Abstract
Background
Despite a remarkable increase in the depth of our understanding and management of breast cancer in the past 50 years, the disease is still a major public health problem worldwide and poses significant challenges. The palpability of breast tumors has facilitated diagnosis and documentation since ancient times. The earliest descriptions of breast cancer date back to around 3500 BCE. For centuries to follow, theories by Hippocrates (460 BCE) and Galen (200 CE), attributing the cause of breast cancer to an “excess of black bile” and treatment options including the use of opium and castor oil, prevailed. Surgical resection was introduced in the 18th century. The advent of modern medicine led to the development of novel treatment options that include hormonal, targeted and chemo-therapies. There are still several therapeutic challenges including the treatment of triple negative breast cancer (TNBC), and overcoming drug resistance.
Scope of review
The increased incidence and awareness of breast cancer has led to significant changes in diagnosis and treatment in recent decades. But, mankind has come a long way. Herein, I have traced how our understanding of breast cancer has evolved from the early description of the disease around 460 BCE as “black bile-containing crab-like tumors” to the conventional as a heterogeneous disease with high degree of diversity between and within tumors, as well as among breast cancer patients. How is breast cancer treated today and how do risk factors, breast cancer subtype and drug resistance contribute to the therapeutic challenges at the turn of the 21st century?
Major conclusions
Breast cancer remains a serious public health issue worldwide. However, appreciable growth in our understanding of breast cancer in the past century has led to remarkable progress in the early detection, treatment and prevention of the disease. The clinical focus is shifting more towards tailored therapy as more targets are characterized and novel highly innovative approaches are developed.
General significance
Tracing the history of breast cancer, highlights how increased awareness of the disease, and progress in research and development have enhance our understanding of the disease.
Keywords: Breast cancer, Hippocrates, Tamoxifen, Angelina Jolie, Herceptin, Radical mastectomy, Mammography, Aromatase inhibitors, BRCA1 and BRCA2, ER, HER2, Triple negative breast cancer (TNBC)
Highlights
-
•
The humoral, lymphatic and anti-hormonal theories of breast cancer
-
•
Introduction of radical mastectomy, radiotherapy, mammography, and targeted therapy
-
•
The introduction of randomized trial
-
•
Breast cancer foundations, awareness and the Angelina Jolie effect
-
•
Promising future for tailored therapy
1. Introduction
Breast cancer is characterized by uncontrolled growth of malignant cells in the mammary epithelial tissue. The disease affects both genders. Breast cancer is the most frequent type of cancer in women worldwide, with an incidence that rises dramatically with age. Breast cancer reportedly accounted for 29% of all new cancer cases and 14% of all cancer-related deaths among women worldwide up to 2012 [1]. This translates into about 1.7 million new cases in women and an estimated 522, 000 deaths in 2012 [2]. As alarming as it may seem, the mortality to incidence ratio for breast cancer is around 0.31, which is far more favorable than pancreas and liver cancers, with ratios of 0.98 and 0.95, respectively [2]. Breast cancer in the 21st century is therefore not necessarily terminal. Better therapeutic options and major improvements in public health and care have resulted in a dramatic reduction in mortality and a major increase in longevity. Breast cancer is rare in males, comprising 1% of all breast cancer diagnoses in the United States and less than 0.1% of cancer-related deaths in men [3].
Since 1999, incidence rates of breast cancer have stabilized among women aged 50 or over, which may reflect trends in mammography screening rates. Epidemiological data from the United States show that breast cancer death rates were stable from 1980 to 1989 for women aged 20 to 64 and increased for women aged 65 and over. In most countries, however, breast cancer incidence rates are increasing, including in countries with historically higher rates, such as those in Europe, as well as regions with historically lower incidence rates like many countries in Latin America, Asia, and Africa [4]. This rise is generally due to changes in reproductive patterns such as age at menarche, age at first pregnancy, number of births, and duration of breastfeeding [5]. The historically higher increase in breast cancer incidence in developed countries is largely due to factors associated with economic development and urbanization including obesity, consumption of processed foods, physical inactivity, and changes in reproductive patterns (delayed childbearing; having fewer children; earlier age at menarche; and shorter duration of breastfeeding). The rising incidence in developing countries is likely due to the increasing adaptation of risk factors associated with the Western lifestyle [6].
Humankind has struggled to understand and treat breast cancer since the earliest documentation more than 3500 years ago. The visible signs and symptoms of breast cancer and the palpability and tangibility of the lumps at later stages of the disease have enabled easy diagnosis by physicians in almost every period of recorded history. Despite the noticeable manifestation of the disease since ancient times, e.g. the Pyramid Era, and the high mortality associated with the advanced form of the disease, cultural and sexual connotations regarding the breast have stigmatized discussions of the disease in the general public and limited its description to clinical journals and textbooks until recently. Besides, from the ancient civilizations and through the 18th and 19th centuries it was recognized that breast cancer could not be cured once the cancer had spread.
In many cultures, breast cancer is still considered a taboo subject. As a result, many patients are reluctant to candidly discuss their disease or its symptoms. However, moral and ethical reforms have been introduced in several societies and breast cancer topics are an open discussion in all forum or media today. Further, with the advent of electronic media and the Internet, online discussions on the awareness of the disease are now accessible even to the remote areas and cultures of the world. The pink symbol of breast cancer, adopted in the early 1990s, represents the international symbol of breast cancer awareness and is used by various breast cancer organizations to promote breast cancer awareness and to support fundraising campaigns.
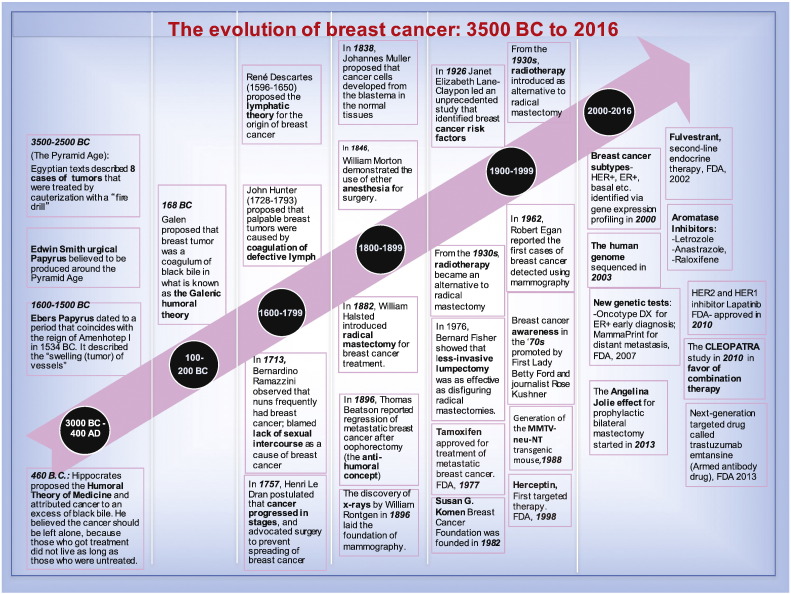
Fig. 1.
Timeline: The evolution of breast cancer: 3000 BCE to 2016.
2. 3500 BCE–400 BCE
2.1. Ancient Egypt and Greece
Origins of the medical papyri: Ancient Egyptians were the first to describe breast tumors around 3500 years ago in two distinct papyri: The Edwin Smith Surgical Papyrus and the Ebers Papyrus. The Edwin Smith Surgical Papyrus (named after an American antiques dealer residing in Cairo in the mid-1800s) is regarded as one of the most important medical documents in the ancient Nile Valley. The papyrus document was produced in the Pyramid Age sometime between 3500 and 2500 BCE that is around the Stonehenge era [7]. The Ebers Papyrus, was named after the Egyptologist George Ebers, who purchased it in 1872 and later published a facsimile with an English-Latin vocabulary of the document in 1875.
Fig. 2.
The Edwin Smith Papyrus: a) Case 39 of the original in hieratic. b) The corresponding modern scientific transcription into hieroglyphics. The images were cropped from the original publication by Breasted. They contain descriptions of tumors with prominent heads in the breast of the patient as transliterated in Harding's publication in 2007. (Harding, F. J. Breast Cancer: Cause, Prevention, Cure. Tekline Publishing, 440 (2007)).
2.2. Hippocrates: The humoral theory of medicine
Fig. 3.
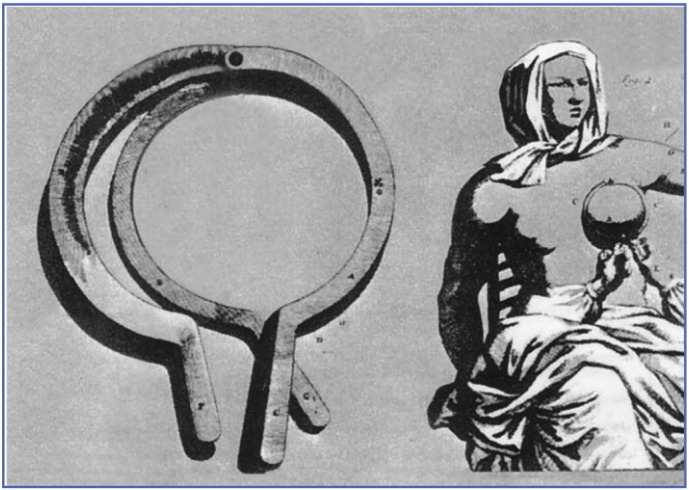
The mastectomy instrument of Gerard Tabor described in 1721: Image adapted from Cotlar, A. M., Dubose, J. J. & Rose, D. M. History of surgery for breast cancer: radical to the sublime. Curr Surg60, 329–337, doi:10.1016/S0149–7944(02)00777-8 (2003).
3. 0–200 CE: Galen
3.1. The Galenic humoral theory
4. 1600–1799
4.1. The lymph theory
Several physicians and philosophers challenged the Galenic humoral theory at the beginning of the 17th century. After the discovery of the lymphatic system by Olof Rudbeck (1630–1708) of Sweden in 1652, René Descartes (1596–1650) (Fig. 4) proposed the lymphatic theory for the origin of breast cancer, which contradicted the prevailing humoral explanation [22]. In 1680, Dutch professor Francois de la Boe Sylvius, also called Franciscus Sylvius (1614–1672), argued that cancer was not caused by accumulation of black bile. Like other diseases caused by chemical imbalances that resulted in excess of acid or an excess of alkali in the blood, cancer was believed to result from the transformation of lymphatic fluids to acidic base [23], [24]. In the 1730s, Paris physician Claude-Deshais Gendron (1663–1750) also opposed the humoral theory of Galen and argued that cancers were “nerve-like” and developed when nerves and the glandular tissue mixed with lymph vessels (The History of Cancer: An Annotated Bibliography, by James Stuart Olson). John Hunter (1728–1793) perpetuated the lymphatic theory of the origin of breast cancer by extrapolating that the coagulation of defective lymph led to palpable breast tumors. Hunter is credited as the father of scientific surgery because he advocated the removal of the cancer along with the lymphatic spread 100 years before William Halsted introduced radical mastectomy in 1882 (see the Radical mastectomy section).
Fig. 4.
Cancer and breast cancer pioneers: 400 CE – 1900. a) Hippocrates (engraving by Peter Paul Rubens, 1638https://en.wikipedia.org/wiki/Hippocrates); b) Galen (Source: http://famousbiologists.org/galen/); c) René Descartes (Source: Hammond, N. (2006). Descartes - The life of Rene Descartes and its place in his times. Tls-Times Lit Suppl, 27–27); d) Bernardino Ramazzini (source: http://www.britannica.com/biography/Bernardino-Ramazzini); e) Henri François Le Dran (source: https://pictures.royalsociety.org/image-rs-10246); f) Johannes Peter Müller (source: http://www.britannica.com/biography/Johannes-Peter-Muller); g) William Stewart Halsted (source: Rutkow, I.M. (2000). William Stewart Halsted - Moments in surgical history. Arch Surg-Chicago 135, 1478–1478.); h) George Thomas Beatson (image from Wellcome Library, London; Photograph by T. & R. Annan & Son; http://wellcomeimages.org/indexplus/page/Home.html).
4.2. Occupational medicine
In 1713, Bernardino Ramazzini (1633–1714) (Fig. 4), credited with establishing the field of occupational medicine [25], noted a higher frequency of breast cancer in nuns in Italy than in married women. He blamed the disparity on celibacy and speculated that the lack of sexual intercourse or the “unnatural” state caused instability of reproductive organs like the breast that may lead to decay and the development of cancers [26]. It is now known from recent epidemiological studies that women who nurse their children are less likely to develop premenopausal breast cancer and therefore the high incidence in nuns reported by Ramazzini was likely because they did not have children and not because of celibacy. Another absurd postulation came from Friedrich Hoffmann of Prussia (1660–1742) and Giovanni Morgagni of Italy (1682–1771). Hoffmann hypothesized that women who had regular sex but still developed cancer were practicing “vigorous” sex that caused lymphatic blockage. Morgagni, who was one of the first to perform an autopsy and to lay the foundation for scientific oncology, theorized that breast cancer was caused by curdled milk. The cause of breast cancer was also blamed on pus-filled inflammations in the breast (Johanes de Gorter, 1689–1762), depressive mental disorders (Claude-Nicolas Le Cat, 1700–1768) and childlessness (Lorenz Heister, 1683–1758).
4.3. Removal of the tumor to prevent “metastasis”
In the mid-18th century, Henri Le Dran (1685–1770) (Fig. 4), a leading French physician, realized that cancer was not a systemic disease but a local affliction that progressed in stages. In 1757, he proposed the surgical removal of the breast tumor before it spread to the lymph nodes of the armpits. This resonated with the views of Claude-Nicolas Le Cat, who argued that surgery was the only method to treat breast cancer. This practice lasted well into the 20th century and eventually led to the application of radical mastectomy or extensive removal of the breast.
5. 1800–1899
5.1. Cancer originates from normal tissue
Major advances in human pathology and safety during surgery as well as progress in oncology marked the 19th century. For instance, in 1838, German pathologist Johannes Muller (1801–1858) (Fig. 4) proposed that cancer cells developed from the blastema between the normal tissues and not from the lymphatic system, and later Rudolph Virchow (1821–1902) demonstrated that tumors were composed of cells (Reviewed in [19]). It was during this time that hand-washing was promoted and the pasteurization technique was invented as a precautionary measure during surgery. Joseph Lister (1827–1912) introduced the concept of surgical antisepsis using carbolic acid spray; aseptic techniques were adopted for the first time by the Baltic German surgeon Ernst von Bergmann (1836–1907); and surgical masks and sterile rubber surgical gloves were also introduced [19]. However, a major progress in surgery occurred on October 16, 1846 when William T. G. Morton (1819–1868) pioneered and publicly demonstrated the use of ether as anesthesia for surgery [19].
5.2. Radical mastectomy
William Halsted (1852–1922) (Fig. 4), a professor of surgery at Johns Hopkins Hospital (Baltimore, USA) was a strong proponent of asepsis during surgical procedures and, like most surgeons at the time, he had embraced the newly discovered anesthetics. He introduced several new surgical techniques, most notably radical mastectomy for breast cancer in 1882. Halsted's operation involved the removal of the whole breast as well as the underlying chest muscle (including both pectoralis major and minor), and the axillary contents. Halsted later extended his operation by removing the supraclavicular lymph nodes after dividing the clavicle. In 1894, he published the results of the 50 patients he treated. He noted a significant decrease in local recurrence to just 6% compared with the 56%–81% reported in Europe at the time [10]. The main achievement of Halsted's operation was the reduction of local recurrence rates compared with other procedures. However, it became clear that the operation did not improve overall survival; many patients who underwent the Halsted radical mastectomy at that time suffered from a relatively advanced stage of cancer. Nevertheless, radical mastectomy became the standard operation for breast cancer worldwide in the late 19th century and remained the gold standard throughout most of the 20th century [10].
5.3. The anti-hormonal theory
An overlooked legacy of the 19th century was the discovery that some breast cancers were hormone-dependent. It had been observed that the growth of breast cancer in patients fluctuated with the menstrual cycle, and that growth of the tumor was slower in postmenopausal women. In a landmark publication in 1896 entitled “On Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases,” Thomas Beatson (1848–1933) (Fig. 4) reported the observation of temporary regression of metastatic breast cancer in two patients treated by surgical oophorectomy. He correctly hypothesized “that internal secretion of the ovaries in some cases favors the growth of the cancer” [27]. This is why he is considered the father of anti-hormonal treatment of breast cancer [10].
6. 1900–1999
The 20th century saw a retreat from radical surgery, the increased value of epidemiological studies and the introduction of radiotherapy, mammography and chemotherapy. Clinical and fundamental breast cancer research led to the molecular and pathological classification of breast cancer, a firm establishment of a hereditary component of breast cancer. Cooperative research groups amassed large cohorts of patients for various studies, and randomized, controlled clinical trials became the norm. Breast cancer was recognized as a major public health issue in the Western world and stimulated a concerted effort by the public at large in the promotion of breast cancer awareness and the fight against the disease.
6.1. Breast cancer risk factors
Although the Halsted operation was revolutionary, this surgical intervention was becoming controversial and losing popularity among some surgeons. In the mid-20th century there was greater awareness of the postoperative morbidity that included disfigurement of the chest, lymphedema of the arm and occasional irradiation-induced sarcomas. Some new breast cancer patients were inclined to choose alternative treatments and even some surgeons were becoming increasingly critical of radical mastectomy although it was considered to be far more successful than any other treatment at the time. The first half of the 20th century also saw an explosion of knowledge about the biology and epidemiology of breast cancer.
6.2. Radiotherapy
In 1896 a German physics professor named Wilhelm Conrad Röentgen (1845–1923) (Fig. 5) described the concept of X-rays, and a few years later X-rays were used for the diagnosis and treatment of cancer [30]. Radiation therapy or radiotherapy began with the use of radium at low levels. By the 1930, radiotherapy was introduced as a welcomed alternative to radical mastectomy despite the scarcity of radioisotopes and its associated hazard. Geoffrey Keynes (1887–1982), surgeon and scholar at St Bartholomew's Hospital in London, developed the idea of treating breast cancer by radium implantation. He reported five-year survival rates of 71% and 29% in stage I and stage II patients, respectively. George Pfahler from the United States also used radiotherapy in the early 1930s and reported a 5-year survival of 80% in patients with the stage I disease. In 1948, Robert McWhirter from Great Britain reported the results of a combined use of simple mastectomy followed by radiotherapy in breast cancer treatment [10]. Advances in radiation physics and computer technology during the last quarter of the 20th century have made it possible to precisely map and target tumors using techniques such as conformal radiation therapy (CRT), intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT), and the use of radio sensitizers to improve sensitivity of tumors to radiation.
Fig. 5.
Cancer and breast cancer pioneers: 20th century. a) Wilhelm Conrad Röntgen (source: http://www.britannica.com/biography/Wilhelm-Rontgen); b) Geoffrey Keynes (Source: http://www.modern-humanities.info/people/Keynes_Geoffrey.htm); c) Janet Elizabeth Lane-Claypon (source: http://www.centenary.mrc.ac.uk/news/tales-from-the-century-janet-lane-claypon-and-epidemiology/); d) Robert Egan. (source: http://onlinelibrary.wiley.com/, in the transcript of an interview titled Mammography and Diseases of the Breast); e) Bernard Fisher (Source: http://drbarronlerner.com/, in an article titled: Bernard Fisher's Battle Against the Radical Mastectomy).
6.3. Mammography
Before the advent of mammography, early diagnosis of breast cancer was limited to palpation or other physical signs such as the retraction or inversion of the nipples. The discovery of X-rays by Röentgen laid the foundations of mammography. In 1913, Albert Salomon, a German surgeon, conducted a roentgeno-histological study on 3000 mastectomies. He found microcalcifications in X-ray images of tumor samples. His mammographs provided substantial information about the pathological differences between cancerous and normal tissues [31]. As revolutionary as these findings were, mammography did not become a common practice until when Raul Leborgne revitalized the interest in mammography decades later, in 1949 [32]. He initiated imaging quality enhancement and emphasized differential diagnosis between benign and malignant calcifications. In 1962, Robert Egan (1920–2002) (Fig. 5) used mammography to diagnose 53 cases of occult breast cancer [33]. The mammography technology has thus evolved from film-screen to computer-aided digital mammography in the beginning of the 21st century.
6.4. Randomized trials
Dr. Bernard Fisher (1918) (Fig. 5), Professor of Surgery at the University of Pittsburgh, is widely credited with bringing clinical trials and statistical methodology to breast cancer research. He expressed the need to critically re-evaluate breast cancer treatment. Like Galen, Fisher asserted that breast cancer was a systemic disease. He hypothesized that viable cancer cells tend to disseminate and travel throughout the circulatory and lymphatic systems, making it impossible to cure cancer by surgery alone. Fisher theorized that stray cancer cells could detach from the original tumor site and migrate through the bloodstream or lymphatic system to other sites in the body. In these cases, the radical mastectomy would not be enough. During his tenure as chairman of the National Surgical Adjuvant Breast and Bowel Project (NSABP) in the early '70s, he challenged Halsted's theory and concluded that less-invasive lumpectomy surgery in combination with radiation treated breast cancer was a more effective option than highly mutilating radical mastectomies. Rose Kushner (see Breast cancer foundations section) and other breast cancer patients rallied behind a trial by Fisher comparing the radical mastectomy to the lumpectomy. Fisher published several randomized trials from the mid-1980s and concluded that radical mastectomy was not necessary given that the survival rates of women receiving lumpectomy with radiation and chemotherapy were the same as women undergoing radical mastectomy [34], [35]. He was famously quoted as saying “for the first time, the treatment of breast cancer was based on science rather than on anatomic and mechanistic principles.” [36].
6.5. Breast cancer awareness
6.6. Breast cancer foundations
Breast cancer advocacy became mainstream in the 1980s and has raised awareness and increased focus on public education through various campaigns ever since. Many changes in breast cancer management and funding have directly resulted from the movement or have been fostered indirectly by it [38]. The Rose Kushner two-step surgical procedures became the norm, and lumpectomies were offered whenever possible. In the early 1980s there was a rapid rise in public awareness campaigns on breast health and breast cancer in the United States and around the world. Public health advocates encouraged women to perform self-examinations of their breasts, and undergo mammography and clinical breast examinations [38].
The Susan G. Komen Breast Cancer Foundation was founded in 1982 in memory of Susan Komen, who died from the disease at age 36 in 1980 (Fig. 6). The signature Komen Race for the Cure was first held in Dallas in 1983. This was the first time that a road race of that magnitude was associated with a cause. The number of participants in the Komen Race for the Cure has grown exponentially since 1983 to millions annually. The mandate of the Komen foundation today is “To save lives and end breast cancer forever by empowering others, ensuring quality care for all and investing in science to find the cures” (http://ww5.komen.org). Similar funding agencies including the Canadian Breast Cancer Foundation (since 1986, http://www.cbcf.org) have been established worldwide to raise awareness, mobilize action on breast cancer and collect funds for breast cancer research. A series of developments championed by the breast cancer advocacy occurred in the early 1990s. For example, the pink ribbon became the symbol for the revolution against breast cancer (1991) (Fig. 6), the U.S. Department of the Army breast cancer research program was established (1992) and the Mammography Quality Standards Act became a law in 1992.
Fig. 6.
Breast cancer advocacy: Key figures. a) Rose Kushner. American journalist patient and pioneering advocate for breast cancer patients (as published in Lerner, B.H. (2002). Breast cancer activism: past lessons, future directions. Nat Rev. Cancer 2, 225–230); b) Betty Ford. First Lady of the United States from 1974 to 1977. Breast cancer patient and advocate (adapted from https://en.wikipedia.org/wiki/Betty_Ford); c) The Susan G. Komen for the Cure logo. Founded in 1982, the “Susan G. Komen for the Cure” is the largest breast cancer foundation in the United States (source: http://ww5.komen.org/); d) The International symbol of breast cancer awareness. The pink ribbon was inspired by the Susan G. Komen for the Cure in 1991 (source: http://www.cancer.org/involved/participate/makingstridesagainstbreastcancer/make-a-pink-ribbon-lapel-pin); e) Angelina Jolie. Hollywood actor. Promoted prophylactic mastectomy (source: http://time.com/3450368/the-angelina-effect/).
6.7. Tamoxifen for breast cancer therapy
The 1980s were a significant era in drug discovery; a mainstay antiestrogen drug Tamoxifen was approved. Estrogens are hormones that regulate mammary gland development and the female menstrual cycle, and are essential for successful reproduction. As described earlier, in the 1890s, before estrogen was discovered in 1929 [39], physicians observed that there were changes in breast tumor mass in premenopausal women with breast cancer during the menstrual cycle. In a series of elegant experiments in the early 1960s, Jensen and colleagues demonstrated that estrogen bound to a molecule [40], which was later identified as the estrogen receptor [41]. These were significant discoveries because in the 1970s it was demonstrated that breast tumors grew in response to estrogen, which intensified the quest for antiestrogen drugs. Scientists at the British pharmaceutical company AstraZeneca first synthesized a drug called tamoxifen in 1962 to exploit its contraceptive effects [42]. However, tamoxifen was later found to exert antiestrogen effects in estrogen receptor-positive breast cancers. It functions by blocking the binding of estrogen to the estrogen receptor. However, because the drug was also shown stimulate estrogen activity in the endometrium, it is considered as a partial antiestrogen and therefore classified as a selective estrogen receptor modulator (SERM) [43]. Following a series of clinical trials in humans, the Food and Drug Administration (FDA) approved tamoxifen for the treatment of metastatic estrogen receptor-positive breast cancer in 1977. The FDA later approved tamoxifen as adjuvant therapy for post-menopausal women (1986) and for prophylactic use in women at high risk of breast cancer (1998) [42]. Almost 40 years since its approval, tamoxifen is still considered as the gold standard for the treatment of all stages of estrogen-receptor-positive breast cancers.
6.8. BRCA1 and BRCA2 mutations
The 1990s and beyond saw a spike in research and development in breast cancer. By that time, it was clear through familiar clustering that first-degree relatives of affected individuals were at a higher risk of developing breast cancer. Several milestones in the ‘90s included the emergence of taxanes as important chemotherapeutic drugs for breast cancer treatment (1994); the introduction of bone-building drugs that helped to reduce complications of breast cancer (1995); the use of sentinel lymph node biopsy to assess breast cancer metastasis (1996); and the approval of capecitabine, an oral chemotherapeutic drug (1998). However, two discoveries in the ‘90s that had a great impact on diagnosis and care were: 1) the identification in 1994 of specific inherited mutations in tumor suppressor genes BRCA1 and BRCA2 that increased the risks of breast and ovarian cancers (Reviewed in [44]); and 2) the development of the first targeted anti-breast cancer drug, trastuzumab (Herceptin) in 1998.
Substantial developments in molecular biology and genetics accelerated in the 1990s. This momentum culminated in numerous genetic discoveries, notably the publication of the identification of the variants of two main breast cancer-associated genes, BRCA1 and BRCA2, published in Science in 1994 by Easton et al. [45] and Wooster et al. [46], respectively. Carriers of these gene mutations have been shown to display between a 40% and 80% risk of developing breast cancer by age 70 (Reviewed in [47]). Families with a history of both breast and ovarian cancers are usually associated with inherited BRCA1 mutations, whereas families that include male breast cancer cases are more commonly linked to BRCA2 mutations. The highest penetrance of these mutations is 36%, found in families of Jewish descent [48]. The three most common mutations in the Ashkenazi Jewish group are BRCA1.185delAG, BRCA1.5382insC, and BRCA2.6174delT. Groups originating from Northern Ireland and the west coast of Scotland tend to harbor a BRCA1 2800delAA mutation, whereas people from the east coast of Scotland commonly display a BRCA2 6503delTT mutation [48].
6.9. Herceptin approval (1998)
1974 marked the identification of a protein called the epidermal growth factor receptor (EGFR). This protein was the first receptor tyrosine kinase discovered by Stanley Cohen et al. [49]. In 1984 and 1986, Ullrich and Coussens (Genentech, USA) and Yamamoto et al. [50] cloned the HER2 (human epidermal growth factor 2) or neu gene. In fact, HER2 is part of a four-member EGFR family, which also includes HER3 and HER4. The neu oncogene was cloned, sequenced and mapped to human chromosome 17 [50]. In 1987, Slamon et al. demonstrated the amplification of the neu oncogene in breast cancer and reported that the amplification correlated with poor prognoses in women with breast cancer [51]. In 1988 William Muller et al. demonstrated that targeting the active form of HER2 to the mouse mammary gland was sufficient to produce malignant tumors that histologically resembled the human breast tumor [52]. It was clear at this point that HER2 amplification was oncogenic in breast cancers.
In 1989, Axel Ullrich and his colleagues at Genentech showed that a monoclonal antibody directed against the extracellular domain of HER2 specifically inhibited the growth HER2-positive breast cancer cells. The humanized antibody against HER2 was produced from Chinese Hamster Ovary (CHO) suspension culture in 1992. This humanized antibody, now called trastuzumab or Herceptin™ entered Phase I clinical trials in 1992 and Phase II in 1993. Phase III was started in 1995 and closed in 1997. In a fast-track process in 1998, the FDA approved Herceptin in combination with paclitaxel as a first-line treatment of HER2-positive metastatic breast cancers, and as a single agent for second and third-line therapy. Herceptin is described officially by the FDA as “a recombinant DNA-derived humanized monoclonal antibody that selectively binds with high affinity in a cell-based assay to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2.” It was the first therapeutic antibody targeted at an oncogene to receive FDA approval. In 2006, Herceptin was also approved as part of adjuvant therapy for women with early-stage HER2-positive breast cancer after it was demonstrated that the drug remarkably reduced the risk of recurrence by more than 50%.
7. 2000–2016
Research in cancer biology in the 1980s and 1990s led to significant progress in cancer prevention, early detection, and treatment. More was learned about cancer in these two decades than in the past centuries. Cancer research in the new millennium is developing on many fronts. The first 15 years in the 21st century have seen progress in gene and protein expression profiling, targeted therapies, immunotherapy, cancer genetics, nanotechnology and robotic surgery. Progress in the understanding of the pathology and molecular biology, as well as the genetic alterations underlying breast cancer development and progression in the 21st century have led to the pathological and molecular classification of breast cancer and development of new genetic tests, for instance, and enabled scientists to develop novel therapeutic drugs and strategies for this disease.
7.1. The human genome project
The “omics” era essentially began in the 21st century, raising expectations that advances in human genomics and related fields such as transcriptomics, proteomics and metabolomics will lead to enhanced progress in diagnosis, therapy and disease prevention. The world”'s largest collaborative biological project was the sequencing of the human genome, which was completed and reported by the International Human Genome Sequencing Consortium on April 14, 2003 (www.genome.gov/11006929). This project steered the genomic revolution, and molecular technologies have diversified since 2003. This has opened up strategies to study cancer and discover novel and more effective diagnostic and therapeutic tools for the disease. Several cancer databases were created in the first decade of the 21st century. For instance, the Cancer Genome Atlas (TCGA) project, founded in the United States in 2005, uses large-scale genome sequencing and bioinformatics to catalogue cancer-related mutations, which has improved our understanding of the molecular basis of cancer (http://cancergenome.nih.gov/abouttcga).
7.2. Breast cancer subtypes identified
The molecular classification of breast cancer based on gene expression profiles reported by a few research groups in the first decade of the 21st century is one of the momentous developments in personalized medicine in recent years. Genome-wide profiling technologies have led to a better understanding of the biological heterogeneity of breast cancer. Gene expression profiling of human breast tumors revealed molecular subtypes with distinct gene signatures and associated clinical outcomes [53], [54], [55], [56]. In 2000 Perou et al. published seminal studies on microarray-based gene expression profiling as a new way of classifying breast cancers. These studies used an “intrinsic” gene list to classify breast cancer into at least four main molecular subtypes: basal-like, HER2 (human epidermal growth factor receptor 2)-positive, luminal A and luminal B. Luminal A and Luminal B types are both estrogen receptor (ER)-positive, whereas the basal-like breast cancers are usually ER-negative, progesterone-receptor (PR)–negative, and HER2-negative tumors (hence, “triple-negative” breast cancers or TNBCs). The basal-like subtype is so defined based on the expression of genes such as cytokeratins 5 and 17, as well as the epidermal growth factor receptor (EGFR/HER1), characteristic of the outer or basally located epithelial layer of the mammary gland. HER2-positive cancers display amplification and high expression of the ERBB2 gene and several other genes of the ERBB2 (HER2) amplicon. All these subtypes are histologically different and tend to vary in their clinical outcomes. The luminal A subtype, for example, is typically histologically low-grade and has the most favorable prognosis, whereas luminal B cancers are often high-grade and therefore more aggressive. HER2-positive cancers are usually high-grade and are associated with poor prognosis. The basal-like tumors are usually the most aggressive and are associated with the shortest relapse-free and overall survival [57]. Basal-like breast cancers account for 56% to 85% of TNBCs [57]. Some TNBCs lack the gene expression profile of the basal-like tumors and are classified as normal-like breast cancers. Normal-like breast tumors are usually small and tend to have a more favorable prognosis [57]. Claudin-low is a more recently described class of breast cancer that is often triple-negative, but distinct in that it expresses low levels of cell-cell junction proteins including claudin and E-cadherin (Reviewed by [58]). A novel molecular stratification of the breast cancer by integrated genomic/transcriptomic analysis revealed 10 integrative breast cancer clusters, typified by well-defined copy number aberrations [56]. All these gene profiling studies have drastically altered our conceptualization of breast cancer and provided a multitude of translational research opportunities to improve diagnosis, prognostic and therapeutic approaches for this disease. In 2012 the International Agency for Research on Cancer (IARC) published the 4th edition of the World Health Organization (WHO) Classification of Tumors of the Breast [59]. This edition included definitions of new histopathological diagnosis classifications complemented by recent descriptions of molecular subtypes, as well as genetics, prognostic and predictive features [60].
7.3. New genetic tests
In 2004, Genomic Health, in Redwood City, California, introduced the Oncotype DX Breast Cancer Assay that enabled a personalized approach to chemotherapy [61]. Steven Shak, the chief medical officer at Genomic Health noted that the days of “one size fits all,” were over and that “the Oncotype DX test now makes it possible to individualize treatments by making an informed decision on whether the benefits of chemotherapy outweigh the risks.” The assay is a commercially available diagnostic multigene expression test. It analyzes the expression pattern or “signature” of 21 genes in breast tumor tissues by using Reverse Transcriptase Polymerase Chain Reaction (RT-PCR). The assay predicts outcomes for patients with breast cancer, including the likelihood of chemotherapy benefit, as well as the probability of breast cancer recurrence and patient survival within 10 years of diagnosis. The test was clinically validated for use in women with early-stage (stage I or II), ER-positive and lymph node-negative breast cancer, which accounts for more than 50% of all women diagnosed with breast cancer [62]. The test results display a Recurrence Score ranging from 0 to 100, where the higher the score, the greater the chances of relapse if a patient is treated with hormonal therapy alone. Thus, with this test, oncologists can identify patients who are in urgent need of lifesaving chemotherapy to prevent cancer recurrence. Low risk patients who do not need additional chemotherapy are thus spared the related side effects and unnecessary additional costs.
Other tumor-based gene expression-based prognostic models have been developed [63]. For instance, the FDA approved the MammaPrint test in February 2007. The MammaPrint test is a 70-gene prognostic signature with high negative predictive value for distant metastasis for lymph node negative breast cancer patients of all ages, irrespective of their ER status, and who have tumors of less than 5 cm. This test would generally spare many older breast cancer patients adjuvant treatments [64].
7.4. New class of drugs
7.4.1. Fulvestrant
Tamoxifen therapy is usually the first treatment for ER-positive patients with locally advanced or metastatic breast cancer. As described earlier, tamoxifen possesses some estrogenic activity, which may cause endometrial hyperplasia or carcinoma and may also predispose patients to thrombosis. Fulvestrant however is essentially a pharmacological antagonist and not a partial agonist like tamoxifen. It is referred to as a “pure” anti-estrogen. The drug is a selective estrogen receptor down-regulator (SERD) so named because it binds directly to the ER, preventing ER dimerization and promoting the rapid degradation of the receptor [65]. In 2002, the FDA approved Fulvestrant, developed by AstraZeneca, as a second-line endocrine therapy “for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy.”
7.4.2. Aromatase inhibitors
The ovaries are the principal source of estradiol (estrogen) in premenopausal women [66]. However, in postmenopausal women the ovaries cease to produce estrogen, and the synthesis of estrogens occurs in a number of extragonadal sites including the adipose tissue of the breast [66]. Estrogens are synthesized in postmenopausal women mainly via conversion of adrenal androgens to estrone and estradiol with estradiol being the predominant physiological hormone. Aromatase is an enzyme that catalyzes the rate-limiting and final step of this estrogen biosynthesis. Aromatase Inhibitors (AIs) block the function of this enzyme, thus limiting the supply of estrogen that fuels the growth of some breast cancers [67]. AIs have been shown to be more effective than the antiestrogen tamoxifen in reducing the risk of breast cancer recurrence and spread, and are well tolerated. In 2005, the FDA approved the drug letrozole (also called Femara), the first in a new class of AI drugs. Letrozole produced by Norvatis was first approved for long-term use in post-menopausal women who have completed five years of tamoxifen treatment. Clinical trials revealed that the drug reduces the risk of breast cancer recurrence and spread even more than tamoxifen alone. Anastrazole (produced by AstraZeneca and marketed under the trade name Arimidex) is another FDA-approved AI that was shown to improve survival and alleviate the symptoms of cancer for post-menopausal women with advanced breast cancer [67]. Raloxifene, a drug that had been used to prevent and treat osteoporosis in postmenopausal women since 1997, was later shown to be as effective as tamoxifen in reducing the risk of developing invasive breast cancer. Raloxifene also functions by blocking the effects of estrogen on breast tissue, but cannot, however, be used to treat invasive breast cancer or to decrease the risk of developing breast cancer [68].
7.4.3. HER2 inhibitors
Lapatinib (also known by its trade name Tykerb by manufacturer GlaxoSmithKline) was FDA-approved in 2007 for HER2-positive patients whose condition was no longer responsive to Herceptin treatment. About 25% of breast cancers overexpress HER2, and as mentioned earlier, this overexpression confers a more aggressive phenotype and is associated with a poor prognosis. HER2 is a receptor tyrosine kinase that transmits signals to promote several cellular processes including cell proliferation. Lapatinib inhibits the tyrosine kinase activity of HER2 and HER1 and thus suppresses signaling by these receptors. The drug was also approved for use in combination with the drug capecitabine for patients with advanced breast cancer whose tumors overexpressed the HER2 protein. In 2010, lapatinib was also approved as an initial therapy in combination with letrozole for patients with HER-2 positive breast cancer (Reviewed in [69]).
8. The Angelina Jolie effect
Women who have inherited mutations in the BRCA1 or BRCA2 genes have a substantially elevated risk of developing breast and ovarian cancer [70]. A multinational study in the first decade of the 21st century was undertaken to determine whether preventive surgery (which includes mastectomy as well as the removal of the fallopian tubes and ovaries - salpingo-oophorectomy) can reduce the cancer and mortality risk in BRCA1/2 mutation carriers. The general consensus from these studies was that the use of preventive surgery significantly reduces the risk of breast cancer as well as the risk of ovarian and fallopian tube cancers [71]. In general, without the surgery, women with inherited mutations in the two BRCA genes have up to an 84% lifetime risk of developing breast cancer [72].
In 2013, Hollywood actor Angelina Jolie, a BRCA1 carrier, revealed in a Time magazine cover story that she had a prophylactic bilateral mastectomy (Fig. 6). The publicizing of her experience raised awareness of BRCA1/2 testing and cancer prevention, and was met with widespread support. Eventually, the so-called Angelina Jolie effect followed, which led to a general increase in referrals to centers for genetic tests [73]. This helped alleviate some of patients' insecurities associated with the loss of their sexual identity post-preventative surgery. The Angelina Jolie effect has underscored the significant impact of a celebrity announcement in the health care sector. Prophylactic mastectomy certainly minimizes the risk of developing breast cancer within 10 years of the surgery [74]. The obvious side effects of mastectomy along with salpingo-oophorectomy for pre-menopausal women are early-onset menopause and infertility.
9. Novel strategies for HER2-positive cancers
9.1. Combination therapy
In 2010, the CLEOPATRA (CLinical Evaluation Of Pertuzumab And TRAstuzumab) study, an international, randomized, double-blind, placebo-controlled phase III trial that involved approximately 800 HER2-positive patients from around 250 centers worldwide, illustrated the effectiveness of combining docetaxel, trastuzumab (Herceptin), and pertuzumab or docetaxel, trastuzumab, and placebo in breast cancer treatment [75], [76]. On June 8, 2012, the FDA approved pertuzumab, developed by Genentech for the treatment of HER2-positive metastatic breast cancer. Pertuzumab represented the first of a new class of drugs called “HER dimerization inhibitors.” This drug binds to HER2 and inhibits its homo- or heterodimerization. The combination of pertuzumab with Herceptin and docetaxel, as first-line treatment for HER2-positive metastatic breast cancer, has been found to substantially slow cancer growth and significantly improve progression-free survival as compared with combining a placebo with Herceptin and docetaxel. Because the use of this regimen was not associated with any serious side effects, this combination therapy represents substantial progress in the standard of care for HER2-positive patients [75], [76]. Additional follow-up studies further supported the positive benefit-to-risk ratio in HER2-positive metastatic breast cancer patients treated with the pertuzumab, Herceptin, and docetaxel regimen [75], [76]. The result of this study has fueled momentum into research exploring the effectiveness of combining two or more drugs that target the same signaling pathway.
9.2. “Armed antibody drug”
Despite the therapeutic advances, the median progression-free survival and overall survival of HER2-positive patients treated with trastuzumab alone or in combination with various chemotherapy-based regimens is 7 months at best. In 2013, the FDA approved a next-generation targeted drug called trastuzumab emtansine (T-DM1) after clinical trials including the pivotal EMILIA study demonstrated that T-DM1 administration extends survival in women with advanced HER2-positive breast cancers that progress despite other standard therapies [77]. T-DM1, also known by its trade name Kadcyla (developed by Genentech) is a conjugation of two anticancer drugs – the HER2-targeted trastuzumab (T) and the chemotherapy drug emtansine, which binds to microtubules and inhibits cell division.[78] T-DM1 specifically targets HER2-positive cells in that trastuzumab selectively binds to subdomain IV of the HER2 receptor at the surface of tumor cells leading to the internalization of this complex by endocytosis and subsequent intra-lysosomal proteolytic degradation and the release of DM1 into the cytoplasm. The action of DM1 is coupled with the anti-tumor activities of trastuzumab, which include antibody-dependent cell-mediated cellular cytotoxicity and inhibition of ligand-independent intracellular HER2 signaling events that promote cell proliferation [79]. The improved outcomes seen with T-DM1 has led to the development of several novel therapies including MM302, an antibody linked to pegylated liposomal doxorubicin (NCT01304797) and MM111, an antibody fusion protein targeting the HER2/HER3 heterodimer [78]. Protein-bound nanoparticle technology is also used now in most cancers. In 2013, the FDA approved Abraxane, a Paclitaxel Albumin-stabilized Nanoparticle Formulation. Abraxane delivery spares patients from the side effects such as severe allergic reactions associated with solvent-delivered taxol.
10. Trends in research and therapy
The therapeutic options for breast cancer patients have increased dramatically in the 21st century with increasing efforts towards the development of more efficient and effective screening, diagnosis and treatment options. The landscape of therapy for patients with metastatic breast cancer is also changing. For instance, many ongoing studies are exploring strategies to overcome endocrine resistance, target TNBC and develop new anti-HER2 therapies. There are promising advances in immunotherapy and new directions in inhibiting aberrant angiogenesis, one of the hallmarks of tumors [80]. Our understanding of the molecular characteristics of tumors in general, coupled with better screening methods has helped shape the recent advances in breast cancer treatment. Neoadjuvant therapy, for example, has become a standard recourse. There are strategies not only for HER2- and ER-positive cancers, but also new options in the management and treatment of TNBC patients [81].
Targeting TNBC has been particularly difficult because of “druggability” challenges (Reviewed by many including [81], [82], [83], [84], [85]). Therefore, chemotherapy is still viewed as the clinical state of the art therapy [83]. Nonetheless, different subsets of TNBC have been distinguished based on various signatures including the expression/activation of certain proteins, transcripts expression, and genomic alterations [84], [85]. Some TNBCs are characterized by a deficiency in homologous recombination, which is partly attributed the loss-of-function of BRCA1 and/or BRCA2 proteins [86]. All these molecular features and well as other cellular characteristics have paved the way for identification of potential options for TNBC “targeted” therapy, some of which are currently being evaluated in various clinical trials [84]. The link between TNBC and germline BRCA1 mutations has led to the investigation of poly-ADP ribose polymerase (PARP) inhibitors in TNBC. Trials of some PARP-inhibitors including Iniparib in TNBCs have shown improved rates of response and progression-free survival [87]. Many cancers, including breast cancer, are driven by a subset of cells termed cancer stem cells. These are tumor-initiating cells that can initiate tumor growth as well as mediate tumor metastasis. Several signaling pathways, such as the STAT3 signaling pathway, are known to regulate breast cancer stem cell (BCSC) self-renewal and may therefore be considered as viable therapeutic targets [88]. Effective targeting of BCSCs would be beneficial for TNBCs, which currently have no specific therapeutic targets. The focus on breast cancer treatment is shifting more towards individualized/tailored therapy, as our understanding of the connection between the characteristics of the individual patient and the respective tumor continues to improve. Advances in high-throughput genomic profiling have enabled researchers to catalogue the spectrum of somatic alterations in breast cancers. Genomic profiling will be valuable for precision and individualized medicine through accurate diagnosis, prognosis, stratification and better dynamic monitoring of treatment response.
Several other therapeutic strategies have attracted attention recently. These include: i) the use of exosomes as potential carriers for delivering drugs to tumor cells [89]; ii) targeting of long noncoding RNAs (lncRNAs), and specifically the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), shown to be also elevated in breast cancers [90], [91]. Some of the recent technological improvements have provided more tools in the fight against breast cancer. These new techniques include marginally invasive treatments such as radiofrequency ablation [92] and cryo-ablation [93]. These technologies are still in their infancy; more research and long-term data collection should clarify their effectiveness.
11. Drug resistance – A major challenge
Current targeted therapies for breast cancer include the use of tamoxifen, fulvestrant and aromatase inhibitors for ER-positive breast cancers, and Herceptin and various HER2 inhibitors for the treatment of HER2-positive breast cancers. Numerous chemotherapeutic drugs have also been approved as adjuvant and neoadjuvant treatment of all breast cancer subtypes including TNBCs. Unfortunately, most patients treated with these drugs eventually develop resistance, often leading to enhanced disease progression and death. Drug resistance is a common manifestation of cancer and is a major factor in the failure of many forms of chemotherapy. The mechanisms underlying drug resistance are poorly understood. This therefore represents a major clinical deficit, especially in the case of a heterogeneous disease like breast cancer wherein a plethora of intrinsic and acquired mechanisms may favor drug resistance. Differential expression of non-coding RNAs in tamoxifen-resistant and aromatase inhibitors-resistant breast cancer cells indicates that microRNAs could be potential therapeutic targets in endocrine-resistant tumors [94].
Research to identify and block these alternative pathways is part of a concerted effort by researchers and clinicians to overcome drug resistance in all cancers including breast cancer (reviewed in [95], [96]).
12. Conclusion
Although described more than 3500 years ago, breast cancer remains a serious public health issue, especially with the dramatic and deleterious shift in lifestyle in most societies. Breast cancer is heterogeneous in nature at both the epidemiological and molecular levels. Clinical and epidemiological evidence has identified many important breast cancer risk factors such as age, family history, early menarche and medical history; factors which are intangible or beyond our control. However, about 70% of breast cancers today arise due to risk factors that we can change or avoid. These include obesity, lack of exercise, smoking, drinking, and diet, along with factors that may negatively influence a woman's hormonal environment such as hormone replacement therapy (HRT) and reproductive history. These rate-limiting steps in the fight against breast cancer must not be overlooked. As highlighted in this review, appreciable growth in the knowledge of cancer biology has led to remarkable progress in the early detection, treatment and prevention of cancers in recent years. The increasing focus on tailored therapy and the integration of cancer stem cell-based targeted therapies and immune therapies, together with existing therapeutic methods, hold promise for the cure of breast cancer.
Hippocrates advocated the importance of tailored therapy over 2500 years ago when he stated that “It is more important to know what sort of person has a disease than to know what sort of disease a person has.”
Conflict of interest
The author is unaware of any affiliations, memberships, or financial holdings that might be perceived as affecting the objectivity of this review.
Transparency document
Transparency document.
Acknowledgements
The author thanks Raghuveera Goel (graduate student, Lukong lab) for his critical review of the manuscript. The author apologizes to those whose work was not included owing to space limitations. The author also thanks The Beatles for inspiring the title. Breast cancer research in the Lukong lab has been supported by funds from various organizations including the Canadian Breast Cancer Foundation (CBCF), (U of S fund no 416170) and Canadian Institutes of Health Research (CIHR), (U of S fund no 411333).
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. (Journal international du cancer) [DOI] [PubMed] [Google Scholar]
- 3.Ferzoco R.M., Ruddy K.J. The epidemiology of male breast cancer. Curr. Oncol. Rep. 2016;18:1. doi: 10.1007/s11912-015-0487-4. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis C.E., Bray F., Ferlay J., Lortet-Tieulent J., Anderson B.O., Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol. Biomark. Prev. 2015;24:1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Jimenez E., Garcia P.A., Aguilar M.J., Padilla C.A., Alvarez J. Breastfeeding and the prevention of breast cancer: a retrospective review of clinical histories. J. Clin. Nurs. 2014;23:2397–2403. doi: 10.1111/jocn.12368. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani S.R., Van De Vijver M.J., Jacquemier J., Anderson T.J., Osin P.P., McGuffog L., Easton D.F. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002;20:2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Helgason C.M. Commentary on the significance for modern neurology of the 17th century B.C. Surgical Papyrus. Can. J. Neurol. Sci. 1987;14:560–563. [PubMed] [Google Scholar]
- 8.Brawanski A. On the myth of the Edwin Smith papyrus: is it magic or science? Acta Neurochir. 2012;154:2285–2291. doi: 10.1007/s00701-012-1523-x. [DOI] [PubMed] [Google Scholar]
- 9.Harding F.J. Tekline Publishing; 2007. Breast Cancer: Cause, Prevention, Cure; p. 440. [Google Scholar]
- 10.Rayter Z., Janine M. Cambridge University Press; 2003. History of Breast Cancer Therapy, Medical Therapy of Breast Cancer. [Google Scholar]
- 11.Crouch M. A short history of breast-cancer - Demoulin, D. Community Health Stud. 1984;8:349–350. [Google Scholar]
- 12.Olch P.D. A short history of breast-cancer – Demoulin, D. J. Hist. Med. Allied Sci. 1984;39:100–101. [Google Scholar]
- 13.Petrakis N.L. A short history of breast-cancer – Demoulin, D, B. Hist. Med. 1984;58:417–418. [Google Scholar]
- 14.Velpeau A. The Sydenham Society; London: 1876. A Treatise on the Diseases of Breast. [Google Scholar]
- 15.H. Amjad, Q.-A. Amjad, History of Breast Cancer, Country and Natural Medicine Newsletter.
- 16.Papavramidou N., Papavramidis T., Demetriou T. Ancient Greek and Greco-Roman methods in modern surgical treatment of cancer. Ann. Surg. Oncol. 2010;17:665–667. doi: 10.1245/s10434-009-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Zhou W.B., Zhao Y., Liu X.A., Ding Q., Zha X.M., Wang S. Bloody nipple discharge is a predictor of breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 2012;132:9–14. doi: 10.1007/s10549-011-1787-5. [DOI] [PubMed] [Google Scholar]
- 18.Galen . Ad glauconeum de methodo medendi. In: Kuhn C.G., editor. Opera Omnia. Lipsiae: Car. Vol. 11. 1826. pp. 139–141. (Cnoblochii). [Google Scholar]
- 19.Donegan W.L. History of breast cancer. Breast Cancer. 2006:1–14. [Google Scholar]
- 20.Galen Galen. De atra bile. In: Kuhn C.G., editor. Vol. 5. 1823. pp. 116–118. (Opera Omnia). [Google Scholar]
- 21.Cotlar A.M., Dubose J.J., Rose D.M. History of surgery for breast cancer: radical to the sublime. Curr. Surg. 2003;60:329–337. doi: 10.1016/S0149-7944(02)00777-8. [DOI] [PubMed] [Google Scholar]
- 22.Chikly B. Who discovered the lymphatic system. Lymphology. 1997;30:186–193. [PubMed] [Google Scholar]
- 23.Haas L.F. Franciscus de le Boe or Sylvius 1614–72. J. Neurol. Neurosurg. Psychiatry. 1992;55:727. doi: 10.1136/jnnp.55.8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tubbs R.S., Linganna S., Loukas M. Franciscus Sylvius (1614-1672): a historical review. Childs Nerv. Syst. 2007;23:1–2. doi: 10.1007/s00381-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 25.Pope M.H. Bernardino Ramazzini: the father of occupational medicine. Spine (Phila Pa 1976) 2004;29:2335–2338. doi: 10.1097/01.brs.0000142437.70429.a8. [DOI] [PubMed] [Google Scholar]
- 26.Mandell J.B. Bathsheba's breast women, cancer & history. J. Clin. Invest. 2005;115 [Google Scholar]
- 27.Boyd S. On oophorectomy in the treatment of cancer. Br. Med. J. 1897;2:890–896. doi: 10.1136/bmj.2.1918.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Press D.J., Pharoah P. Risk factors for breast cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiology. 2010;21:566–572. doi: 10.1097/EDE.0b013e3181e08eb3. [DOI] [PubMed] [Google Scholar]
- 29.Wainwright J.M. A comparison of conditions associated with breast cancer in Great Britain and America. Am. J. Cancer. 1931;15 [Google Scholar]
- 30.Dunn P.M. Wilhelm Conrad Roentgen (1845–1923), the discovery of x rays and perinatal diagnosis. Arch. Dis. Child. Fetal Neonatal Ed. 2001;84:F138–F139. doi: 10.1136/fn.84.2.F138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard J.D. History of mammography. Bull. Acad. Natl Med. 1998;182:1613–1620. [PubMed] [Google Scholar]
- 32.Bassett L.W., Gold R.H. The evolution of mammography. AJR Am. J. Roentgenol. 1988;150:493–498. doi: 10.2214/ajr.150.3.493. [DOI] [PubMed] [Google Scholar]
- 33.Kalaf J.M. Mammography: a history of success and scientific enthusiasm. Radiol. Bras. 2014;47:VII–VIII. doi: 10.1590/0100-3984.2014.47.4e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher B., Bauer M., Margolese R., Poisson R., Pilch Y., Redmond C., Fisher E., Wolmark N., Deutsch M., Montague E. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N. Engl. J. Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B., Redmond C., Fisher E.R., Bauer M., Wolmark N., Wickerham D.L., Deutsch M., Montague E., Margolese R., Foster R. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N. Engl. J. Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B. The evolution of paradigms for the management of breast cancer: a personal perspective. Cancer Res. 1992;52:2371–2383. [PubMed] [Google Scholar]
- 37.Lerner B.H. No shrinking violet: Rose Kushner and the rise of American breast cancer activism. West. J. Med. 2001;174:362–365. doi: 10.1136/ewjm.174.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun S. The history of breast cancer advocacy. Breast J. 2003;9(Suppl. 2):S101–S103. doi: 10.1046/j.1524-4741.9.s2.13.x. [DOI] [PubMed] [Google Scholar]
- 39.Tata J.R. One hundred years of hormones. EMBO Rep. 2005;6:490–496. doi: 10.1038/sj.embor.7400444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen E.V., Jacobson H.I., Walf A.A., Frye C.A. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol. Behav. 2010;99:151–162. doi: 10.1016/j.physbeh.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog. Horm. Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- 42.Jordan V.C. Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 43.Plouffe L., Jr. Selective estrogen receptor modulators (SERMs) in clinical practice. J. Soc. Gynecol. Investig. 2000;7:S38–S46. doi: 10.1016/s1071-5576(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 44.Narod S.A., Foulkes W.D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 45.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P.A., Harshman K. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 46.Wooster R., Bignell G., Lancaster J., Swift S., Seal S. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 47.Cornejo-Moreno B.A., Uribe-Escamilla D., Salamanca-Gomez F. Breast cancer genes: looking for BRACA's lost brother. Isr. Med. Assoc. J. 2014;16:787–792. [PubMed] [Google Scholar]
- 48.B.B.C. Scottish/Northern Irish BRCA1 and BRCA2 mutations in Scotland and Northern Ireland. Br. J. Cancer. 2003;88:1256–1262. doi: 10.1038/sj.bjc.6600840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carpenter G., Cohen S. Epidermal growth factor. Annu. Rev. Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986;319:230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- 51.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire R.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 52.Muller W.J., Sinn E., Pattengale P.K., Wallace R., Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 53.Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., Fluge O., Pergamenschikov A., Williams C., Zhu S.X., Lonning P.E., Borresen-Dale A.L., Brown P.O., Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 54.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Thorsen T., Quist H., Matese J.C., Brown P.O., Botstein D., Eystein Lonning P., Borresen-Dale A.L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sotiriou C., Neo S.Y., McShane L.M., Korn E.L., Long P.M., Jazaeri A., Martiat P., Fox S.B., Harris A.L., Liu E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., Graf S., Ha G., Haffari G., Bashashati A., Russell R., McKinney S., Caldas C., Aparicio S., Brenton J.D., Ellis I., Huntsman D., Pinder S., Purushotham A., Murphy L., Bardwell H., Ding Z., Jones L., Liu B., Papatheodorou I., Sammut S.J., Wishart G., Chia S., Gelmon K., Speers C., Watson P., Blamey R., Green A., Macmillan D., Rakha E., Gillett C., Grigoriadis A., di Rinaldis E., Tutt A., Parisien M., Troup S., Chan D., Fielding C., Maia A.T., McGuire S., Osborne M., Sayalero S.M., Spiteri I., Hadfield J., Bell L., Chow K., Gale N., Kovalik M., Ng Y., Prentice L., Tavare S., Markowetz F., Langerod A., Provenzano E., Borresen-Dale A.L. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012 doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rakha E.A., Ellis I.O. Triple-negative/basal-like breast cancer: review. Pathology. 2009;41:40–47. doi: 10.1080/00313020802563510. [DOI] [PubMed] [Google Scholar]
- 58.Prat A., Perou C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lakhani S.R., Ellis I.O., Schnitt S.J., Tan P.H., van de Vijver M.J. vol. 4. 2012. WHO Classification of Tumours; p. 240. (IARC WHO Classification of Tumours, No 4). [Google Scholar]
- 60.Sinn H.P., Kreipe H. Focusing on Issues and Updates from the 3rd Edition. fourth ed. Vol. 8. 2013. A brief overview of the WHO classification of breast tumors; pp. 149–154. (Breast Care (Basel)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirk R. Risk factors. Oncotype DX assay predicts local recurrence in breast cancer, Nat rev. Clin. Oncol. 2010;7:300. doi: 10.1038/nrclinonc.2010.75. [DOI] [PubMed] [Google Scholar]
- 62.Carlson B. Oncotype DX test offers guidance for women debating chemotherapy. Biotechnol. Healthc. 2006;3:12–14. [PMC free article] [PubMed] [Google Scholar]
- 63.Bao T., Davidson N.E. Gene expression profiling of breast cancer. Adv. Surg. 2008;42:249–260. doi: 10.1016/j.yasu.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris S.R., Carey L.A. Gene expression profiling in breast cancer. Curr. Opin. Oncol. 2007;19:547–551. doi: 10.1097/CCO.0b013e3282f0ada3. [DOI] [PubMed] [Google Scholar]
- 65.Wakeling A.E. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr. Relat. Cancer. 2000;7:17–28. doi: 10.1677/erc.0.0070017. [DOI] [PubMed] [Google Scholar]
- 66.Samavat H., Kurzer M.S. Estrogen metabolism and breast cancer. Cancer Lett. 2015;356:231–243. doi: 10.1016/j.canlet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chumsri S., Howes T., Bao T., Sabnis G., Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011;125:13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke B.L., Khosla S. New selective estrogen and androgen receptor modulators. Curr. Opin. Rheumatol. 2009;21:374–379. doi: 10.1097/BOR.0b013e32832ca447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rimawi M.F., Schiff R., Osborne C.K. Targeting HER2 for the treatment of breast cancer. Annu. Rev. Med. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 70.Paoletti C., Hayes D.F. Molecular testing in breast cancer. Annu. Rev. Med. 2014;65:95–110. doi: 10.1146/annurev-med-070912-143853. [DOI] [PubMed] [Google Scholar]
- 71.Advani P., Moreno-Aspitia A. Vol. 6. Dove Med Press; 2014. Current Strategies for the Prevention of Breast Cancer; pp. 59–71. (Breast Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domchek S.M., Friebel T.M., Singer C.F., Evans D.G., Lynch H.T., Isaacs C., Garber J.E., Neuhausen S.L., Matloff E., Eeles R., Pichert G., Van T'veer L., Tung N., Weitzel J.N., Couch F.J., Rubinstein W.S., Ganz P.A., Daly M.B., Olopade O.I., Tomlinson G., Schildkraut J., Blum J.L., Rebbeck T.R. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Evans D.G., Barwell J., Eccles D.M., Collins A., Izatt L., Jacobs C., Donaldson A., Brady A.F., Cuthbert A., Harrison R., Thomas S., Howell A., F.H.S. Group, R.G.C. Teams, Miedzybrodzka Z., Murray A. The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014;16:442. doi: 10.1186/s13058-014-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narod S.A. The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Res. Treat. 2011;128:581–583. doi: 10.1007/s10549-011-1479-1. [DOI] [PubMed] [Google Scholar]
- 75.Swain S.M., Baselga J., Kim S.B., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.M., Schneeweiss A., Heeson S., Clark E., Ross G., Benyunes M.C., Cortes J., C.S. Group Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swain S.M., Kim S.B., Cortes J., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.M., Schneeweiss A., Knott A., Clark E., Ross G., Benyunes M.C., Baselga J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J., Song P., Schrieber S., Liu Q., Xu Q., Blumenthal G., Amiri Kordestani L., Cortazar P., Ibrahim A., Justice R., Wang Y., Tang S., Booth B., Mehrotra N., Rahman A. Exposure-response relationship of T-DM1: insight into dose optimization for patients with HER2-positive metastatic breast cancer. Clin. Pharmacol. Ther. 2014;95:558–564. doi: 10.1038/clpt.2014.24. [DOI] [PubMed] [Google Scholar]
- 78.Lambert J.M., Chari R.V. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J. Med. Chem. 2014;57:6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 79.Martinez M.T., Perez-Fidalgo J.A., Martin-Martorell P., Cejalvo J.M., Pons V., Bermejo B., Martin M., Albanell J., Lluch A. Treatment of HER2 positive advanced breast cancer with T-DM1: a review of the literature. Crit. Rev. Oncol. Hematol. 2015 doi: 10.1016/j.critrevonc.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z., Dabrosin C., Yin X., Fuster M.M., Arreola A., Rathmell W.K., Generali D., Nagaraju G.P., El-Rayes B., Ribatti D., Chen Y.C., Honoki K., Fujii H., Georgakilas A.G., Nowsheen S., Amedei A., Niccolai E., Amin A., Ashraf S.S., Helferich B., Yang X., Guha G., Bhakta D., Ciriolo M.R., Aquilano K., Chen S., Halicka D., Mohammed S.I., Azmi A.S., Bilsland A., Keith W.N., Jensen L.D. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015 doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Reilly E.A., Gubbins L., Sharma S., Tully R., Guang M.H., Weiner-Gorzel K., McCaffrey J., Harrison M., Furlong F., Kell M., McCann A. The fate of chemoresistance in triple negative breast cancer (TNBC) BBA Clin. 2015;3:257–275. doi: 10.1016/j.bbacli.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gluz O., Liedtke C., Gottschalk N., Pusztai L., Nitz U., Harbeck N. Triple-negative breast cancer--current status and future directions. Ann. Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 83.von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., Gerber B., Eiermann W., Hilfrich J., Huober J., Jackisch C., Kaufmann M., Konecny G.E., Denkert C., Nekljudova V., Mehta K., Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 84.Denkert C., Liedtke C., Tutt A., von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2016 doi: 10.1016/S0140-6736(16)32454-0. [DOI] [PubMed] [Google Scholar]
- 85.Miah S., Banks C.A., Adams M.K., Florens L., Lukong K.E., Washburn M.P. Advancement of mass spectrometry-based proteomics technologies to explore triple negative breast cancer. Mol. BioSyst. 2016;13:42–55. doi: 10.1039/c6mb00639f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crown J., O'Shaughnessy J., Gullo G. Emerging targeted therapies in triple-negative breast cancer. Ann. Oncol. 2012;23(Suppl. 6):vi56–vi65. doi: 10.1093/annonc/mds196. [DOI] [PubMed] [Google Scholar]
- 87.Liang H., Tan A.R. Iniparib, a PARP1 inhibitor for the potential treatment of cancer, including triple-negative breast cancer. IDrugs. 2010;13:646–656. [PubMed] [Google Scholar]
- 88.Carrasco E., Alvarez P.J., Prados J., Melguizo C., Rama A.R., Aranega A., Rodriguez-Serrano F. Cancer stem cells and their implication in breast cancer. Eur. J. Clin. Investig. 2014;44:678–687. doi: 10.1111/eci.12276. [DOI] [PubMed] [Google Scholar]
- 89.Wu C.Y., Du S.L., Zhang J., Liang A.L., Liu Y.J. Exosomes and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Cancer Gene Ther. 2016 doi: 10.1038/cgt.2016.69. [DOI] [PubMed] [Google Scholar]
- 90.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S., Kim Y., MacLeod A.R., Krainer A.R., Norton L., Brogi E., Egeblad M., Spector D.L. Differentiation of mammary tumors and reduction in metastasis upon Malat1 LncRNA loss. Cancer Res. 2016;76 doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mendell J.T. Targeting a long noncoding RNA in breast cancer. N. Engl. J. Med. 2016;374:2287–2289. doi: 10.1056/NEJMcibr1603785. [DOI] [PubMed] [Google Scholar]
- 92.Le A., Stine Z.E., Nguyen C., Afzal J., Sun P., Hamaker M., Siegel N.M., Gouw A.M., Kang B.H., Yu S.H., Cochran R.L., Sailor K.A., Song H., Dang C.V. Tumorigenicity of hypoxic respiring cancer cells revealed by a hypoxia-cell cycle dual reporter. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1402012111. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lanza E., Palussiere J., Buy X., Grasso R.F., Beomonte Zobel B., Poretti D., Pedicini V., Balzarini L., Cazzato R.L. Percutaneous image-guided cryoablation of breast cancer: a systematic review. J. Vasc. Interv. Radiol. 2015;26(11):1652–1657. doi: 10.1016/j.jvir.2015.07.020. e1651. [DOI] [PubMed] [Google Scholar]
- 94.Hayes E.L., Lewis-Wambi J.S. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40. doi: 10.1186/s13058-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Q., Yang Z., Nie Y., Shi Y., Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 96.Rondon-Lagos M., Villegas V.E., Rangel N., Sanchez M.C., Zaphiropoulos P.G. Tamoxifen resistance: emerging molecular targets. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17081357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.