plant Physiol. 1982;70: 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak JM, Mori IC, Pei ZM, Leonhard N, Angel Torres M, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22: 2623–2633. 10.1093/emboj/cdg277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Miao YC, An GY, Zhou Y, Shangguan ZP, Gao JF, et al. K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res. 2001;11: 195–202. 10.1038/sj.cr.7290086 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005;138: 2337–2343. 10.1104/pp.105.063503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.