Abstract
No-caloric sweeteners, such as aspartame, are widely used in various food and beverages to prevent the increasing rates of obesity and diabetes mellitus, acting as tools in helping control caloric intake. Aspartame is metabolized to phenylalanine, aspartic acid, and methanol. Our aim was to study the effect of chronic administration of aspartame on glutathione redox status and on the trans-sulphuration pathway in mouse liver. Mice were divided into three groups: control; treated daily with aspartame for 90 days; and treated with aspartame plus N-acetylcysteine (NAC). Chronic administration of aspartame increased plasma alanine aminotransferase (ALT) and aspartate aminotransferase activities and caused liver injury as well as marked decreased hepatic levels of reduced glutathione (GSH), oxidized glutathione (GSSG), γ-glutamylcysteine (γ-GC), and most metabolites of the trans-sulphuration pathway, such as cysteine, S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH). Aspartame also triggered a decrease in mRNA and protein levels of the catalytic subunit of glutamate cysteine ligase (GCLc) and cystathionine γ-lyase, and in protein levels of methionine adenosyltransferase 1A and 2A. N-acetylcysteine prevented the aspartame-induced liver injury and the increase in plasma ALT activity as well as the decrease in GSH, γ-GC, cysteine, SAM and SAH levels and GCLc protein levels. In conclusion, chronic administration of aspartame caused marked hepatic GSH depletion, which should be ascribed to GCLc down-regulation and decreased cysteine levels. Aspartame triggered blockade of the trans-sulphuration pathway at two steps, cystathionine γ-lyase and methionine adenosyltransferases. NAC restored glutathione levels as well as the impairment of the trans-sulphuration pathway.
Keywords: Aspartame, Cysteine, S-adenosylmethionine, N-acetylcysteine
Highlights
-
•
Aspartame down-regulates glutamate cysteine ligase and decreased cysteine levels.
-
•
Aspartame blockades the trans-sulphuration pathways.
1. Introduction
Nowadays, no-caloric sweeteners are widely used to prevent the increasing rates of obesity and diabetes mellitus and to handle these patients, acting as critical tools in helping control caloric intake. Among them, aspartame stands out from all the others [1]. Aspartame is a dipeptide derivative (L-aspartyl L-phenylalanine methyl ester) that is used in a foods and beverages worldwide [2]. After its oral ingestion, aspartame is absorbed from the intestinal lumen and hydrolyzed to phenylalanine (50%) -the precursor for two neurotransmitters of the catecholamine family-; aspartic acid (40%) -an excitatory amino acid-; and methanol (10%) -which is oxidized to cytotoxic formaldehyde and formic acid- [3]. Although the Food and Drug Administration (FDA) approved aspartame consumption, its use has been controversial as it has been associated with several adverse effects as hyperglycemia [4], [5], neurologic and behavioral disturbances [6] and hepatocellular lesions [7]. Most of them were ascribed to the generation of aspartame metabolites, particularly to methanol metabolites as formaldehyde and formate.
Methanol levels were found elevated after aspartame administration to humans [8] and rats [8], [9], [10]. However, there are some species differences in the metabolism of methanol because humans metabolize methanol to formaldehyde through alcohol dehydrogenase, whereas rodents use catalase, which also has antioxidative activity [11]. Formaldehyde is converted to formate through a similar mechanism in both species via formaldehyde dehydrogenase, which is a glutathione-dependent enzyme [12]. Then, formate in metabolized to carbon dioxide through a tetrahydrofolate-dependent pathway [13].
Aspartame-derivative methanol has been linked to depletion of reduced glutathione [9], [10]. Indeed, GSH depletion in brain, liver, and erythrocytes is a common feature of the long-term administration of aspartame [5], [7], [9], [10], [14], [15]. The aim of this work was to determine the effect of chronic administration of aspartame on glutathione redox status and on the trans-sulphuration pathway in mouse liver.
2. Materials and methods
2.1. Animals
Male Swiss mice (30±6 g b.w.) were obtained from Central Animal Facility of the Federal University of Santa Maria (Brazil). They were fed on a standard rodent chow (Supra, São Leopoldo, Brazil) and tap water ad libitum, in temperature- and humidity- controlled animal quarters under a 12-h light-dark cycle. The Ethics Committee of the Federal University of Santa Maria (Brazil) approved the study protocol (#001/2015).
2.2. Experimental protocol
Mice (n =18) were divided into three groups with six animals each one: control; aspartame (Sigma-Aldrich, St Louis, USA); and aspartame treated with N-acetylcysteine (NAC) (Sigma-Aldrich, St Louis, USA). Control group received vehicle (0.9% NaCl) by gavage for 90 days, whereas aspartame and aspartame treated with NAC groups received aspartame (80 mg/kg, 2.5 ml/kg, prepared in 0.9% NaCl solution). From day 60 until the 90 days immediately after administration of aspartame, the mice of the third group received NAC (163 mg/kg, pH 6.8–7.2) intraperitoneally, whereas the others received its vehicle intraperitoneally.
All treatments were prepared daily prior to administration. Prior to sacrifice, mice were anesthetized with isoflurane inhaled at 3%, blood was collected in heparinized tubes and subsequently the animals were sacrificed through exsanguination 3 h after the last treatment.
2.3. Assays
2.3.1. Alanine amino transferase and aspartate amino transferase activities
ALT and AST activities were determined in plasma using commercial kits (Labtest, Lagoa Santa, Brazil). Results were expressed as UI/L.
2.3.2. Histology
Liver samples were fixed 10% formaldehyde and embedded in paraffin. Next, 6 µm thick histological sections were cut and stained with hematoxilin-eosin to detect microarquitecture and morphological alterations.
2.3.3. Determination of sulfur-containing amino acids
Frozen liver samples were homogenized in 400 μl of phosphate saline buffer containing 11 mM N-ethyl maleimide (NEM). Perchloric acid (PCA) was then added to obtain a final concentration of 4% and centrifuged at 15,000g for 15 min at 4 °C. The concentrations of GSH, oxidized glutathione (GSSG), glutamylcysteine (γ-GC), cysteine, cystathionine, homocysteine, S-adenosyl homocysteine (SAH), S-adenosyl methionine (SAM) and methionine were determined in the supernatants by high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS). The chromatographic system consisted of a Micromass QuatroTM triple-quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with a Zspray electrospray ionization source operating in the positive ion mode with a LC-10A Shimadzu (Shimadzu, Kyoto, Japan) coupled to the MassLynx software 4.1 for data acquisition and processing. Samples were analyzed by reversed-phase HPLC with a C18 Mediterranea SEA column (Teknokroma, Barcelona, Spain) (5.060.21 cm) with 3 mm particle size. In all cases, 20 μl of the supernatant were injected onto the analytical column. The mobile phase consisted of the following gradient system (min/%A/%B) (A, 0.5% formic acid; B, isopropanol/acetonitrile 50/50; 0,5% formic acid): 5/100/0, 10/0/100, 15/0/100, 15.10/100/0, and 60/100/0. The flow rate was set at 0.2 ml/min. Positive ion electrospray tandem mass spectra were recorded with the electrospray capillary set at 3 keV and a source block temperature of 120 °C. Nitrogen was used as the drying and nebulizing gas at flow rates of 500 and 30 L/h, respectively. Argon at 1.5610–3 mbar was used as the collision gas for collision-induced dissociation. An assay based on LC-MS/MS with multiple reaction monitoring was developed using the transitions m/z, cone energy (V), collision energy (eV) and retention time (min) for each compound that represents favorable fragmentation pathways for these protonated molecules. Calibration curves were obtained using six-point (0.01–100 mmol/l) standards (purchased from Sigma-Aldrich, St Louis, USA) for each compound. The concentrations of metabolites were expressed as nmol/mg of protein.
2.3.4. RT-PCR
A small piece of liver was excised and immediately immersed in RNA-later solution (Ambion, Thermo Fisher Scientific, Waltham, USA) to stabilize the RNA. Total RNA was isolated using Trizol (Sigma-Aldrich, St Louis, USA). The cDNA for amplification in the PCR assay was constructed by reversion transcription reaction using Revertaid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, USA). Real time-PCR was performed using SYBR Green PCR Master Mix (Takara, Kusatsu, Japan) in an iQ5 real-time PCR detection system (BioRad, Hercules, USA). Each reaction was performed in duplicate and the melting curves were constructed to ensure that a single product was amplified. GAPDH was analyzed as real time RT-PCR control. The following primers were used: Gclc: forward 5′-CCATCACTTCATTCCCCAGA-3′ and reverse 5′-GATGCCGGATGTTTCTTGTT-3′; Cth: forward 5′- TCGTTTCCTGCAGAATTCACT −3′ and reverse 5′-CTGCTCTTTCAGGGCCTCTT-3′; Gapdh: forward 5′-GGGCATCTTGGGCTACAC-3′ and reverse 5′-GGTCCAGGGTTTCTTACTCC-3′. The threshold cycle (CT) was determined and the relative gene expression was expressed as follows: fold change =2-Δ (ΔCT), where ΔCT = CTtarget – CThousekeeping, and Δ(ΔCT) =ΔCTtreated - ΔCTcontrol.
2.3.5. Western blotting
Frozen liver samples were homogenized on ice in Hepes lysis buffer (100 mg/mL) containing 75 mM NaCl, 750 μM magnesium chloride, 25 mM Hepes (pH 7.4), 500 μM EGTA, 5% glycerol, 0,5% Igepal, 1 mM dithiothreitol, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, and 1 mM sodium orthovanadate. A protease inhibitor cocktail (Sigma-Aldrich, St Louis, USA) was added before its use at a concentration of 5 μL/mL. All debris was removed through centrifugation at 15,000g at 4 °C for 15 min, and the supernatant obtained was used for analysis. Fifty micrograms of protein were separated in Criterion Gel 4–15% (BioRad, Hercules, USA) by electrophoresis and transferred to Trans-Blot Turbo Nitrocellulose membranes (BioRad, Hercules, USA). Western blotting and chemiluminescence detection using Luminata Clássico Western HRP Substrate (Millipore, Billerica, USA) were utilized to determine the catalytic subunit of glutamate cysteine ligase (GCLc), cystathionine gamma-lyase (CTH) and methionine adenosyltransferase 1A (MAT1A) and 2A (MAT2A) and GAPDH. The following antibodies were used: antibody against GCLc (1/1000) (Abcam, Cambridge, UK), antibody against CTH (1/500) (Abcam, Cambridge, UK), antibody against MAT1A (1/500) (Abcam, Cambridge, UK), antibody against MAT2A (1/500) (Abcam, Cambridge, UK) and antibody against GAPDH (1/1000) (Cell Signaling Technology, Danvers, USA).
2.4. Statistical analysis
The data were compared by one-way ANOVA followed by the Tukey-Kramer Multiple Comparisons Test. Results are reported as mean±standard deviation (SD) and differences were considered to be significant at P<0.05.
3. Results
3.1. Effect of aspartame on plasma aminotransferase activities
Chronic aspartame administration produced an elevation in ALT and AST activities in the plasma. ALT activity returned to normal levels after chronic NAC treatment. All data are shown in Table 1.
Table 1.
ALT and AST activities in plasma of aspartame-treated mice. Effect of NAC.
The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows:
P<0.05 versus control.
P<0.05 versus ASP. ASP, aspartame; NAC, N-acetylcysteine.
3.2. Effect of aspartame on the liver histology
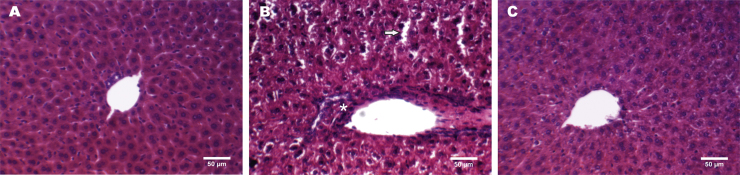
Aspartame administration increased hepatocellular injury, triggering leukocyte infiltration, reduction in nuclear area, and degeneration of hepatocytes with increased liver sinusoidal diameter in different areas of the liver (Fig. 1B). NAC treatment restored the normal histological architecture, showing preserved hepatocyte and sinusoidal morphology after aspartame treatment (Fig. 1C), which was similar to the hepatic histology of control mice (Fig. 1A). Hence, chronic aspartame may lead to toxic hepatitis.
Fig. 1.
Representative images of hematoxylin-eosin histological staining in liver of control (A), aspartame-treated mice (B) and mice treated with aspartame plus NAC (C). Leukocyte infiltration (asterisk), reduction in nuclear volume and degeneration of hepatocytes (arrows) are shown in aspartame-treated mice (B).
3.3. Effect of aspartame on the glutathione levels and redox status in the liver
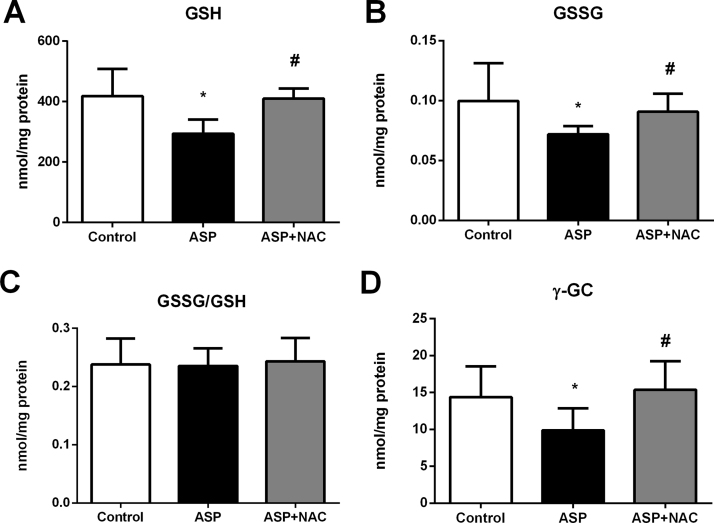
Aspartame administration caused a 30% decrease in GSH levels in the liver, which was prevented by NAC treatment (Fig. 2A). However, GSH depletion was not associated with glutathione oxidation in the liver as aspartame also induced a parallel GSSG depletion maintaining the normal GSSG/GSH ratio (Fig. 2B,C).
Fig. 2.
GSH (A), GSSG (B), GGSG/GSH*1000 ratio (C) and γ-GC (D) levels in liver of aspartame-treated mice. Effect of NAC. The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows: *, P<0.05 versus control; #, P<0.05 versus ASP. Abbreviations: ASP, aspartame; NAC, N-acetylcysteine; GSH, reduced glutathione; GSSG, oxidized glutathione; γ-GC, γ-glutamylcysteine.
3.4. Effect of aspartame on γ-glutamylcysteine and the glutamate cysteine ligase
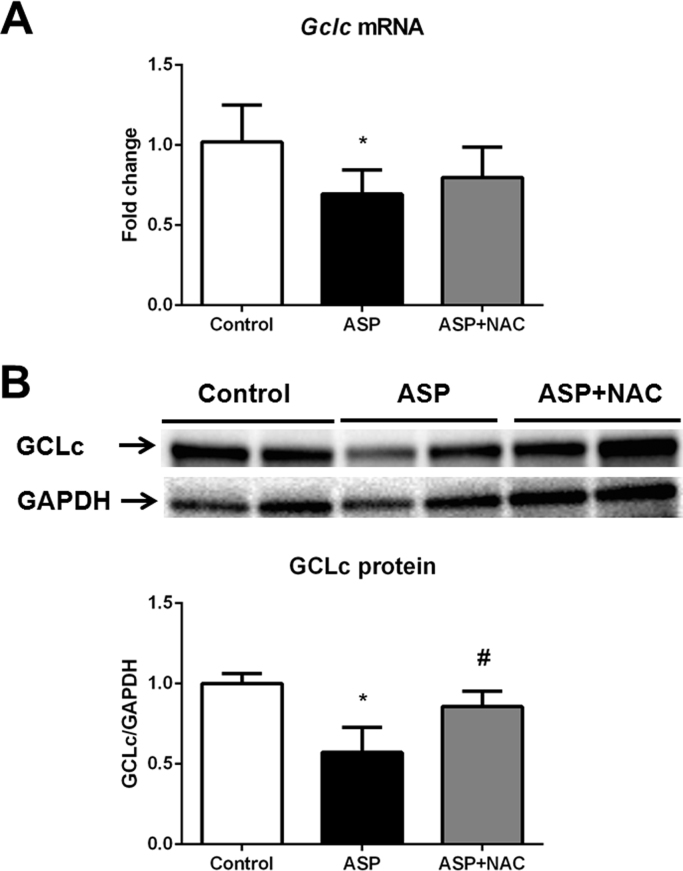
The levels of γ-GC were decreased in the liver upon administration of aspartame (Fig. 2D). NAC administration restored the normal γ-GC hepatic levels. Aspartame administration also triggered down-regulation of both the gclc mRNA (Fig. 3A) and GCLc protein (Fig. 3B,C) levels in the liver. GCLc protein levels returned to the control levels after treatment with NAC (Fig. 3B).
Fig. 3.
Gclc mRNA relative expression versus gapdh (A), representative Western blotting image and densitometry of GCLc protein relative expression versus GAPDH protein (B) in liver of aspartame-treated mice. Effect of NAC. The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows: *, P<0.05 versus control; #, P<0.05 versus ASP. Abbreviations: ASP, aspartame; NAC, N-acetylcysteine; GCLc, catalytic subunit of glutamate cysteine ligase.
3.5. Effect of aspartame on the trans-sulphuration pathway in the liver
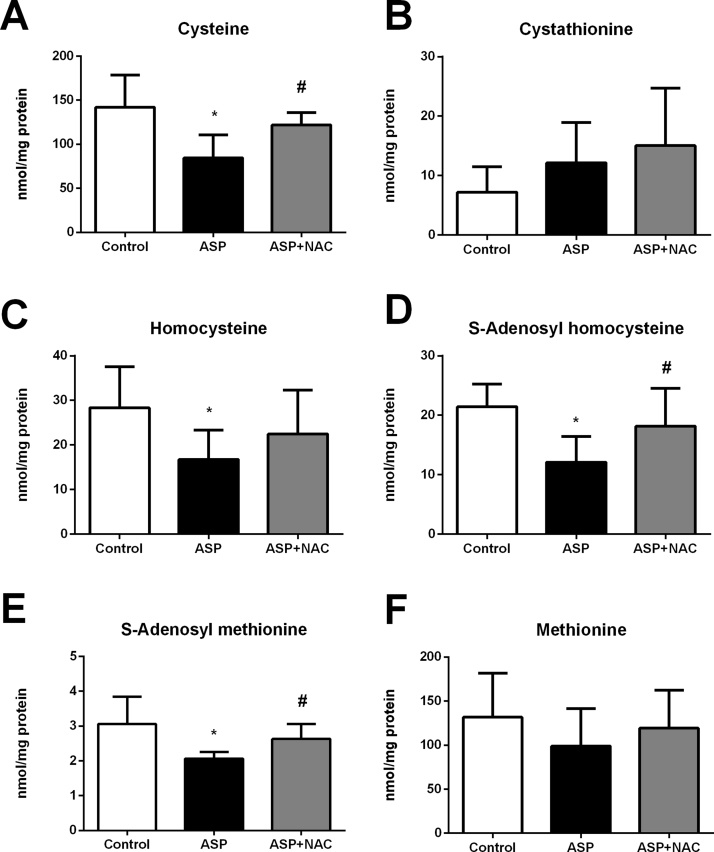
Aspartame administration caused a severe depletion in most metabolites of the trans-sulphuration pathway in the liver. Indeed, cysteine (Fig. 4A), homocysteine (Fig. 4C), SAH (Fig. 4D), and SAM (Fig. 4E) hepatic levels were severely depleted by aspartame, whereas methionine (Fig. 4F) and cystathionine levels (Fig. 4B) did not change. NAC administration restored these changes in aspartame-treated mice leading to normal levels of all metabolites of the trans-sulphuration pathways.
Fig. 4.
Cysteine (A), cystathionine (B), homocysteine (C), SAH (D), SAM (E) and methionine (F) levels in liver of aspartame-treated mice. Effect of NAC. The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows: *, P<0.05 versus control; #, P<0.05 versus ASP. Abbreviations: ASP, aspartame; NAC, N-acetylcysteine; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine.
3.6. Effect of aspartame on expression of cystathionine gamma-lyase and methionine adenosyltransferases in the liver
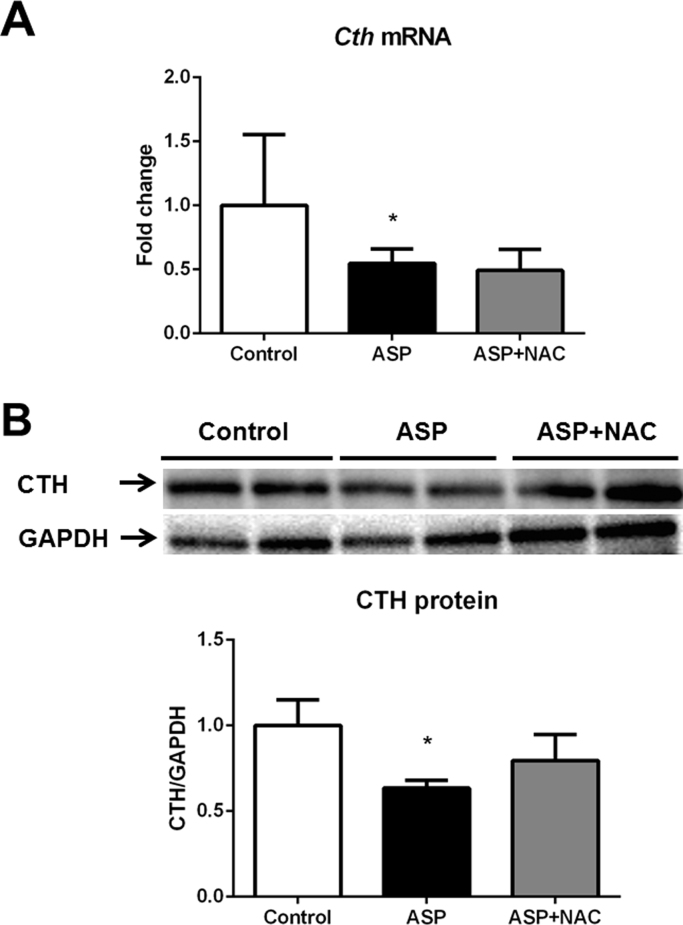
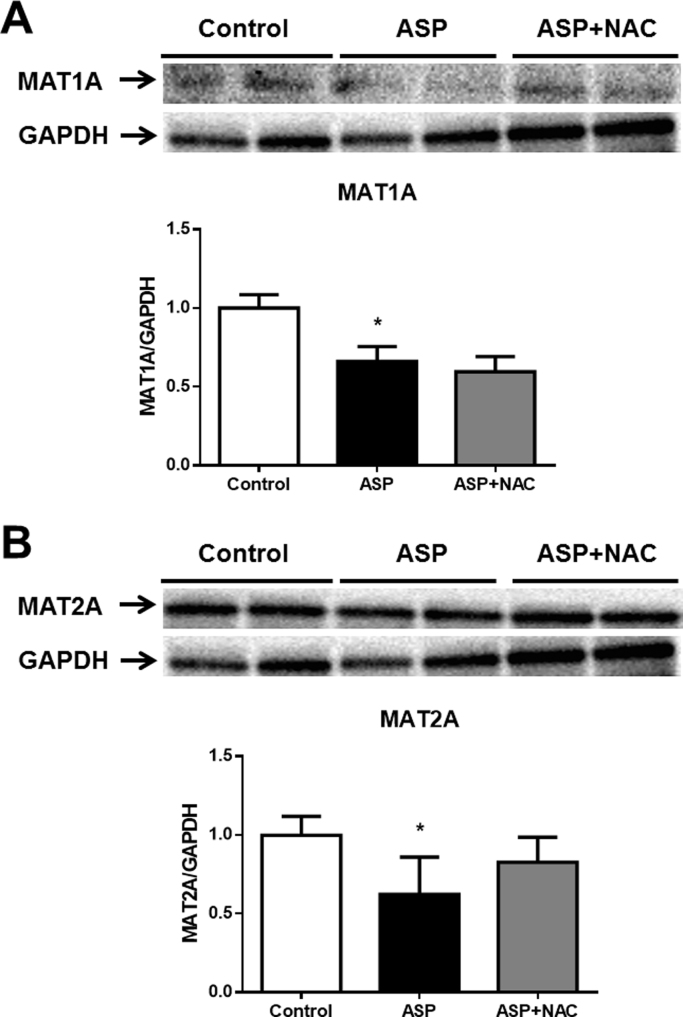
Aspartame administration led to a decrease in the cth mRNA (Fig. 5A) and CTH (Fig. 5B) protein levels as well as in MAT1A (Fig. 6A) and MAT2A (Fig. 6B) protein levels in the liver.
Fig. 5.
Cth mRNA relative expression versus gapdh (A), representative Western blotting image and densitometry of CTH protein relative expression versus GAPDH protein (B) in liver of aspartame-treated mice. Effect of NAC. The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows: *, P<0.05 versus control; #, P<0.05 versus ASP. Abbreviations: ASP, aspartame; NAC, N-acetylcysteine; CTH, cystathionine γ-lyase.
Fig. 6.
Representative Western blotting image and densitometry of MAT1A (A) and MAT2A (B) protein relative expression versus GAPDH protein in liver of aspartame-treated mice. Effect of NAC. The number of mice per group was 6. Results are expressed as mean±SD. The statistical difference is indicated as follows: *, P<0.05 versus control. Abbreviations: ASP, aspartame; NAC, N-acetylcysteine; MAT1A, methionine adenosyltransferase 1A; MAT2A, methionine adenosyltransferase 2A.
4. Discussion
Aspartame is present in more than 6000 food products around the world [2]. However, most aspartame consumers are unaware about its potentially detrimental metabolites and controversial safety [7]. Aspartame was also related to non-alcoholic fatty liver disease associated to metabolic syndrome [16], [17]. Aspartame and other artificial sweeteners may drive the development of glucose intolerance, fatty liver disease linked to metabolic syndrome through induction of compositional and functional alterations of the intestinal microbiota in mice and humans [17]. Aspartame adverse effects also have been ascribed to the toxic actions of methanol and its metabolites generated from aspartame metabolism [18], [19]. Indeed, methanol levels were elevated in rat plasma after long-term intake of aspartame at high doses [12], and even at similar doses to those expected in humans [10]. Methanol metabolism produces formaldehyde, which was found to be even more toxic than methanol itself and formate [19]. Formaldehyde can form adducts with proteins and nucleic acids, which accumulate in rat liver during chronic treatment with high doses of aspartame [18]. Formaldehyde also decreases hepatic GSH content and induces cell death in rat thymocytes, being pointed as possible responsible at least for part of aspartame toxicity [19].
Aspartame-induced liver inflammation and necrosis is associated with GSH depletion and a decrease in glutathione peroxidase and glutathione reductase activities [7]. Aspartame also provokes adrenal cell apoptosis in vitro [20] and brain apoptosis in vivo [10] via mitochondrial oxidative stress. Hence, GSH depletion and changes in GSH-related enzymes are considered main features linked to aspartame-induced oxidative stress and injury [5], [7], [9], [10], [14], [15]. Moreover, methanol may cause oxidative stress and is considered the major contributor to it upon aspartame administration.
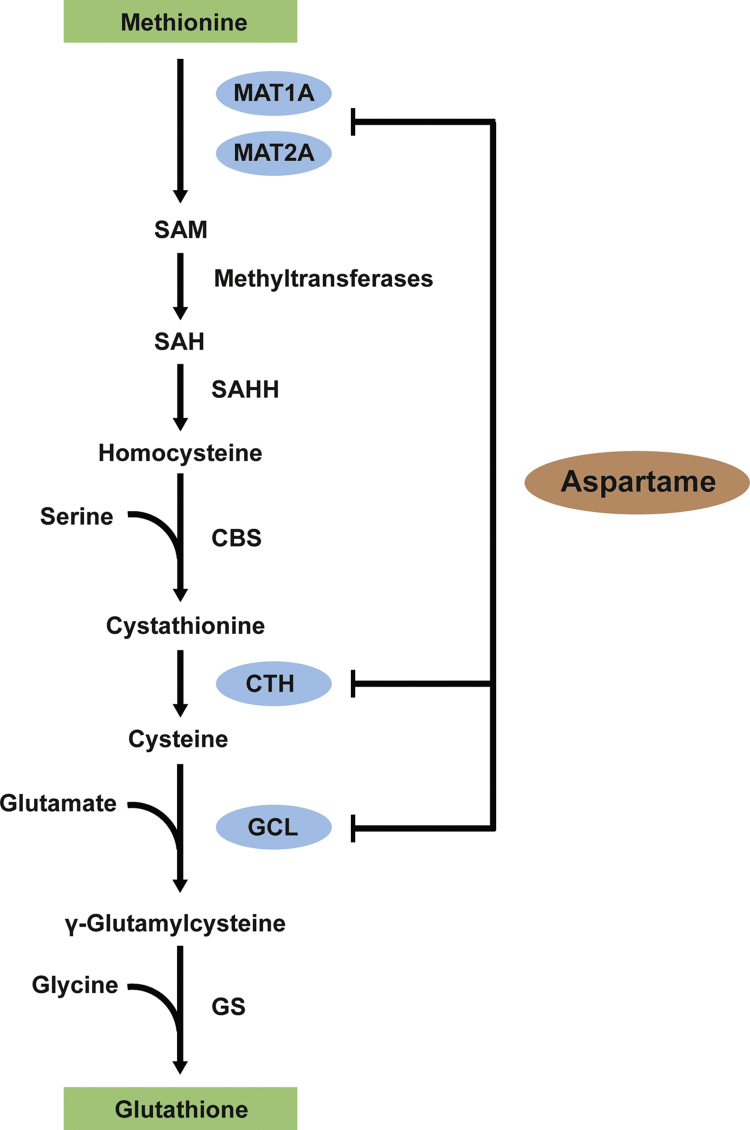
Nevertheless, the effects of chronic aspartame administration on glutathione redox status and on the trans-sulphuration pathway (Fig. 7) were still unknown. The aspartame doses used here in this research (80 mg/kg), when extrapolated to human equivalent doses using the body surface area normalization method [21], correspond to 6 mg/kg i.e., the daily intake of 360 mg of aspartame or 360 mL of soft drink (2 cans of 355 ml), by a 60 kg individual.
Fig. 7.
The trans-sulphuration pathway coupled with glutathione synthesis. Abbreviations: MAT1A, methionine adenosyltransferase 1A; MAT2A, methionine adenosyltransferase 2A; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SAHH, S-adenosylhomocysteine hydrolase; CBS, cystathionine β-synthase; CTH, cystathionine γ-lyase; GS, glutathione synthetase.
Hepatocytes are those cells with the most active trans-sulphuration pathway able to use methionine for GSH synthesis through this pathway (Fig. 7), where methionine is converted to cysteine and thus is used for GSH synthesis [22], [23], [24]. Under normal physiological conditions the rate of GSH synthesis in the liver is mainly determined by two factors: the activity of GCL [25] and the availability of its substrate cysteine [26]. Aspartame-induced GSH depletion should be ascribed to both events (Fig. 7), since we report here a decrease in Gclc mRNA and GCLc protein levels as well as a severe reduction in cysteine levels, which is likely to be due to a marked decrease in Cth mRNA and CTH protein levels. The cystathionine levels were preserved likely due to decrease in its hydrolysis as cystathionine gamma-lyase, which converts cystathionase into cysteine, exhibited a marked decrease in both its mRNA and protein levels after aspartame administration.
Another important metabolite of the trans-sulphuration pathway (Fig. 7) is SAM, which is synthetized by MAT1A (liver-specific) and MAT2A (non-liver-specific) enzymes. It has been shown a reduction in MAT1A in the liver induces GSH depletion [27], [28]. We show here that aspartame triggers downregulation of both enzymes, MAT1A and MAT2A, and induces a marked decrease in SAM levels. MAT1A is considered crucial for hepatic function, since its absence in mice liver produces marked depletion of hepatic SAM levels, making the liver more susceptible to damage [28]. Therefore, since SAM is involved in methylating reactions and in the critical protection of hepatocytes, our findings open a new scenario to explore further consequences of aspartame side effects that may better explain its adverse effects.
Considering that GSH plays an important role in detoxification of electrophilic xenobiotics and in the protection against oxidative stress, and taking into account the aspartame-induced depletion of GSH levels, pharmacological strategies to maintain GSH levels may be important for people who regularly need to consume foods and beverages with sweeteners. A compound widely used in the clinic to protect against acetaminophen-induced GSH depletion and toxicity is NAC [5]. NAC is also used as a mucolytic agent and in the treatment of diseases such as cystic fibrosis, chronic obstructive pulmonary disease, diabetes mellitus, and immunodeficiency virus/AIDS [29]. The bioavailability of NAC (below 5%) is related to its N-deacetylation in the intestinal mucosa and first-pass metabolization in the liver [30]. This last process releases cysteine [31], [32], which may taken up by epithelial cells and maintain GSH synthesis [33]. Therefore, the beneficial effects of NAC have been ascribed to its capability to scavenge reactive oxygen species and increase cellular GSH levels, since NAC is a precursor for cysteine whose availability is a limiting factor for GSH synthesis. Our findings suggest that administration of N-acetyl cysteine may be beneficial in subjects consuming regularly foods and beverages with sweeteners.
In conclusion, chronic administration of aspartame caused marked hepatic GSH depletion, which should be ascribed to GCLc down-regulation and decreased cysteine levels. Aspartame triggered blockade of the trans-sulphuration pathway at two steps (Fig. 7), cystathionine γ-lyase and methionine adenosyltransferases. NAC restored glutathione levels as well as the impairment of the trans-sulphuration pathway.
Conflict of interest
The authors declare that there are no conflict of interest.
Acknowledgements and funding
Grant SAF2015-71208-R with FEDER funds from the Spanish Ministry of Economy and Competitiveness supported this work. I.F. received a fellowship from Programa de Pós-Doutorado no Exterior, which belongs to the Conselho Nacional de Desenvolvimento Científico e Tecnológico. C.A.B. and C.E.B received a fellowship from Programa de Demanda Social, which belongs to the Comissão de Aperfeiçoamento de Pessoal de Nível Superior.
References
- 1.Magnuson B.A., Carakostas M.C., Moore N.H., Poulos S.P., Renwick A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016;74:670–689. doi: 10.1093/nutrit/nuw032. [DOI] [PubMed] [Google Scholar]
- 2.Butchko H.H., Stargel W.W., Comer C.P., Mayhew D.A., Benninger C., Blackburn G.L., de Sonneville L.M.J., Geha R.S., Hertelendy Z., Koestner A., Leon A.S., Liepa G.U., McMartin K.E., Mendenhall C.L., Munro I.C., Novotny E.J., Renwick A.G., Schiffman S.S., Schomer D.L., Shaywitz B.A., Spiers P.A., Tephly T.R., Thomas J.A., Trefz F.K. Aspartame: review of safety. Regul. Toxicol. Pharmacol. RTP. 2002;35:S1–93. doi: 10.1006/rtph.2002.1542. [DOI] [PubMed] [Google Scholar]
- 3.Ranney R.E., Oppermann J.A., Muldoon E., McMahon F.G. Comparative metabolism of aspartame in experimental animals and humans. J. Toxicol. Environ. Health. 1976;2:441–451. doi: 10.1080/15287397609529445. [DOI] [PubMed] [Google Scholar]
- 4.Collison K.S., Makhoul N.J., Zaidi M.Z., Al-Rabiah R., Inglis A., Andres B.L., Ubungen R., Shoukri M., Al-Mohanna F.A. Interactive effects of neonatal exposure to monosodium glutamate and aspartame on glucose homeostasis. Nutr. Metab. 2012;9:58. doi: 10.1186/1743-7075-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finamor I.A., Ourique G.M., Pês T.S., Saccol E.M.H., Bressan C.A., Scheid T., Baldisserotto B., Llesuy S.F., Partata W.A., Pavanato M.A. The protective effect of N-acetylcysteine on oxidative stress in the brain caused by the long-term intake of aspartame by rats. Neurochem. Res. 2014;39:1681–1690. doi: 10.1007/s11064-014-1360-9. [DOI] [PubMed] [Google Scholar]
- 6.Coulombe R.A., Sharma R.P. Neurobiochemical alterations induced by the artificial sweetener aspartame (NutraSweet) Toxicol. Appl. Pharmacol. 1986;83:79–85. doi: 10.1016/0041-008x(86)90324-8. [DOI] [PubMed] [Google Scholar]
- 7.Abhilash M., Paul M.V.S., Varghese M.V., Nair R.H. Effect of long term intake of aspartame on antioxidant defense status in liver. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011;49:1203–1207. doi: 10.1016/j.fct.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Davoli E., Cappellini L., Airoldi L., Fanelli R. Serum methanol concentrations in rats and in men after a single dose of aspartame. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1986;24:187–189. doi: 10.1016/0278-6915(86)90227-9. [DOI] [PubMed] [Google Scholar]
- 9.Iyyaswamy A., Rathinasamy S. Effect of chronic exposure to aspartame on oxidative stress in the brain of albino rats. J. Biosci. 2012;37:679–688. doi: 10.1007/s12038-012-9236-0. [DOI] [PubMed] [Google Scholar]
- 10.Ashok I., Sheeladevi R. Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biol. 2014;2:820–831. doi: 10.1016/j.redox.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cederbaum A.I., Qureshi A. Role of catalase and hydroxyl radicals in the oxidation of methanol by rat liver microsomes. Biochem. Pharmacol. 1982;31:329–335. doi: 10.1016/0006-2952(82)90179-4. [DOI] [PubMed] [Google Scholar]
- 12.Harris C., Wang S.-W., Lauchu J.J., Hansen J.M. Methanol metabolism and embryotoxicity in rat and mouse conceptuses: comparisons of alcohol dehydrogenase (ADH1), formaldehyde dehydrogenase (ADH3), and catalase. Reprod. Toxicol. 2003;17:349–357. doi: 10.1016/s0890-6238(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 13.Tephly T.R. The toxicity of methanol. Life Sci. 1991;48:1031–1041. doi: 10.1016/0024-3205(91)90504-5. [DOI] [PubMed] [Google Scholar]
- 14.Prokic M.D., Paunovic M.G., Matic M.M., Djordjevic N.Z., Ognjanovic B.I., Stajn A.S., Saicic Z.S. Prooxidative effects of aspartame on antioxidant defense status in erythrocytes of rats. J. Biosci. 2014;39:859–866. doi: 10.1007/s12038-014-9487-z. [DOI] [PubMed] [Google Scholar]
- 15.Abhilash M., Sauganth Paul M.V., Varghese M.V., Nair R.H. Long-term consumption of aspartame and brain antioxidant defense status. Drug Chem. Toxicol. 2013;36:135–140. doi: 10.3109/01480545.2012.658403. [DOI] [PubMed] [Google Scholar]
- 16.Nseir W., Nassar F., Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World J. Gastroenterol. 2010;16:2579–2588. doi: 10.3748/wjg.v16.i21.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., Kuperman Y., Harmelin A., Kolodkin-Gal I., Shapiro H., Halpern Z., Segal E., Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 18.Trocho C., Pardo R., Rafecas I., Virgili J., Remesar X., Fernández-López J.A., Alemany M. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 1998;63:337–349. doi: 10.1016/s0024-3205(98)00282-3. [DOI] [PubMed] [Google Scholar]
- 19.Oyama Y., Sakai H., Arata T., Okano Y., Akaike N., Sakai K., Noda K. Cytotoxic effects of methanol, formaldehyde, and formate on dissociated rat thymocytes: a possibility of aspartame toxicity. Cell Biol. Toxicol. 2002;18:43–50. doi: 10.1023/a:1014419229301. [DOI] [PubMed] [Google Scholar]
- 20.Horio Y., Sun Y., Liu C., Saito T., Kurasaki M. Aspartame-induced apoptosis in PC12 cells. Environ. Toxicol. Pharmacol. 2014;37:158–165. doi: 10.1016/j.etap.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 22.Kaplowitz N., Aw T.Y., Ookhtens M. The regulation of hepatic glutathione. Annu. Rev. Pharmacol. Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 23.Lu S.C. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 24.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Díaz D., Krejsa C.M., Kavanagh T.J. Expression of glutamate-cysteine ligase during mouse development. Mol. Reprod. Dev. 2002;62:83–91. doi: 10.1002/mrd.10076. [DOI] [PubMed] [Google Scholar]
- 26.Tateishi N., Higashi T., Shinya S., Naruse A., Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J. Biochem. 1974;75:93–103. doi: 10.1093/oxfordjournals.jbchem.a130387. [DOI] [PubMed] [Google Scholar]
- 27.Lu S.C. S-Adenosylmethionine. Int. J. Biochem. Cell Biol. 2000;32:391–395. doi: 10.1016/s1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 28.Lu S.C., Alvarez L., Huang Z.Z., Chen L., An W., Corrales F.J., Avila M.A., Kanel G., Mato J.M. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc. Natl. Acad. Sci. USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Holdiness M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Whillier S., Raftos J.E., Chapman B., Kuchel P.W. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep. Commun. Free Radic. Res. 2009;14:115–124. doi: 10.1179/135100009X392539. [DOI] [PubMed] [Google Scholar]
- 32.Radtke K.K., Coles L.D., Mishra U., Orchard P.J., Holmay M., Cloyd J.C. Interaction of N-acetylcysteine and cysteine in human plasma. J. Pharm. Sci. 2012;101:4653–4659. doi: 10.1002/jps.23325. [DOI] [PubMed] [Google Scholar]
- 33.Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]