Abstract
The authors summarized data from a group of physicians with experience using high-dose rate electronic brachytherapy for the treatment of nonmelanoma skin cancer. The data have been published or presented in abstract format at national dermatology and radiation oncology meetings. The data included 1,822 treated lesions from 2009 to 2014 in patients ranging in age from 52 to 104 years. Most lesions were basal cell carcinoma (57%) or squamous cell carcinoma (38%) less than 2cm in size (97%). Median follow-up at the various centers ranged from 4 to 16 months, and results yielded an extremely low recurrence rate of less than one percent. Results show that within the confines of this follow up period, electronic brachytherapy is an effective, convenient, nonsurgical treatment option for patients with nonmelanoma skin cancer with few recurrences and excellent cosmetic results.
NONMELANOMA SKIN CANCERS (NMSC) are a global problem and represent the most common cancers diagnosed in the United States each year with an estimated 3.5 million new cases.1 Cancers of the skin (most of which are basal and squamous cell skin cancers) are by far the most common of all types of cancer. According to one estimate, about 5.4 million basal and squamous cell skin cancers are diagnosed each year (occurring in about 3.3 million Americans, as some people have more than one). About 8 out of 10 of these are basal cell cancers. Squamous cell cancers occur less often.2 This has translated to a significant increase in the number of new skin cancer procedures being performed per year (estimated 5% growth per year).3 Fortunately, most NMSC lesions are detected at an early stage that is amenable to very effective local therapy and thus are highly curable.
Treatment paradigms. Primary care physicians and general dermatologists manage most NMSC in their office. The specific modality chosen is dependent on a combination of clinical and pathologic factors and has varying degrees of effectiveness. These include specific squamous or basal cell histologic subtypes, size, lesion grade, location, treatment history, and patient preference. Historically, most patients with early stage skin cancer have been treated with destruction, including cryosurgery, electrodessication and curettage (ED&C), and simple excision. Although several topical chemotherapeutic options are available, as is immunotherapy for basal cell carcinoma (BCC), these have not been widely employed because of concerns over effective cure rates or tolerance of side effects.
Mohs micrographic surgery, an advancement in surgical technique specific to NMSC, removes cancerous tissue in a precise fashion utilizing histologic margin control. The surgical margins are carefully evaluated in a staged fashion, with meticulous inking and mapping. In addition, the tissue specimen is sectioned horizontally allowing for complete circumferential peripheral and deep margin assessment. The result is preservation of healthy tissue integrity compared to removal of larger volumes with simple excision. The long-term clearance rates for this “microscopic” or “micrographic” procedure are generally considered to be excellent with five-year clearance rates that approach 99 percent for primary BCC4–7 and 97 percent for primary squamous cell carcinoma (SCC)8 and can be as low as 93 percent for large and aggressive or recurrent BCC9–11 or 90 percent for locally recurrent (previously treated) SCC, and 68 percent for poorly differentiated SCC.12
This specific technique requires specialized training gained either through fellowship or postgraduate training.13 Among the drawbacks for this procedure are specialized equipment, staff, training, and facilities.
The Mohs surgical procedure typically takes several hours, usually on a single day, for the removal of the cancerous tissue and subsequent tissue repair. On occasion, the repair may be accomplished at a later date by the Mohs surgeon or by another surgeon, usually a plastic surgeon, when it is considered optimal for patient care.
Cryotherapy, photodynamic therapy (PDT), or topical chemotherapeutic agents represent other treatment alternatives. These treatments have inherent technical and logistical limitations in target coverage that have resulted in higher local recurrence rates including an inability to penetrate deeper layers and patient nonadherence. Also, PDT is not United States Food and Drug Administration (FDA) approved for the treatment of NMSC; topical chemotherapy with 5% fluorouracil (FU) suspension is not generally considered very effective therapy, even though FDA approved for BCC; and imiquimod, approved for nonfacial superficial BCC, is not widely used due to lower cure rates and tolerability issues, including systemic “flu like” effects.14–16
Radiation therapy. When determining therapeutic options for NMSC, the treating practitioner and patient weigh various considerations in the risk/benefit discussion. These include, among others, functional concerns, post-treatment cosmesis, and scarring. Patient belief systems, social concerns, previous experience, and overall mental and physical health need to be considered. Radiation therapy may be the primary treatment of choice in order to achieve optimal overall results as determined in consensus decision-making. Therapeutic x-rays have been used for the primary treatment for skin cancer for nearly a century. First-generation machines were costly to maintain and resulted in more collateral exposure of nearby healthy tissues. Newer superficial radiation therapy machines have been developed; however, this technique still delivers greater doses to tissues at depth thereby typically requiring a lengthy 15 to 30 daily treatment course. This is especially problematic for surface lesions in cosmetically sensitive locations or those with nearby critical structures, such as the face, head, and neck areas.
Modern electron beam radiation therapy is more readily available and allows three-dimensional treatment planning. However, there are challenges with dosimetry of irregular or curved surfaces and the technique requires a broader surface area to be irradiated over long courses than with more targeted techniques, such as HDR brachytherapy.17–19
High-dose rate surface brachytherapy. High-dose rate (HDR) surface brachytherapy can be delivered with either a radioactive source (typically iridium-192) or with an electronic, miniaturized x-ray source. The brachytherapy source is placed in close proximity to the target lesion using specialized applicators directing the radiation flow from a machine called a remote afterloader, for the iridium-192 isotope. Treatments use a controller that provides the energy and controls the treatment for electronic brachytherapy treatments. The size of the applicators and shape of the target area can be customized to the size, shape, complexity, and extent of the skin lesion. This technique can be used for all NMSC subtypes as well as keloidal scarring.
A key advantage of HDR brachytherapy is that it focuses the ionizing radiation on the selected tumor bed and maximally spares adjacent, deeper healthy tissues due to a rapid falloff of the customized, surface radiation dose. Therefore, the treatment courses are typically much shorter, with faster individual treatment sessions, which can be delivered in treatment areas that are more patient friendly. The treatment is noninvasive and therefore does not require anesthesia or needles, cutting, or sutures. This causes less scarring in sensitive facial areas, such as the nose, ear, lip, or eyelid, and less need for reconstructive procedures post-tumor removal. This is a distinct advantage especially in those selected patients with a history of difficulty in wound healing; on medications that interfere with tissue repair; who are elderly, have diabetes, peripheral vascular disease, or are on anticoagulants; or those with neurologic or psychiatric comorbidities.
An innovative type of HDR surface brachytherapy is HDR electronic brachytherapy (eBx), which combines the benefits of traditional isotopic brachytherapy with those of low-energy X-ray radiotherapy. In this technique, a high-dose rate X-ray source is placed directly into a skin applicator close to the surface and provides a homogenous dose pattern in the treatment area to a specified depth, typically 3mm, with a range of 0.3mm to 0.7mm. In contrast, iridium-based HDR surface brachytherapy using the Leipzig applicator requires increased margin to adequately treat the lesion due to its larger penumbra. This can be critically important for treating sensitive locations, such as the oral commissures, nasal tip, nasal ala and os, glabella, lateral and medial canthi, conchal bowl and external auditory canal, and other areas where there is minimal space to allow for additional margin.
HDR electronic brachytherapy allows delivery outside of radiation oncology facilities due to minimal shielding requirements since isotopes or megavoltage linear accelerators are not used. The mobility of the system increases access for patients as it can be transported between multiple rooms or facilities.
The short, few-minute HDR electronic brachytherapy treatments are typically delivered over an accelerated 8- to 10-treatment course twice weekly to 40Gy. The treatments are typically pain-free and well-tolerated with fast recovery times relative to protracted external beam radiotherapy (EBRT) during which schedules are frequently 20 to 30 daily fractions to 50 to 60Gy.
METHODS
A group of physicians using the Xoft (San Jose, California) electronic brachytherapy (EBT) system contributed their data to this aggregate look at their overall experience.20–25 Some of the data has been published in part or have been presented in abstract format at national dermatology meetings. A total of 1,259 patients with 1,822 lesions were described in the six publications/presentations. The treatment and outcome datafor patients seen in private practice at eight clinical sites between July 2009 and August 2014 were collected retrospectively and are summarized.
RESULTS
Lesions in this aggregate dataset were treated to 40 to 45Gy using 3 to 8 fractions with the majority being treated with eight fractions. Applications occurred 2 or 3 times a week with 36 to 48 hours between treatments. Surface applicator sizes of 10, 20, 35, and 50mm are available for treatment to allow for complete coverage of target lesion with acceptable margin.
Table 1 details the combined demographic data. Patients ranged from 52 to 104 years old. The mean age reported ranged from 70 to 83 years. The majority of the lesions were BCC (57%) or SCC (38%) and less than 2cm in size (97%). Lesions were located on the head or extremities with the most commonly treated area being the face (33%). Figures 1, 2, 3, and 4 show the baseline lesions and the outcomes at follow-up for three patients. Figure 1 shows SCC pre-treatment and at one-year follow-up. Figure 2 shows BCC pre-treatment and at one-year follow-up. Figure 3 shows baseline BCC and 23-month follow-up. Figure 4 shows baseline SCC and then at 24-month follow-up. Patients were pleased with the cosmetic outcome and those sites where patients graded lower scores for cosmetic outcomes experienced hypopigmentation, rash dermatitis, or erythema. The lesions were treated to a depth of 3mm or less below the surrounding skin surface in 90 percent of the cases. Table 2 details the treatment methodologies. Table 3 summarizes the follow-up experience. Patients were followed from 2 to 16.5 months post-treatment and 14 lesions were reported to have recurrence of cancer (0.97%).
TABLE 1.
Demographics
| DEMOGRAPHICS | |
|---|---|
| Treatment period | 2009–2014 |
| # Patients | 1259 |
| # Lesions | 1822 |
| Age (Years) | N=1259 |
| Mean, range | 77 (70–83) |
| Average of Medians | 80 |
| Histopathology | N=1748 |
| BCC | 990 (57%) |
| SCC | 670 (38%) |
| Other | 88 (5%) |
| Lesion Size (cm) | N=1427 |
| <1cm | 798 (56%) |
| ≥1<2cm | 584 (41%) |
| ≥2<3cm | 34 (2%) |
| ≥3<4cm | 6 (0.4%) |
| ≥4<5cm | 5 (0.4%) |
| Lesion Location | N=1822 |
| Face* | 596 (33%) |
| Nose | 393 (22%) |
| Upper/lower extremities | 309 (17%) |
| Ear | 169 (9%) |
| Trunk | 135 (7%) |
| Scalp | 134 (7%) |
| Hand/wrist | 58 (3%) |
| Neck | 16 (1%) |
| Lip | 7 (0.4%) |
| Eye | 7 (0.4%) |
May include forehead, temple, eyelid, cheek, glabella, lips, chin, sideburn, and ear
Figure 1.
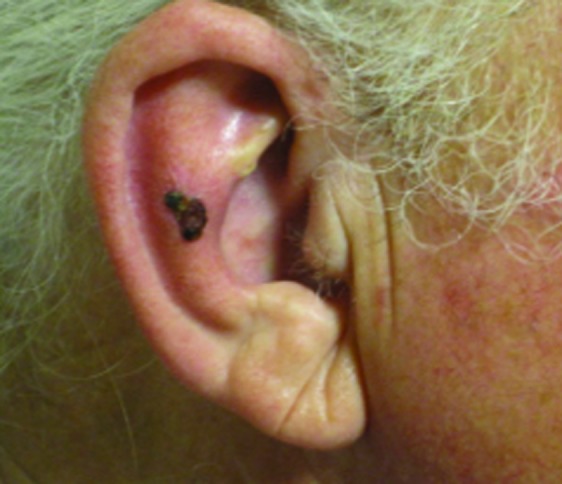

Squamous cell carcinoma on right antihelix treated with 40Gy to a 3mm depth. (A) Pre-treatment; (B) 1 year
Figure 2.
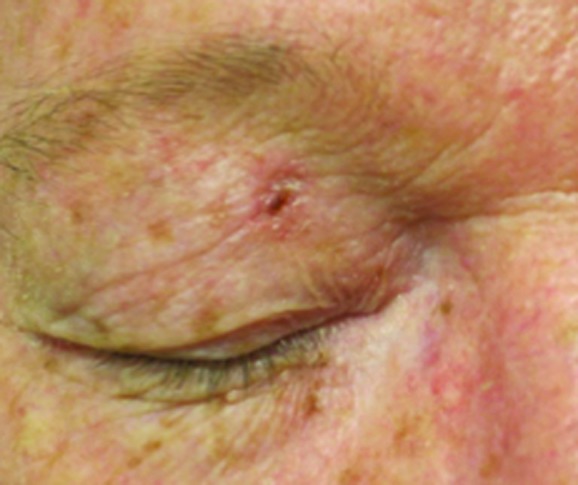
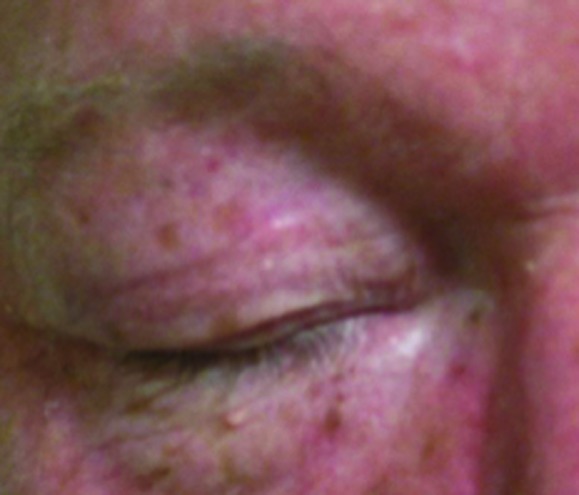
Basal cell carcinoma on right upper eyelid treated with 40Gy to a 3mm depth. (A) Pre-treatment; (B) 1 year
Figure 3.
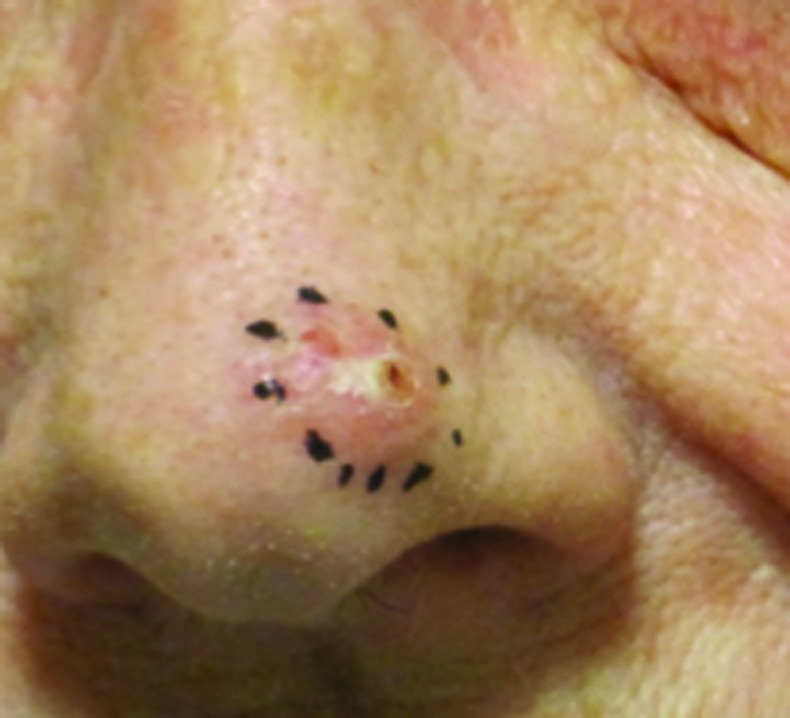
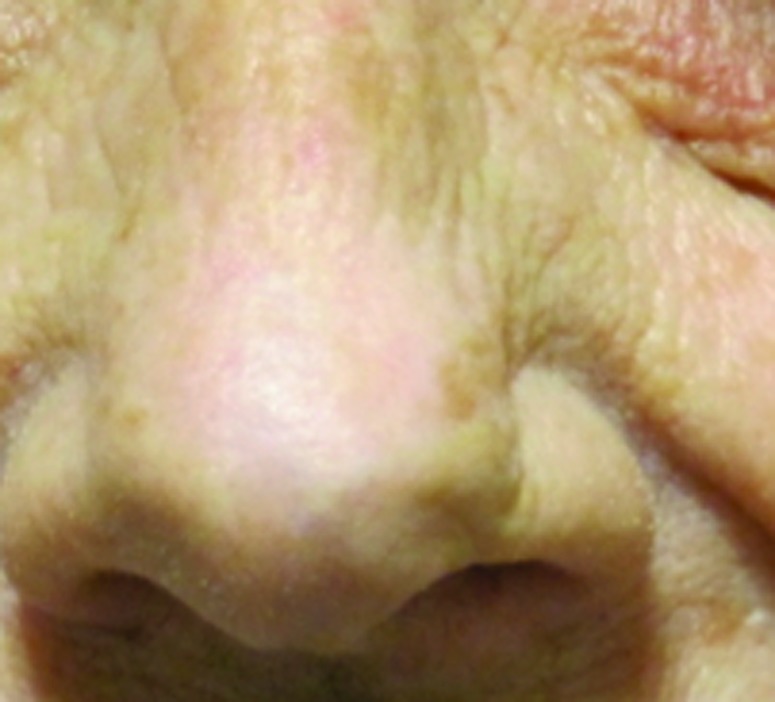
Basal cell carcinoma on left tip of nose treated with 40Gy to a 3mm depth. (A) Pre-treatment; (B) 23 months
Figure 4.
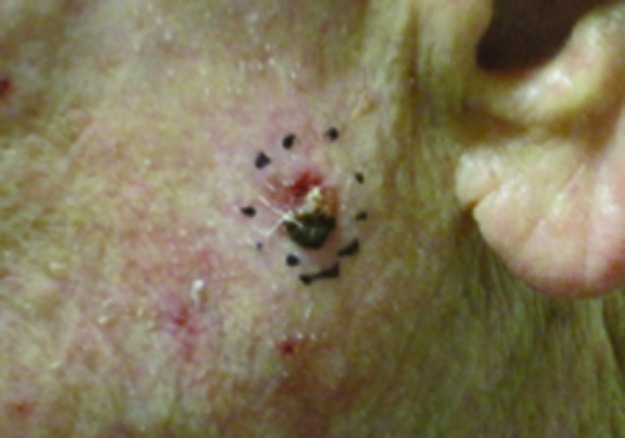
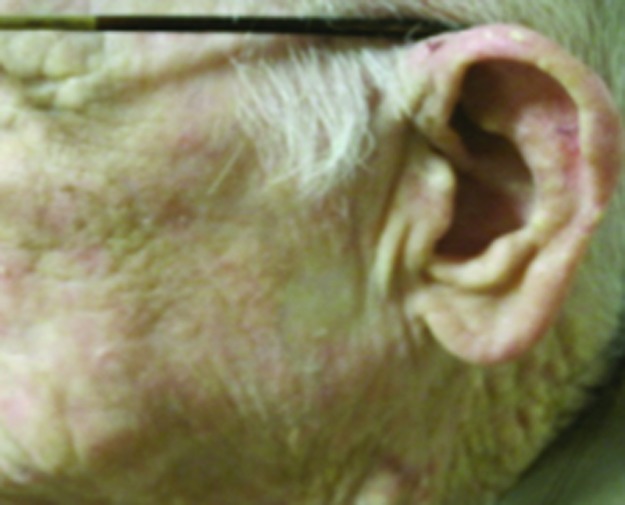
Squamous cell carcinoma on left cheek treated with 40Gy to a 5mm depth. (A) Pre-treatment; (B) 1 year
TABLE 2.
Treatment data
| TREATMENT | |
|---|---|
| Total dose (Gy) | 40–45 |
| Fractions | 8–10 |
| Treatment depth (mm) | N=1451 |
| ≤2mm | 1315 (90.6%) |
| 3mm | 130 (9.0%) |
| ≥4mm | 2 (0.1%) |
TABLE 3.
Follow-up results
| FOLLOW-UP | N=1440 |
|---|---|
| Lesion follow-up range (months) | 0–63 |
| <1year | 926 |
| ≥1<2 years | 366 |
| ≥2<3 years | 101 |
| ≥3<4 years | 35 |
| ≥4≤5 years | 12 |
| Recurrence | 0.97% |
DISCUSSION
HDR electronic brachytherapy is a highly effective nonsurgical alternative for a broad array of patients with NMSC. It is essential for the dermatologist and radiation oncologist to collaborate on reviewing tumor pathology, determine potential treatment options, select the ideal patients, enable precise treatment planning, and in turn offer high-quality eBx delivery with optimal outcomes and clinical efficiency. There are clear benefits when both the dermatologist and radiation oncologist are involved with this modality utilizing their unique expertise respectively.
The dermatologist helps to identify potential candidates for electronic brachytherapy and can often help delineate the skin target lesion. The radiation oncologist is critical in delivering this treatment and properly counseling the patient on the risks and benefits as well as obtaining informed consent for this procedure. This collaborative approach allows for optimal patient care and patient satisfaction. HDR surface brachytherapy has demonstrated excellent local control rates for BCC and SCC in appropriately selected patients.26–29
This includes complex lesion locations in addition to those in cosmetically sensitive areas of the face (nose, eyelids, lips, ears), such as in parts of the body with thin, delicate tissue that could lead to delayed healing postoperatively (e.g., the pretibial area of the leg, the dorsum of the hand, or in those areas with special site considerations). These lesions typically are less than 4cm in diameter and less than 5mm in depth.
HDR electronic brachytherapy may also be preferable in elderly patients with health issues, such as peripheral vascular disease, diabetes mellitus, or those on anticoagulants in which case anesthesia and surgery may be contraindicated. For patients with dementia, psychiatric illness, and anxiety disorders and for those with personal objections that may interfere with prolonged in-office surgical procedures, HDR electronic brachytherapy can be performed as primary radiation therapy or can be used as an adjunct to surgery in certain situations such as for higher risk lesions.
The control rates of NMSC after HDR electronic brachytherapy, with both isotopic and electronic sources, cited in the literature are generally very high with minimal toxicity for favorable patients.30,31 During the treatment course, patients may develop mild erythema with pruritus, superficial desquamation, and induration limited to the lesion area. They typically resolve within a couple weeks of completing therapy. The most common long-term skin surface change is a relative hypopigmentation.
CONCLUSION
All of the papers/presentations reported that patients and physicians were pleased with the convenience of this nonsurgical treatment which allows for sensitive areas to be treated without the need for tissue excision and grafting. The outcomes, as measured by patient satisfaction, tumor clearance rates, and treatment complications, of the electronic brachytherapy are comparable to standard treatment, and recurrence rates were extremely low.
ACKNOWLEDGMENTS
The authors would like to acknowledge Eminence Clinical Research, Inc., for assistance with writing and statistical support of this manuscript.
Footnotes
Disclosure:Dr. Bhatnagar is a consultant for Icad. Drs. Patel, Werschler, Ceilley, and Strimling report no relevant conflicts of interest.
REFERENCES
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. [Accessed on June 3, 2016]. http://www.cancer.org/cancer/skincancer-basalandsquamouscell/detailedguide/skin-cancer-basal-and-squamous-cell-key-statistics
- 3.National Cancer Institute. [Accessed on March 15, 2016]. http://www.cancer.gov/cancertopics/types/skin
- 4.Hruza GJ. Mohs micrographic surgery local recurrences. J Dermatol Surg Oncol. 1994;20:573–577. doi: 10.1111/j.1524-4725.1994.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohs FE. Micrographic surgery for the microscopically controlled excision of eyelid cancers. Arch Ophthalmol. 1986;104:901–909. doi: 10.1001/archopht.1986.01050180135046. [DOI] [PubMed] [Google Scholar]
- 6.Resti GA, et al. Outcome of 110 basal cell carcinoma of the eyelid treated with frozen section-controlled excision: mean follow-up over 5 years. Eur J Ophthalmol. 2014;24:476–482. doi: 10.5301/ejo.5000405. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra R. The Australian Mohs database, part II: periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology. 2004;111:631–636. doi: 10.1016/j.ophtha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Leibovitch I, Huilgol SC, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:253–260. doi: 10.1016/j.jaad.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 9.Smeets NW, Kuijpers DL, Nelemans P, et al. Mohs micrographic surgery for treatment of basal cell carcinoma of the face-results of a retrospective study and review of the literature. Br J Dermatol. 2004;151:141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 10.Wennberg AM, Larkö O, Stenquist B. Five-year results of Mohs micrographic surgery for aggressive facial basal cell carcinoma in Sweden. Acta Derm Venereol. 1999;79:370–372. doi: 10.1080/000155599750010292. [DOI] [PubMed] [Google Scholar]
- 11.Smeets NW, Krekels GA, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766–1772. doi: 10.1016/S0140-6736(04)17399-6. [DOI] [PubMed] [Google Scholar]
- 12.Rowe DE, Carroll RJ, Day CL., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–990. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 13.Cumberland L, Dana A, Liegeois N. Mohs micrographic surgery for the management of nonmelanoma skin cancers. Facial Plast Surg Clin North Am. 2009;17:325–335. doi: 10.1016/j.fsc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Basset-Seguin N, Ibbotson SH, Emtestam L, et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol. 2008;18:547–553. doi: 10.1684/ejd.2008.0472. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes LE, de Rie MA, Leifsdottir R, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol. 2007;143:1131–1136. doi: 10.1001/archderm.143.9.1131. [DOI] [PubMed] [Google Scholar]
- 16.Berking C, Hauschild A, Kölbl O, Mast G, Gutzmer R. Basal cell carcinoma—treatments for the commonest skin cancer. Dtsch Arztebl Int. 2014;111:389–395. doi: 10.3238/arztebl.2014.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovett RD, Perez CA, Shapiro S, Garcia DM. External radiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys. 1990;19:235–e242. doi: 10.1016/0360-3016(90)90529-s. [DOI] [PubMed] [Google Scholar]
- 18.Silva JJ, Tsang RW, Panzarella P, et al. Results of radiotherapy for epithelial skin cancer of the pinna: the Princess Margaret Hospital experience, 1982-1993. Int J Radiat Oncol Biol Phys. 2000;47:451–e459. doi: 10.1016/s0360-3016(00)00410-7. [DOI] [PubMed] [Google Scholar]
- 19.Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol Biol Phys. 2001;51:748–e755. doi: 10.1016/s0360-3016(01)01656-x. [DOI] [PubMed] [Google Scholar]
- 20.Doggett S, Willoughby M, Willoughby C, et al. Incorporation of electronic brachytherapy for skin cancer into a community dermatology practice. J Clin Aesthet Dermatol. 2015;8:28–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Paravati AJ, Hawkins PG, Martin AN, et al. Clinical and cosmetic outcomes in patients treated with high-dose-rate electronic brachytherapy for nonmelanoma skin cancer. Pract Radiat Oncol. 2015;5:e659–e664. doi: 10.1016/j.prro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Bhatnagar A. Electronic brachytherapy for the treatment of non-melanoma skin cancer: results up to 4 years. 73rd Annual Meeting of the American Academy of Dermatology; San Francisco, CA. March 20–24, 2015. [Google Scholar]
- 23.Patel R, Wong G, Minn Y, et al. Initial experience with electronic brachytherapy for skin cancer: clinical and dosimetric characteristics. Poster PO44 American Brachytherapy Society, Annual Meeting; San Diego, CA. April 3–5, 2014. [Google Scholar]
- 24.Sinha R, Kurtzman S, McIlmoil N, Morganroth G California Skin Institute; Western Radiation Oncology. One-Year Experience of Electronic Brachytherapy for the Treatment of Nonmelanoma Skin Cancer
- 25.Strimling R. Initial experience of electronic brachytherapy for the treatment of 494 non-melanoma skin cancer (NMSC) lesions in 297 patients. 73rd Annual Meeting of the American Academy of Dermatology; San Francisco, CA. March 20–24, 2015. [Google Scholar]
- 26.Petrovich Z, Kuisk H, et al. Treatment results and patterns of failure in 646 patients with carcinomas of the eyelids, pinna and nose. Am J Surg. 1987;154:447–450. doi: 10.1016/0002-9610(89)90022-6. [DOI] [PubMed] [Google Scholar]
- 27.Gauden R, Pracy M, Avery AM, Hodgetts I, Gauden S. HDR brachytherapy for nonmelanoma skin cancers. J Med Imaging Radiat Oncol. 2013;5792:212–217. doi: 10.1111/j.1754-9485.2012.02466.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhatnagar A. Nonmelanoma skin cancer treated with electronic brachytherapy: Results at 1 year. Brachytherapy. 2013;12:134–140. doi: 10.1016/j.brachy.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Caccialanza M, Piccinno R, Kolesnikova L, Gnecchi L. Radiotherapy of skin carcinomas of the pinna: a study of 115 lesions in 108 patients. Int J Dermatol. 2005;44(6):513–517. doi: 10.1111/j.1365-4632.2005.02103.x. [DOI] [PubMed] [Google Scholar]
- 30.Olschewski T, Bajor K, Lang B, Lang E, Seegenschmiedt MH. Radiotherapy of basal cell carcinoma of the face and head: Importance of low dose per fraction on long-term outcome. J Dtsch Dermatol Ges. 2006;4:124–130. doi: 10.1111/j.1610-0387.2006.05880.x. [DOI] [PubMed] [Google Scholar]
- 31.Guix B, Finestres F, Tello J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys. 2000;47:95–102. doi: 10.1016/s0360-3016(99)00547-7. [DOI] [PubMed] [Google Scholar]


