Abstract
Background: Vitiligo vulgaris, an acquired disease related to autoimmune activity directed against melanocytes, is a common disorder of pigmentation affecting up to one percent of the population. Several autoimmune disorders are reported to improve during pregnancy—a state of relative immunosuppression. Objectives: To assess self-reported changes in vitiligo disease activity that occurred during pregnancy. Methods: A survey of 24 subjects with vitiligo was conducted by telephone using questions that pertained to vitiligo history, pregnancy history, and vitiligo disease activity prior to, during, and after pregnancy. Additional questions pertained to demographics, history of other autoimmune disease, and family history of vitiligo. Results: 18 of 24 subjects (75%) reported stable or improvement in vitiligo activity during pregnancy. Of these 18 subjects, five (27%) had discontinued vitiligo therapy during their pregnancies. Conclusion: The majority of patients surveyed reported either stable or improved vitiligo activity during pregnancy. These results support a protective effect exerted by the state of pregnancy against changing or progressing depigmentation characteristic of vitiligo vulgaris in the non-pregnant state.
VITILIGO VULGARIS (VV), A condition characterized by extensive loss of melanocytes, is widely accepted as an autoimmune disease1,2 that affects up to one percent of individuals.3 Moreover, pregnancy is considered to be a state of suppression of the maternal adaptive immune system.4,5 Autoimmune diseases, such as rheumatoid arthritis (RA)6 and multiple sclerosis (MS),7 are often reported to become quiescent during pregnancy. However, the impact of pregnancy on VV disease activity has not been adequately explored. Given the autoimmune pathogenesis underlying VV, an alteration of disease activity, whether regression or exacerbation of hypopigmented vitiliginous lesions, might be expected during pregnancy. The primary aim of this study was to assess disease activity in pregnant women previously diagnosed with VV.
MATERIALS AND METHODS
Following Institutional Review Board (IRB) approval, this singlecenter, survey-based study was conducted at an urban academic medical center. Eligible subjects were identified using the Northwestern Medicine Enterprise Data Warehouse (NMEDW) repository for electronic medical records. Two distinct searches were run within the NMEDW to detect eligible patients. The first search detected patients with VV who had a documented clinic encounter and pregnancy within the prior two years. The second search detected all females age 18 to 55 with VV who had a clinic encounter within the past two years.
Subjects included in the study were women ≥18 and ≤55 years of age with a diagnosis of VV who either subsequently became pregnant or delivered a child during the period September 1, 2011 to September 1, 2013. Subjects who were pregnant at the time of the survey were in their second or third trimesters of pregnancy.
After providing consent, subjects responded to 19 questions pertaining to their VV history, including treatment regimens, pregnancy history, and VV disease activity prior to, during, and after pregnancy. Additional questions pertained to demographics, history of autoimmune disease, and family history of vitiligo.
RESULTS
The searches yielded 230 patients; 113 met inclusion criteria and 24 consented to participate in the study. The large majority of excluded IRB-approved subjects (of the 113 meeting inclusion criteria) were not included due to non-availability for consent and survey by telephone. Seventeen subjects were self-reported as Caucasian/white (70.8%), three were Asian/Pacific islander, two were Hispanic/Latino, one was African American/black, and one was of mixed ethnicity. Twenty patients (83%) were reporting about a prior pregnancy while four (17%) were pregnant at the time of survey.
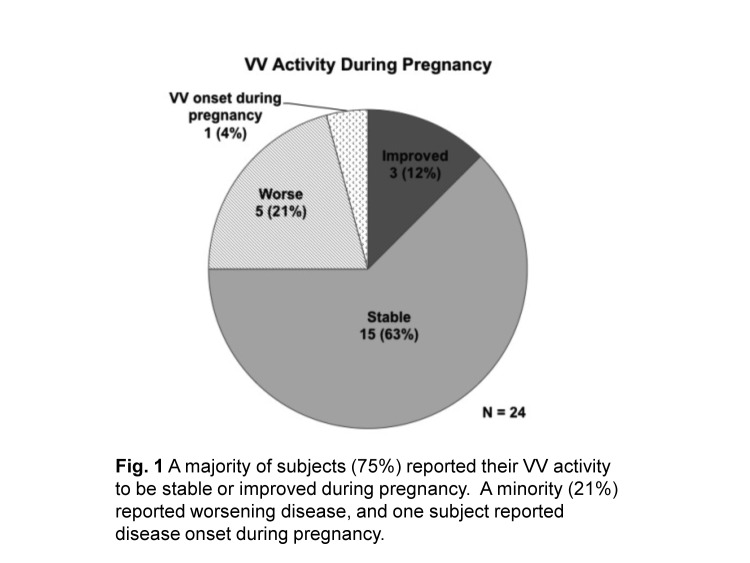
Three subjects (12.5%) reported VV improvement during a prior pregnancy (Figure 1). These three all described one or more lesions decreasing in size with the remaining lesions neither improved nor worsened during prior pregnancy. Fifteen subjects (62.5%) reported that their VV was stable with no new lesions or changes to lesions preexisting prior to pregnancy.
Figure 1.
A majority of subjects (75%) reported their VV activity to be stable or improved during pregnancy. A minority (21%) reported worsening disease and one subject reported disease onset during pregnancy.
Of the 18 subjects (75%) who reported stable or improved VV during pregnancy, five (28%) were receiving topical and/or phototherapy treatment prior to pregnancy, but had suspended all therapy during pregnancy. One additional subject who received both phototherapy and topical treatment prior to pregnancy stopped the topical treatment, but continued phototherapy during pregnancy. Two subjects (11%) received phototherapy treatment prior to and during pregnancy. Ten subjects (56%) received no VV treatment prior to or during pregnancy.
Five (20.8%) of 24 subjects reported worsening VV during pregnancy (Figure 1). Four of these five subjects reported an increase in size of one or more of their preexisting VV lesions during pregnancy, and four reported the appearance of new lesions during pregnancy (Table 1). Three of the five subjects were treated with topical therapy prior to pregnancy and suspended treatment during pregnancy.
Table 1.
Characterization of VV lesions in five subjects reporting worsening VV during pregnancy
| SUBJECT | SURVEY QUESTIONS | |
|---|---|---|
| How did your preexisting vitiligo spots change in size during your pregnancy? | Did you acquire any new vitiligo spots during pregnancy | |
| 1 | Some spots increased in size while others decreased in size | Yes |
| 2 | One or more lesions increased in size and the rest stayed the same | Yes |
| 3 | One or more lesions increased in size and the rest stayed the same | No |
| 4 | All spots increased in size | Yes |
| 5 | Not applicable; no changes in preexisting lesions | Yes |
One subject (4.2%) reported onset of VV for the first time during a prior pregnancy (Figure 1) with VV progression for five years following her diagnosis until she became pregnant again during the time period for this study. Throughout her second pregnancy, her VV activity was stable despite suspending topical corticosteroid treatment during this pregnancy. Notably, however, she reported her VV activity as worsened in the postpartum period.
Moreover, in the postpartum period (≤3 months postdelivery), 16 subjects (66%) reported stable VV, four subjects (17%) reported worsening VV, and four (17%) were pregnant at the time of survey, so postpartum VV activity was not assessed.
DISCUSSION
The prevalence of VV in young women (age <30 years),8 its autoimmune nature, and changes in maternal immune activity that occur during pregnancy, all suggest that individuals with VV might experience changes in VV activity during pregnancy. To the authors’ knowledge, no research has adequately assessed changes in VV activity during pregnancy.
In this survey of 24 subjects with histories of VV prior to pregnancy, the authors found that 18 (75%) reported either stable or improved VV activity during pregnancy. Rather than a destabilizing effect, these findings imply that, for VV patients who become pregnant, there is no effect or there is a possible stabilizing phenomenon for VV activity from a state of pregnancy. Changes underlying this observation are likely multifactorial, but the immunological alterations and physiological changes that occur during pregnancy may play significant roles.
Cortisol levels increase to nearly three times baseline levels during the second and third trimesters.9 Additionally, a positive feedback loop with progesterone increases levels of interleukin-10 (IL-10), an anti-inflammatory regulator.10,11 IL-10 is secreted from the trophoblast cells of the placenta and has been cited as a possible contributor to the remission of RA during and the exacerbation of systemic lupus erythematous during pregnancy.12,13 These changes parallel concepts used in the current medical management of VV. Low-dose systemic corticosteroid therapy is reported to prevent progression and promote repigmentation in patients with actively spreading VV.14 Tacrolimus, a calcineurin inhibitor that blocks cutaneous T cell activation and increases levels of IL-10 in VV lesions, is reported to decrease lesion size and promote repigmentation when applied topically.15
Likewise, the increase in IL-10 during pregnancy has been cited as an explanation for the quiescence of RA during pregnancy.10 Tofacitinib, a Janus kinase (JAK) inhibitor used in the treatment of rheumatoid arthritis,16 indirectly increases IL-10 levels via suppression of interferon-γ.17,18 It is not surprising, therefore, that there has been report of concurrent improvement in VV for patients with RA being treated with tofacitinib.19
Given that six of 24 subjects (25%) reported worsening VV and Subject 1 developed VV in the authors’ study population, the inherent stresses placed on the body during pregnancy should be considered as contributory. The odds of the onset or exacerbation of VV in patients with stressful events (such as changes in life conditions, changes in sleep, and a new person in the family) are reported to be nearly seven times higher than in patients without such stressful events.20
While literature reporting about VV activity related to pregnancy is sparse, there are two published case reports of women developing VV during pregnancy. One patient developed VV on her areolae bilaterally during the third month of her first pregnancy.21 A second patient experienced onset of VV on the neck and arms symmetrically late in her pregnancy and gave birth to a male child with congenital vitiligo.22 In the authors’ study population, one subject developed de novo VV during pregnancy.
This study is limited by the potential for recall bias inherent to the survey design. Moreover, subjects had no quantifiable measurements of their VV lesions during pregnancy, so it is possible that VV activity was not accurately reported. Additionally, several subjects stopped all medications during pregnancy. This might confound the authors’ results as it cannot be determined if worsened VV activity was attributable to physiological or immunologic changes during pregnancy or to the cessation of medications (whether for VV or for other disorders), or a combination of these confounders. Further exploration of these issues is warranted to determine a causal relationship between pregnancy and changes in VV activity.
Vitiligo is the most common depigmenting disorder. In the authors’ survey of 24 patients with vitiligo vulgaris, a majority reported stable or improved disease during pregnancy. This suggests a stabilizing or protective effect of pregnancy against vitiligo disease progression. Immunological, hormonal, or other physiological changes of pregnancy may contribute to these findings. Further investigation with a larger cohort is warranted to even better characterize VV activity during pregnancy.
KEY POINTS
The majority of study subjects in this cohort reported either stable or improved VV activity during pregnancy. These findings may be explained by immunological, hormonal, or other physiological changes of pregnancy. These results are consistent with a protective effect exerted by the state of pregnancy against changing or progressing depigmentation characteristic of VV in the non-pregnant state.
ACKNOWLEDGMENTS
Northwestern Medicine Enterprise Data Warehouse (NMEDW) was supported, in part, by the Northwestern University Clinical and translational science Institute, funded, in part, by Grant Number UL1TR000150 from the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Clinical and Translational Science Award (CTSA) is a registered trademark of DHHS.
Footnotes
Disclosure:The authors report no relevant conflicts of interest. All study subjects provided verbal informed consent.
REFERENCES
- 1.Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity. 2001;34(1):65–77. doi: 10.3109/08916930108994127. [DOI] [PubMed] [Google Scholar]
- 2.Ongenae K, Van Geel N, Naeyaert J-M. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16(2):90–100. doi: 10.1034/j.1600-0749.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Taïeb A, Picardo M VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20(1):27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 4.Clemens LE, Siiteri PK, Stites DP. Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol. 1979;122(5):1978–1985. [PubMed] [Google Scholar]
- 5.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 6.Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37(5):631–44. doi: 10.1080/08820130802205886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339(5):285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 8.Kyriakis KP, Palamaras I, Tsele E, et al. Case detection rates of vitiligo by gender and age. Int J Dermatol. 2009;48(3):328–329. doi: 10.1111/j.1365-4632.2009.03770.x. [DOI] [PubMed] [Google Scholar]
- 9.Soldin OP, Guo T, Weiderpass E, et al. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84(3):701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63(6):482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H, Mosmann TR, Guilbert L, et al. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 12.Hanna N, Hanna I, Hleb M, et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164(11):5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 13.Huizinga TW, van der Linden MW, Deneys-Laporte V, Breedveld FC. Interleukin-10 as an explanation for pregnancy-induced flare in systemic lupus erythematosus and remission in rheumatoid arthritis. Rheumatology. 1999;38(6):496–498. doi: 10.1093/rheumatology/38.6.496. [DOI] [PubMed] [Google Scholar]
- 14.Kim SM, Lee HS, Hann SK. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patients. Int J Dermatol. 1999;38(7):546–550. doi: 10.1046/j.1365-4362.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 15.Taher ZA, Lauzon G, Maguiness S, Dytoc MT. Analysis of interleukin-10 levels in lesions of vitiligo following treatment with topical tacrolimus. Br J Dermatol. 2009;161(3):654–659. doi: 10.1111/j.1365-2133.2009.09217.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 17.Maeshima K, Yamaoka K, Kubo S, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–1798. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Paik PK, Chen J, et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24(5):563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015 Jun 24. [cited 2015 Oct 8]. http://dx.doi.org/10.1001/jamadermatol.2015.1520 [Internet] [DOI] [PubMed]
- 20.Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21(7):921–928. doi: 10.1111/j.1468-3083.2006.02106.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein FK. Zentralblatt Für Gynäkol. 1958;80(41):1616–1620. [Vitiligo in pregnancy] [PubMed] [Google Scholar]
- 22.Kedward AL, Gawkrodger DJ. Congenital stable symmetrical type vitiligo in a patient whose mother developed vitiligo during pregnancy. Eur J Dermatol. 2008;18(3):353. doi: 10.1684/ejd.2008.0413. [DOI] [PubMed] [Google Scholar]