1. Introduction
Influenza A (H1N1), described as epidemic in June 2009 [1], was declared the first pandemic of this century, due to reports of high morbidity and mortality, and sustained transmission in many countries [2], [3], [4]. Alerts about the increased risk factors (e.g., pregnancy, coexisting diseases, childhood, age, and inability to perform self-care) were also assessed. Physiological and anatomical changes that occur during pregnancy can affect the known clinical presentation of respiratory signs and symptoms, masking the adequate diagnosis, and delaying the treatment [5], [6]. In addition, pregnancy may increase the risk of severe influenza-associated complications, supporting to the recommendation to promptly treat pregnant women with H1N1 infection [7]. In severe cases of Influenza A (H1N1) infection, admission to an intensive care unit (ICU) is recommended. Approximately 9–31% of the hospitalized patients were admitted to an ICU, with a mortality rate ranging from 14 to 46% [3], [4], [8], [9]. From July 2009 to January 2, 2010, 44,544 cases of the disease and 2051 deaths were reported in Brazil [10]. H1N1 infection is therefore a possible cause of acute respiratory distress syndrome (ARDS).
The prevalence of ARDS during pregnancy has been estimated as 16 to 70 cases per 100,000 pregnancies [11]. Non-obstetric causes of ARDS include sepsis, intracerebral hemorrhage, blood transfusion, trauma, and also H1N1 infection. Overall mortality for both the mother and fetus is high, and significant morbidity can persist even after recovery. Mortality due to ARDS during pregnancy is not significantly different than that in non-pregnant patients (23%–39%), and is associated with marked perinatal morbidity and a high rate of fetal loss (23%) [11].
Treating ARDS during pregnancy follows that for the general population and includes providing supportive care while identifying and treating the underlying cause. Once conventional lung-protective mechanical ventilation fails, alternative approaches including the use of high-frequency oscillatory ventilation, lung recruitment maneuvers, prone positioning, and inhaled nitric oxide can be used, without reducing mortality in the general population [11]. However, strategies commonly used in non-pregnant patients might not be acceptable during pregnancy [12]. Extracorporeal membrane oxygenation (ECMO) can be used in patients with ARDS and refractory hypoxemia as salvage therapy [13]. The benefit of ECMO over lung-protective strategies using conventional ventilation remains controversial [14], [15], and there are no high-quality data on its use in pregnancy. Observational data from the 2009 H1N1 pandemic suggested that ECMO may play a crucial role in younger patients with refractory hypoxemia resistant to conventional lung-protective mechanical ventilation strategies [16].
Here, we report the maternal clinical course, treatment, and fetal outcome of an H1N1 infected pregnant woman with severe outcomes, and the successful use of ECMO.
2. Case report
Previously healthy 30-year-old white Brazilian woman (G1P0), at 27 weeks of gestation, attended in the emergency department with a 5-day history of progressive dyspnea, lethargy, and fever.
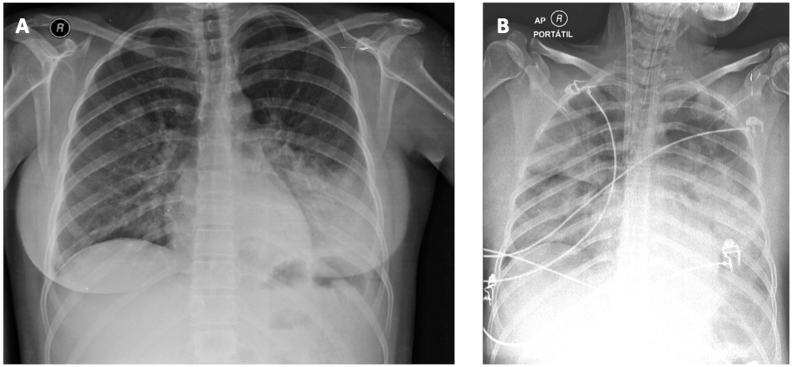
Clinical examination revealed a gravid uterus, consistent with gestational age, initially treated as bacterial pneumonia, with coverage for H1N1 (Amoxicillin plus Clavulanate 1g TID (three times a day), Clarithromycin 500mg BID (twice daily), and Oseltamivir 75mg BID, after allocated in ward. She had no auscultatory findings, and chest X-ray showed consolidation in the base of the left hemithorax (Fig. 1a). Fetal ultrasound had no alteration.
Fig. 1.
A - Chest X-ray on admission day, before ICU admission. B - Chest X-ray 24 hours after hospital admission, under mechanical ventilation and veno-venous extracorporeal membrane oxygenation (cannulation of right internal jugular vein).
About 4 hours after hospitalization, due to worsening of dyspnea, associated with an increased demand of supplemental oxygen, the patient was transferred to the ICU, and started continuous non-invasive ventilation (NIV) using a full-face mask (10 L/min O2). Since there was an unsatisfactory clinical and laboratorial response after 3 hours under NIV, we chose for elective endotracheal intubation. After 12 hours of ICU admission, the patient presented severe hipoxemia, (PaO2/FiO2 <80), setting ARDS criteria (PaO2/FiO2 <200) [17] [Fig. 1b]. It was performed, after neuromuscular blockade, alveolar recruitment, but there was no adequate response. It was also attempted a semi-pronation position (900) to the left, with new alveolar recruitment. Both attempts did not show satisfactory improvement in oxygenation.
After 24 hours hospitalization, having exhausted the ventilatory strategies to improve blood oxygenation, we indicated veno-venous ECMO installation, through cannulation of the right internal jugular vein and the right femoral vein. On that moment, the patient presented a preserved cardiac function, through echocardiogram, corroborating the choice of venous-venous ECMO.
Prior to ECMO installation, along with the obstetrician, and the ECMO team, we decided not to interrupt the gestation. The fetus viability was daily monitored (daily cardiac rhythm evaluation and bedside ultrasound) and it was administrated 48 hours of betamethasone, for fetal lung maturation, just in case of an emergency delivery became needed.
After 4 days under ECMO, the patient presented increased white blood cell count, and new culture samples were obtained. The previous antibiotics were replaced by Piperacillin-Tazobactam, and Oseltamivir was continued. Tracheal aspirates showed an Amoxicillin and Clavulanate resistant Enterobacter. Seven days after ECMO installation the patient still presented with an important impairment of lung function, and we associated Methylprednisolone (2mg/kg), maintained until extubation.
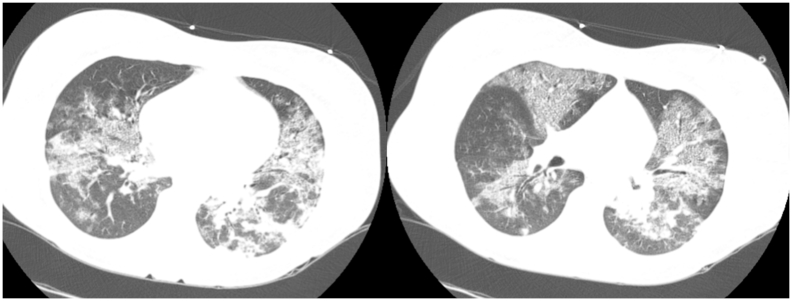
The patient remained 9 days under ECMO and 11 days under mechanical ventilation, achieving a significant improvement in lung function [Fig. 2]. Forty-eight hours after ECMO cessation, patient was extubated, still requiring intermittent NIV during the 6 following days, until ICU discharge. The patient was discharged after 21 days of hospitalization, with the current pregnancy (30 weeks), with fetal ultrasound demonstrating normodramnia and fetal biometry compatible with the gestational age.
Fig. 2.
Chest computed tomography, performed when the patient had achieved clinical conditions to do the exam, ten days after hospital admission. The patient had significant improvement in lung function, without ECMO use, but still under mechanical ventilation. Chest computed tomography shows diffuse and bilateral interstitial infiltrates.
Following hospital discharge, the antenatal evaluation was performed regularly, uneventfully. Caesarean section was electively performed at 38 weeks of gestation, resulting in the delivery of a healthy male infant.
3. Discussion
The recent case report illustrates a severe course of an H1N1 infection. This provides further evidence to the notion that pregnant women are at a high risk for dangerous and complicated course of H1N1 infection. H1N1 has a predilection for younger women (median age 26 years), multiparous, in the first or second trimester of pregnancy. Comorbidities may increase the risk for a severe course of the H1N1 infection [18].
In this case, five days prior to hospital admission the patient already presented an evolution of symptoms without suspicion of H1N1 infection, delaying antiviral therapy institution, which may have contributed to the severity of the case. It is well known that early introduction of antiviral therapy (oseltamivir or zanamivir) improves the chances for successful treatment. The time from symptom onset to initial presentation for clinical care usually ranges from 1 to 7 days [18]. The median start of antiviral treatment is at 6 days and the delayed treatment is associated with admission to ICU and death [19]. Severe pneumonia and ARDS are the most important complications of influenza, and 19% of the ICU admissions in pregnant women are due to ARDS ( [18], [20]. ARDS secondary to H1N1 infection is characterized by severe hypoxemia and need for mechanical ventilation [21]. The mortality rate among pregnant women with H1N1 infection was 25%, and was also observed an increased risk of H1N1 influenza infection during the third trimester of pregnancy [22].
The obstetric and neonatal consequences of H1N1 infection significantly increase the need for cesarean section, mainly due to worsening maternal conditions. In a review of 28 pregnant women with ARDS, Catanzarite et al. suggested that delivery is indicated during the third trimester of pregnancy or in case of deteriorating maternal conditions [23]. Other authors caution against routine delivery, emphasizing that the risks associated with labor or cesarean delivery may be unacceptably high [24] [25]. Cesarean section did not seem to worsen maternal conditions, because intensive management for maternal hypoxia, including ECMO, also led to better than expected outcomes when compared with the usual 30–35% mortality rate reported for ARDS.
The neonatal ICU admission rate also increased, mainly because of preterm deliveries that comprised almost exclusively fetuses from the most severely ill pregnant women, but neonatal morbidity and mortality have remained extremely low. These data are very important because they were collected from infants delivered by mothers treated with Oseltamivir, thereby reinforcing data on the safety of this antiviral during pregnancy [26].
Experience with ECMO in pregnancy is limited. Before the influenza pandemic, ECMO had been described only in a few obstetric patients with ARDS from different causes. In the Australian and New Zealand Intensive Care Society (ANZIC) group experience, 9 of the 64 (14%) critically ill pregnant women received ECMO, and 6 of them (67%) survived [27]. The same Australian group analyzed retrospectively the clinical course of 12 pregnant or postpartum women treated with ECMO in seven tertiary centers, reporting a high rate of hemorrhagic complications that caused the death of three women, whereas ECMO circuit-related complications were rare; 66% of these patients survived, and the infants' survival rate was 71% [28].
The main technical problems expected with ECMO in pregnancy are the difficult blood drainage because of caval compression by the gravid uterus, which may require the placement of additional venous cannulas, and the need of an emergency delivery [29]. In our patient a femorojugular bypass was used, blood was drained through a femoral cannula of very large caliber (25 F), and the patient was kept preferentially in left lateral decubitus, allowing an effective ECMO.
Patients on ECMO need to be systemically anticoagulated. Heparin has no effect on the fetus because it does not cross the placental barrier, whereas the risk of obstetric hemorrhagic complications is increased.
The efficacy and safety of corticosteroids in patients with serious respiratory complications from influenza virus is unclear. A recent study showed an improved outcome of patients with ARDS from H1N1 or type B influenza virus receiving early corticosteroids [30]. Despite this, current meta-analysis does not recommend adjuvant steroid administration [31].
The present case and literature review confirm ECMO feasibility during pregnancy, as an effective and relatively safe tool for the mother and fetus, with better outcomes than those achieved with standard of care. In our patient, emergency delivery was considered, but no signs of fetal distress were evident while the risk of a surgical procedure was extremely high. Therefore, we decided to postpone the delivery, considering ECMO support the best option to warrant the highest chance of survival to the mother and fetus.
Given the complexity of this clinical scenario (interplay of mother's disease, fetal conditions, and ECMO-related morbidity), issues remain about the timing of ECMO implantation and the management of gestation. Thus, it is clear that this kind of decision is extremely challenging and has to be made on a case-by-case basis, in conjunction with the obstetric, neonatal, and critical care teams. The present case confirms the importance in considering H1N1 infection in pregnant women, and the feasibility of ECMO as a possible treatment strategy.
Competing interests
The authors declare that they have no competing interests and no specific funding was received.
Contributors
RTA, CA and VSD participated in patient care.
RTA and VCS wrote and revised the manuscript.
All authors read and approved the final manuscript.
Acknowledgements
We express our thanks to the board of directors of the Hospital e Maternidade Santa Lucia, for all the necessary support. To Alexandre Sciliano, medical doctor responsible for ECMO, and all your team. To the multiprofessional team that composes the staff of the ICU of the Hospital e Maternidade Santa Lucia, for their commitment and dedication to the patients. To the patient itself, and their family, by the confidence in the team. Luciana Tuccori for the text revision.
References
- 1.Chang L.-Y., Shih S.-R., Shao P.-L., Huang D.T.-N., Huang L.-M. Novel swine-origin influenza virus A (H1N1): the first pandemic of the 21st century. J. Formos. Med. Assoc. Taiwan Yi Zhi. 2009 Jul;108(7):526–532. doi: 10.1016/S0929-6646(09)60369-7. [DOI] [PubMed] [Google Scholar]
- 2.Domínguez-Cherit G., Lapinsky S.E., Macias A.E., Pinto R., Espinosa-Perez L., de la Torre A. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009 Nov 4;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 3.Jain S., Kamimoto L., Bramley A.M., Schmitz A.M., Benoit S.R., Louie J. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 2009 Nov 12;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Loeches I., Díaz E., Vidaur L., Torres A., Laborda C., Granada R. Pandemic and post-pandemic influenza A (H1N1) infection in critically ill patients. Crit. Care Lond. Engl. 2011;15(6):R286. doi: 10.1186/cc10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J.A., Zumla A., Gautret P., Gray G.C., Hui D.S., Al-Rabeeah A.A., Memish Z.A. Surveillance for emerging respiratory viruses. Lancet Infect. Dis. 2014 Oct;14(10):992–1000. doi: 10.1016/S1473-3099(14)70840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta N., Chen K., Hardy E., Powrie R. Respiratory disease in pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2015 Jul;29(5):598–611. doi: 10.1016/j.bpobgyn.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet Lond. Engl. 2009 Aug 8;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 8.Louie J.K., Acosta M., Winter K., Jean C., Gavali S., Schechter R. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009 Nov 4;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009 Nov 4;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 10.Yokota R.T.C., Skalinski L.M., Igansi C.N., de Souza L.R.O., Iser B.P.M., Reis P.O. Risk factors for death from pandemic (H1N1) 2009, southern Brazil. Emerg. Infect. Dis. 2011 Aug;17(8):1467–1471. doi: 10.3201/eid1708.101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte A.G. ARDS in pregnancy. Clin. Obstet. Gynecol. 2014 Dec;57(4):862–870. doi: 10.1097/GRF.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 12.Guntupalli K.K., Karnad D.R., Bandi V., Hall N., Belfort M. Critical illness in pregnancy: part II: common medical conditions complicating pregnancy and puerperium. Chest. 2015 Nov;148(5):1333–1345. doi: 10.1378/chest.14-2365. [DOI] [PubMed] [Google Scholar]
- 13.MacLaren G., Combes A., Bartlett R.H. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012 Feb;38(2):210–220. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 14.Bein T., Grasso S., Moerer O., Quintel M., Guerin C., Deja M. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016 May;42(5):699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guérin C., Reignier J., Richard J.-C., Beuret P., Gacouin A., Boulain T. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013 Jun 6;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 16.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A., Jones D., Bailey M., Beca J., Bellomo R. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009 Nov 4;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 17.Definition Task Force A.R.D.S., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 Jun 20;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.Callaghan W.M., Creanga A.A., Jamieson D.J. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet. Gynecol. 2015 Sep;126(3):486–490. doi: 10.1097/AOG.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oboho I.K., Reed C., Gargiullo P., Leon M., Aragon D., Meek J., Anderson E.J., Ryan P., Lynfield R., Morin C., Bargsten M., Zansky S.M., Fowler B., Thomas A., Lindegren M.L., Schaffner W., Risk I., Finelli L., Chaves S.S. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J. Infect. Dis. 2016 Aug 15;214(4):507–515. doi: 10.1093/infdis/jiw033. [DOI] [PubMed] [Google Scholar]
- 20.Schwaiberger D., Karcz M., Menk M., Papadakos P.J., Dantoni S.E. Respiratory failure and mechanical ventilation in the pregnant patient. Crit. Care Clin. 2016 Jan;32(1):85–95. doi: 10.1016/j.ccc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Jahromi G.S., Zand F., Khosravi A. Acute respiratory distress syndrome associated with H1N1 influenza during pregnancy. Int. J. Obstet. Anesth. 2010 Oct;19(4):465–466. doi: 10.1016/j.ijoa.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Puvanalingam A., Rajendiran C., Sivasubramanian K., Ragunanthanan S., Suresh S., Gopalakrishnan S. Case series study of the clinical profile of H1N1 swine flu influenza. J. Assoc. Phys. India. 2011 Jan;59 14–6, 18. [PubMed] [Google Scholar]
- 23.Catanzarite V., Willms D., Wong D., Landers C., Cousins L., Schrimmer D. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet. Gynecol. 2001 May;97(5 Pt 1):760–764. doi: 10.1016/s0029-7844(00)01231-x. [DOI] [PubMed] [Google Scholar]
- 24.Grisaru-Granovsky S., Ioscovich A., Hersch M., Schimmel M., Elstein D., Samueloff A. Temporizing treatment for the respiratory-compromised gravida: an observational study of maternal and neonatal outcome. Int. J. Obstet. Anesth. 2007 Jul;16(3):261–264. doi: 10.1016/j.ijoa.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Madsen K., Strange D.G., Hedegaard M., Mathiesen E.R., Damm P. Maternal and fetal recovery after severe respiratory failure due to influenza: a case report. BMC Res. Notes. 2013;6:62. doi: 10.1186/1756-0500-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wollenhaupt M., Chandrasekaran A., Tomianovic D. The safety of oseltamivir in pregnancy: an updated review of post-marketing data. Pharmacoepidemiol. Drug Saf. 2014 Oct;23(10):1035–1042. doi: 10.1002/pds.3673. [DOI] [PubMed] [Google Scholar]
- 27.Influenza Investigators A.N.Z.I.C., Maternity Australasian. Outcomes Surveillance System. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair P., Davies A.R., Beca J., Bellomo R., Ellwood D., Forrest P. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011 Apr;37(4):648–654. doi: 10.1007/s00134-011-2138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad A.F., Rahman M., Maybauer D.M., Fraser J.F., Costantine M.M., Pacheco L.D. Extracorporeal Membrane oxygenation in pregnant and postpartum women with H1N1-related acute respiratory distress syndrome: a systematic review and meta-analysis. Obstet. Gynecol. 2016 Feb;127(2):241–247. doi: 10.1097/AOG.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 30.Huang S.F., Fung C.P., Perng D.W., Wang F.D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. J. Microbiol. Immunol. Infect. 2015 Sep 9 doi: 10.1016/j.jmii.2015.08.016. pii: S1684-1182(15)00839-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Sun W., Svendsen E.R., Tang S., MacIntyre R.C., Yang P. Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit. Care Lond. Engl. 2015;19:46. doi: 10.1186/s13054-015-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]