Abstract
Vitiligo is one of the most common cutaneous disorders of depigmentation. Although its underlying causes are still being studied and no definitive cure currently exists, recent research has provided insight into pathogenic mechanisms and new treatment options. Objective: The aim of this paper is to provide a comprehensive overview of the medical and surgical therapies for vitiligo with emphasis on the most recent treatment modalities. Design: This review was conducted through a literature search using PubMed and the National institutes of Health’s clinicalTrials.gov databases from January 2010 to July 2015. This yielded 86 studies, 12 of which were excluded, and 74 of which were reviewed. Results: Recent studies and ongoing clinical trials indicate that there are many promising new medical and surgical treatment modalities for this chronic condition. Conclusion: A combination of traditional and newer treatments may work synergistically to provide additional improvement in patients’ disease state and quality of life.
VITILIGO IS AN ACQUIRED disease with a variable course. It is characterized clinically by well-defined depigmented macules or patches thought to occur secondary to melanocyte dysfunction and loss. It is the most common depigmentation disorder, affecting approximately 0.5 to 2.0 percent of the population and has no predilection for gender or race.1 Vitiligo is categorized into nonsegmental (NSV) and segmental (SV) subtypes, the latter occurring in a minority (5–16%) of patients.2 Onset and disease course may vary by subtype. In addition, individuals with vitiligo may experience significant psychosocial manifestations, including low self-esteem and depression.3
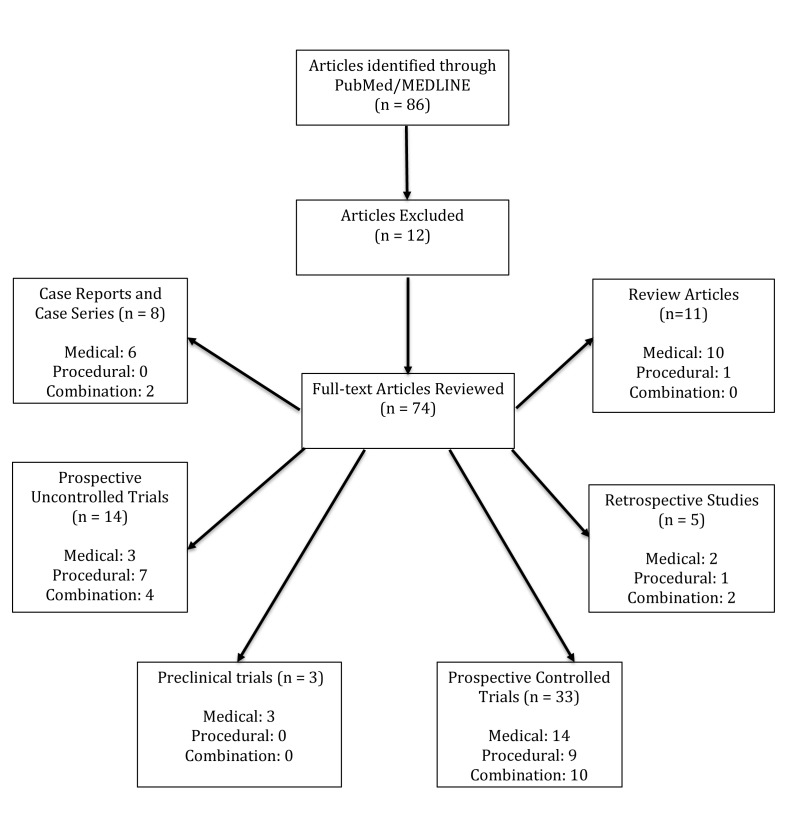
Pathogenic causes are likely multifactorial, including genetic influences, dysfunctional biochemical pathways, autoimmune processes, melanocyte adhesion deficits, and nervous system imbalances.4,5 Traditional vitiligo treatments include topical and oral immunomodulators and phototherapy. On rare occasions, depigmentation creams may also be used when repigmentation therapies prove inadequate and greater than 50 percent of body surface area (BSA) is involved. Advancements in understanding of the pathogenesis of vitiligo have contributed to newer promising therapeutic options. This paper aims to provide a comprehensive overview of these newer treatment options. A review of PubMed and the National Institutes of Health’s ClinicalTrials.gov database from January 2010 to July 2015 using the search phrases “vitiligo,” “vitiligo pathogenesis,” “vitiligo treatments,” and “vitiligo + [drug class or treatment technique]” was performed and clinical trials, case studies, case series, and reviews were included (Figure 1). Studies on alternative or holistic vitiligo treatments were excluded.
Figure 1.
A review of PubMed and the National Institute of Health’s ClinicalTrials.gov database from January 2010 to July 2015 using the search phrases “vitiligo,” “vitiligo pathogenesis,” “vitiligo treatments,” and “vitiligo + [drug class or treatment technique]” was performed and clinical trials, case studies, case series, and reviews were included.
MEDICAL TREATMENTS
Topical creams. First-line vitiligo treatment includes moderate-to-high strength topical corticosteroids and calcineurin inhibitors, both of which dampen the cellular immune response (Table 1).6 Several recent studies comparing the use of topical steroids to calcineurin inhibitors have found topical steroids (mometasone 0.1% or clobetasol 0.05% daily) similar in efficacy to calcineurin inhibitors (tacrolimus 0.1% or pimecrolimus 1.0% BID), both with tolerable adverse drug reaction (ADR) rates.7,8 A study by Kose et al8 showed mean repigmentation rates of 65 percent with mometasone and 42 percent with pimecrolimus after three months of daily treatment (p=0.154). The authors concluded that pimecrolimus may be preferable for localized facial vitiligo due to the potential for steroid-induced cutaneous atrophy, telangiectasia, and striae formation.
Table 1.
Medical treatments (Randomized and prospective controlled trials for medical treatments for vitiligo from January 2010 to July 2015. Case reports, case series, retrospective studies, pilot studies, preclinical studies, and uncontrolled trials were excluded from this table)
| AUTHOR | YEAR | INTERVENTION | PATIENTS (N) | TREATMENT PERIOD (MONTHS) | PRIMARY OUTCOMES | ADVERSE EFFECTS |
|---|---|---|---|---|---|---|
| Topical therapies | ||||||
| Ho et al | 2011 | Tacrolimus vs. clobetasol vs. placebo | 100 | 6 | Successful response defined as repigmentation >50%; tacrolimus was successful in 58% of patients (n=55) with facial vitiligo and 23% (n=45) with non-facial vitiligo; clobetasol yielded comparable results | None reported |
| Kose et al | 2010 | Mometasone vs. pimecrolimus | 40 | 3 | 65% repigmentation rate with mometasone, 42% repigmentation rate with pimecrolimus | Mometasone: atrophy, telangiectasia, and/or erythema (10%); pimecrolimus: burning sensation and/or pruritis (10%) |
| Systemic therapies | ||||||
| Singh et al | 2015 | Methotrexate vs. oral minipulse dexamethasone | 52 | 6 | 6 of 25 patients in the methotrexate group developed new lesions, while 7 of 25 in the steroid group developed new lesions. Methotrexate was found to be equally effective as oral minipulse steroids in controlling unstable vitiligo | Methotrexate: 20% developed nausea, Oral minipulse steroids: 20% developed weight gain and acneiform eruption |
| Singh et al | 2014 | Minocycline vs. mini pulse dexamethasone | 50 | 6 | Minocycline was found comparably effective to oral mini pulse corticosteroids in halting actively-spreading disease | Minocycline: facial hyperpigmentation (8%), oral mucosal hyperpigmentation (12%), nausea, and vomitting (12%); Dexamethasone: weight gain, headache, and/or weakness (28%) |
| Ultraviolet (UV) light therapy | ||||||
| Bansal et al | 2013 | Psoralen + NB-UVB vs. NB-UVB | 45 | 5 | Psoralen + NB-UVB hastens repigmentation rates and yields greater repigmentation and decreased VASI scores vs. NB-UVB alone (29.2 vs. 21.7% at 5 months, p=0.043) | P-NBUVB: nausea (45%), hyperpigmentation (25%); both arms: phototoxicity (5%), depigmented macuoles (10%) |
| El Mofty et al | 2013 | PUVA vs. BB-UVA | 45 | 5 | Mean repigmentation with PUVA comparable to BB-UVA suggesting the latter might be useful when oral psoralens are contraindicated | PUVA: phototoxicity (61.5%), thickening (23.1%); BB-UVA: phototoxicity (21.4%) |
| El Mofty et al | 2013 | NB-UVB vs. BB-UVA | 45 | 5 | NB-UVB yield significantly greater repigmentation rates than BB-UVA (64% ± 27.4 vs 44% ± 29.8 at 4 months, p=0.047) | NB-UVB: burning sensation and/or erythema (15%) |
| El Zawahry et al | 2012 | NB-UVB vs. UVA1 | 40 | 3 | NB-UVB superior to UVA1 based on percentage change in VASI score (median: -6.7% vs. 0%, P<0.001) and change in VETF area score (-4.4% vs. 0%, P=0.001) | NB-UVB: phototoxicity (5%), koebnerization (5%) |
| Sapam et al | 2012 | NB-UVB vs. PUVA | 56 | 6 | No significant difference in mean degree of repigmentation between the NB-UVB group and PUVA group (45% vs. 40%, respectively) | NB-UVB: pruritis (7.1%); PUVA: pruritus (19.2%), thickening (15.4%), hyperpigmentation (7.7%), giddiness (7.7%), erythema (7.7%), nausea (7.7%) |
| Siadat et al | 2014 | NB-UVB vs. oral minocycline | 42 | 3 | NB-UVB therapy reduced disease activity by 76.2% vs. 33.9% with minocycline (p<0.05), and was better able to reduce lesion diameters (p=0.031) | NB-UVB: pruritus, erythema (% not reported); minocycline: oral mucosal hyperpigmentation, GI complaints, and/or headache (14.2%) |
| Singh et al | 2013 | PUVA-Sol vs. PUVA | 35 | 9 | PUVA-Sol fell short of PUVA in repigmentation and quality of life (QOL) improvement outcomes (26% vs. 46% repigmentation at 9 months, respectively, p=0.06; mean QOL metric post-PUVA-sol treatment was a third of that post-PUVA at the same time point, p=0.04) | PUVA-Sol: phototoxicity (64.7%); PUVA: phototoxicity (100%) |
| Monochromatic excimer light laser (MEL) therapy | ||||||
| Le Duff et al | 2010 | MEL vs. excimer lamp | 20 | 3 | Both treatments showed similar efficacy with >50% mean repigmentation; the lamp induced more erythema than the laser | Both arms: erythema (“majority of patients”) |
| Shi et al | 2013 | MEL vs. excimer lamp | 14 | 2 | Both treatments exhibited similar efficacies in treating vitiligo; >50% in 79% of patches treated by laser and 87.5% of patches treated by lamp | MEL laser: erythema (92.9%); excimer lamp: erythema (85.7%) |
| Verhaeghe et al | 2011 | MEL vs. NB-UVB | 11 | 3 | No vitiliginous patches achieved >50% repigmentation after 3 months of MEL. 20% of lesions treated with NB-UVB achieved repigmentation scores >50% | MEL laser: erythema (82%), burning sensation (27%); NB-UVB: erythema (82%), burning sensation (18%) |
| Combination UV and topical or systemic therapies | ||||||
| Akdeniz et al | 2014 | Topical calcipotriol + NB-UVB + betamethasone vs. NB-UVB + topical calcipotriol vs. NB-UVB | 45 | 6 | Significantly greater repigmentation at 6 months observed with topical calcipotriol + NB-UVB + betamethasone therapy compared to NB-UVB alone (63.3 ± 7.6% vs 46.7 ± 8.0%, p = 0.0048). No other significant differences reported | None reported |
| Anbar et al | 2014 | Latanoprost + NB-UVB vs. Latanoprost vs. NB-UVB | 22 | 3 | At 6 month follow-up, latanoprost-induced repigmentation was comparable to that of the NB-UVB treatment. The latanoprost-NB-UVB combination was superior to other treatment arms, with 75% of patients retaining their repigmentation at 6 month follow-up | None reported |
| Baldo et al | 2014 | Topical tacrolimus + NB-UVB | 48 | 9 | NB-UVB therapy was deemed comparable to 0.1% topical tacrolimus, with at least partial repigmentation rates of ~70% | Erythema and/or folliculitis (16%) |
| Lim et al | 2015 | NB-UVB + percutaneous afamelanotide vs. NB-UVB | 55 | 6 | Afamelanotide + NB-UVB, vs. NB-UVB alone, yielded faster repigmentation of facial (41 vs. 61 days, p=0.001) and upper extremity lesions (46 vs. 69 days, p=0.003), and greater 6-month repigmentation rates (48.6% vs. 33.3%) | Combination: erythema (68%), nausea (18%), pruritis (7%), hyperpigmentation (7%) NB-UVB: erythema (82%), pruritis (7%), burning sensation (7%) |
| Nordal et al | 2011 | Topical tacrolimus + NB-UVB vs. NB-UVB | 40 | 3 | 42.1% repigmentation observed with tacrolimus + NB-UVB vs. 29% with NB-UVB monotherapy. A correlation between the number of topical tacrolimus applications and the repigmentation response was observed (p=0.044) | None reported |
| Combination MEL laser (MEL) and topical therapies | ||||||
| Hui-Lan et al | 2009 | Topical pimecrolimus + MEL laser treatment vs. MEL | 48 | 4 | Topical pimecrolimus + MEL laser treatment yielded significantly greater repigmentation at 7.5 months than MEL laser treatment alone (71% vs. 50% of subjects with >50% repigmentation, p=0.001) | Combination: burning sensation (16.7%), pruritis (14.6%) MEL laser: erythema (“common”), burning sensation (12.5%) |
| Nistico et al | 2012 | Topical tacrolimus + MEL laser treatment vs. MEL laser treatment | 52 | 3 | Repigmentation rates of the MEL laser treatment + tacrolimus (+vitamin E) vs. MEL laser treatment (+vitamin E) not statistically significant (p=0.36) at 4 months (70% vs. 55% with > 50% repigmentation) | Combination: erythema and/or burning sensation (25%) MEL laser: erythema and/or burning sensation (30%) |
PUVA=psoralen UVA; PUVA sol=psoralen UVA (sunlight as PUVA source); NB-UVB=narrowband UVB; MEL=meditec excimer light laser; QOL=quality of life; BB-UVB=broadband UVB; VASI=vitiligo area scoring index
Comparing tacrolimus response among vitiligo subtypes, Udompataikul et al9 found that patients with NSV, both generalized and focal, experienced higher response rates than those with SV and acrofacial NSV (94% vs. 77% vs. 56%, respectively). Children displayed nine times higher odds of response than adults (95% CI: 1.09–1.88). Disease duration of less than five years also correlated with an improved response. A randomized Phase 4 trial, using köebnerized lesions as a model for early stage disease, aims to compare topical tacrolimus to pimecrolimus and topical mometasone (NCT01082393).10
Systemic medications. Systemic corticosteroids are generally employed in rapidly progressive cases to help with disease stabilization. In a large, retrospective study, Kanwar et al11 found that low-dose oral dexamethasone mini pulse therapy (2.5mg/day on 2 consecutive days/week) halted progressive vitiligo in 91.8 percent of subjects at a mean of 13.2±3.1 weeks. Some degree of repigmentation was observed in all lesions at a mean of 16.1±5.9 weeks, and relapse occurred in 12.3 percent of patients at an average of 55.7±26.7 weeks post-treatment. Lee et al12 also reported favorable results with a combination of topical and systemic immunosuppressants, including two patients with focal NSV who experienced complete remission within 2 to 3 months of treatment with 0.03% topical tacrolimus and oral prednisone (20mg) daily.12
The oral antibiotic minocycline has also shown promise in the treatment of vitiligo due to its anti-inflammatory free-radical scavenging properties that confer a protective effect on melanocytes against H2O2-induced apoptosis.13 A preliminary study assessing the efficacy of oral minocycline (100mg daily) in progressive, slowly spreading vitiligo showed initial arrest of disease progression in 91 percent (29/32) of patients and arrest of re-progression in 10 patients after one month.14 A larger randomized controlled trial (RCT) further demonstrated that six months of 100mg minocycline daily was comparable to oral mini pulse corticosteroids (2.5mg for 2 consecutive days/week x 6 months) in halting actively spreading disease.15
Statins, best known for combating atherosclerosis, have also garnered interest in vitiligo treatment due to their immunomodulatory activity. Statins downregulate expression of a variety of adhesion molecules involved in the cellular immune response, as well as MHC II in antigen presenting cells (APCs), T cell chemokine receptors, and inflammatory cytokines including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, and IL-2. They are also antioxidants, blocking nitric oxide synthase and increasing the production of regulatory markers IL-12 and TGF-β.16,17 Interest in statins as a treatment for vitiligo began with a case report of vitiligo regression in a patient taking simvastatin 80mg daily.18 Preclinical data suggests statins prevent and reverse depigmentation by halting the influx and proliferation of cutaneous autoreactive cytotoxic T cells and by decreasing production of IFN-γ.19 Based on these promising findings, a Phase 2 RCT is evaluating the efficacy of simvastatin 80mg daily in adult vitiligo patients, including its effect on peripherally circulating autoreactive cytotoxic T cells (NCT01517893).10
Methotrexate, known for its use in inflammatory and immune-mediated conditions, such as psoriasis and inflammatory bowel disease, has also been studied in treating vitiligo. Sandra et al20 reported the first case in the literature of methotrexate being used in a patient with rapidly progressive vitiligo in 1998. The depigmentation halted the vitiligo from spreading and within three months of initiating treatment at 7.5mg weekly, substantial repigmentation was seen. This led to a pilot study of six patients with stable vitiligo by Alghamdi et al,21 in which patients were treated with methotrexate 25mg weekly for six months. No improvement was seen in any patient. Singh et al22 recently conducted a randomized, open-label trial of methotrexate (10mg weekly) compared with oral mini-pulse steroid therapy in 52 patients with unstable vitiligo, and found no difference in effectiveness between the two treatment modalities in halting the spread of depigmentation.22
Biologics, used to combat other immune-mediated diseases, such as psoriasis and rheumatoid arthritis, are also gaining attention as potential treatment options for vitiligo. A recent case report describes the successful use of tofacitinib citrate, an oral janus kinase 1/3 inhibitor, to treat a woman with generalized vitiligo affecting nearly 10 percent of her total BSA. She was treated for five months and had significant repigmentation with no adverse effects.23 Ruxolitinib, a janus kinase 1/2 inhibitor, was also described in a case report of a patient with concurrent vitiligo and alopecia areata, who had substantial repigmentation after 20 weeks of therapy. Unfortunately, most of the repigmented areas had depigmented within 12 weeks of drug cessation.24 A pilot study will assess the efficacy of another biologic, abatacept, a fusion protein that prevents co-stimulatory activation of T cells by APCs, in the treatment of vitiligo (NCT02281058).10
Ultraviolet light therapy. Ultraviolet (UV) light therapy represents another staple treatment of vitiligo, with both innate and cellular immunosuppressive as well as mitogenic and melanogenic properties that promote melanocyte proliferation and melanin synthesis. A 2012 study by Anbar et al25 showed that UVA light therapy combined with psoralen (PUVA) helps reverse melanocyte and keratinocyte degeneration in and around lesions of NSV. The sun can also be the source of UVA light in PUVA—a technique termed PUVA sol—which utilizes fewer healthcare resources. A nonrandomized comparative trial, however, suggests that it falls short of PUVA in repigmentation rates (46% vs. 26% repigmentation at 9 months, respectively, p=0.06) and quality of life improvement outcomes.26 El Mofty et al27 showed that broadband (BB)-UVA produces similar repigmentation rates to PUVA, along with lower rates of phototoxicity, making it a viable alternative in situations where psoralen cannot be used.
UVB therapy is classified as narrowband (NB-UVB, 311–313nm) or broadband (BB-UVB, 280–320nm). While randomized, controlled data suggest BB-UVB may more effectively stimulate repigmentation than does NB-UVB, use of the latter predominates due to its exclusion of more harmful wavelengths.28 Psoralen has also been shown to potentiate NB-UVB therapy. At five months follow-up, Bansal et al29 noted a reduction in Vitiligo Area Severity Index (VASI), scores of 29.2 vs. 21.7 percent in patients using psoralen and NB-UVB compared to NB-UVB alone (p=0.043).
UVA-1 has also been studied in the treatment of vitiligo, and randomized controlled data suggests that NB-UVB yields superior results to UVA-1 therapy with comparable ADRs.30 NB-UVB also yields comparable repigmentation rates to PUVA therapy with better color matching and significantly fewer ADRs, making it a preferred treatment option.31 Furthermore, in a recent RCT, NB-UVB therapy has been shown to outperform minocycline, reducing disease activity by 76.2 vs. 33.9 percent over three months (p<0.05) with both resulting in minimal ADRs.32
Monochromatic excimer light (MEL) laser therapy. MEL laser therapy is akin to focused, high-intensity UVB light therapy using a wavelength of 308nm.33 Excimer lamp, with an equivalent wavelength, has been shown to yield results and ADRs comparable to MEL laser therapy with one study reporting mean repigmentation rates >50 percent in 79 percent of patches treated by laser and 87.5 percent patches treated by the excimer lamp (p>0.05).34 A retrospective review evaluating the effects of MEL therapy in segmental vitiligo observed that higher repigmentation rates correlated positively with treatment duration (r=0.315, p=0.004) and cumulative UV dosage (r=0.366, p=0.001) and correlated negatively with disease duration (r=-0.265, p=0.017).35 A separate small RCT (n=11) observed zero lesions with >50-percent repigmentation after three months of MEL therapy, underscoring the outcome variability of this treatment modality.36
Combination UV and topical therapies. NB-UVB has been studied as a monotherapy and in combination with topical therapies including immunomodulators and steroids. One 48-subject, intrapatient comparison study found NB-UVB comparable to 0.1% topical tacrolimus, with at least partial repigmentation rates of 69 and 71 percent, respectively.37 Majid et al38 further observed that the combination of NB-UVB and tacrolimus 0.1% yielded faster and greater repigmentation compared to NB-UVB alone (71% vs. 60.5%, p<0.05). Nordal et al39 since noted a cumulative dose-dependent relationship between topical tacrolimus and repigmentation in NSV patients treated with NB-UVB and found this treatment combination superior to NB-UVB alone (median reduction in target lesions were 42.1% vs. 29% respectively at three months, p=0.005).
A 45-patient RCT comparing NB-UVB alone to NB-UVB with topical calcipotriol, and NB-UVB with topical calcipotriol and betamethasone observed significantly greater repigmentation at six months with the topical steroid combination therapy compared to NB-UVB alone (63.3±7.6% vs. 46.7±8.0%, p=0.0048), and no other significant differences between treatment arms.40
The combination of MEL and calcineurin inhibitors has also been studied. An RCT comparing the combination of topical tacrolimus, MEL therapy, and vitamin E versus MEL therapy and vitamin E versus vitamin E alone in 52 vitiligo patients showed significantly greater repigmentation in the combination arms compared to the vitamin-only arm at four months (70% and 55% vs. 0% with >50% repigmentation, p<0.001). No statistical difference between the combination therapy groups was observed (p=0.36).41 In contrast, Hui-Lan et al42 compared MEL therapy and topical 0.1% pimecrolimus BID to MEL therapy alone in 48 patients and found that the combination yielded significantly greater repigmentation at 7.5 months (71 vs. 50% of subjects with >50% repigmentation, p=0.001). A recent pilot study reported comparable repigmentation success with combined MEL therapy, topical 0.1% tacrolimus, and low-dose oral prednisolone (0.3mg/kg daily) in 14 patients with recent-onset localized vitiligo at less than half the follow-up time (5 segmental, 9 focal; 71.4% with >50% repigmentation at 3 months).43 Initial repigmentation was observed within two weeks in most patients. Larger RCTs in the future might further explore the efficacy of triple therapy compared to MEL and topical treatments. Other studies on the horizon are assessing the efficacy of topical compounds that convert sunlight into NB-UVB (NCT01992185) and comparing the combination of NB-UVB and PUVA to NB-UVB alone (Phase 4, NCT01732965).10
Afamelanotide. Vitiligo patients are known to have melanocortin system defects including reduced serum and cutaneous lesion alpha-melanocyte stimulating hormone (α-MSH).44,45 Afamelanotide is a longer lasting synthetic analogue of α-MSH that binds to the melanocortin-1 receptor, stimulating melanocyte proliferation and melanogenesis. A small pilot study of afamelanotide (16mg implant) implanted monthly in four patients, one month post-initiation of triweekly NB-UVB, resulted in significant repigmentation within a month and eventual diffuse hyperpigmentation of the GV lesions of all four patients.44 Subsequently, a 55-patient Phase 1/2 study comparing combination 7 to 10 day release afamelanotide 16mg bioresorbable implants with NB-UVB to NB-UVB alone found combination therapy yielded faster repigmentation of facial (41 vs. 61 days, p=0.001) and upper extremity lesions (46 vs. 69 days, p=0.003), and greater six-month repigmentation rates in NSV (48.6 vs. 33.3%).45 Though subjects’ Fitzpatrick skin types (FSTs) ranged from III to VI, the differences were significant only for FSTs IV to VI. ADRs included nausea, abdominal pain, and hyperpigmentation. Of note, afamelanotide-induced hyperpigmentation of normal skin can accentuate the differential pigmentation between normal and lesional skin. This may be more problematic in lighter skin types, and must be taken into account when considering this treatment option.46
Latanoprost. Latanoprost (LT) is a prostaglandin F2alpha analogue that can induce skin pigmentation, a side effect discovered through its use in glaucoma therapy.47–49 It upregulates tyrosinase and promotes melanocyte proliferation. A recent, 22-patient, randomized, placebo-controlled trial comparing topical LT to NB-UVB and to the combination of the two, reported that the LT and NB-UVB combination was superior to NB-UVB therapy alone (62.5 vs. 12.5% with >50% repigmentation at 6 months, p<0.05).47 LT alone yielded comparable results to NB-UVB (42.9 vs. 28.6% with >50% repigmentation at 6 months, p>0.05) and superior outcomes to placebo (42.9 vs. 0% with >50% repigmentation at 6 months, p<0.05). A Korean case series reported three patients with periorbital vitiligo who experienced 20, 50, and greater than 90-percent repigmentation after two months of topical LT therapy.49 Likewise, a Phase 4 clinical trial in India investigating topical bimatoprost 0.03% solution twice daily observed 50 to 100-percent repigmentation in 7 of 10 patients after four months. Results were first visible at two months, and patients with recalcitrant, focal vitiligo as well as those with disease duration less than six months tended to respond best.48 These promising results clearly warrant further investigation into LT’s safety and efficacy for the treatment of vitiligo.
PROCEDURAL TREATMENTS
Erbium laser-assisted dermabrasion. Various lasers and surgical procedures have also been studied in the treatment of vitiligo (Table 2).50,51 In a randomized, 18-patient, intra-patient controlled study, Bayoumi et al50 compared the efficacy of erbium laser-assisted dermabrasion followed by topical steroid use for three weeks, a one-week break, and then three months of NB-UVB treatment to the same regimen without laser dermabrasion. Nearly 50 percent of lesions in the laser cohort achieved >50-percent repigmentation versus 4.2 percent of paired lesions without laser treatment (p<0.0001). Despite the greater repigmentation rates in the laser cohort, tolerance to laser therapy was approximately half that of the non-laser regimen due to adverse effects including pain, edema, delayed healing, and hypertrophic scar formation.
Table 2.
Procedural and combination treatments (Randomized and prospective controlled trials for procedural and combination [medical and procedural] treatments for vitiligo from January 2010 to July 2015. Case reports, case series, retrospective studies, pilot studies, preclinical studies, and uncontrolled trials were excluded from this table)
| AUTHOR | YEAR | INTERVENTION | PATIENT # | TREATMENT PERIOD (MONTHS) | PRIMARY OUTCOMES | ADVERSE EFFECTS |
|---|---|---|---|---|---|---|
| Lasers | ||||||
| Bayoumi et al | 2012 | Laser dermabrasion + topical hydrocortisone 17-butyrate + NB-UVB vs. topical hydrocortisone 17-butyrate + NB-UVB | 18 | 4 | 50% of lesions in the laser cohort achieved >50% repigmentation vs. 4.2% of paired lesions without laser treatment (treated only with the topical immunosupressant + NB-UVB) (p<0.0001); tolerance of laser therapy was about half that of the non-laser regimen | Laser dermabrasion: pain, edema, delayed healing, and/or hypertrophic scar formation (50%) |
| Vachiramon et al | 2016 | Fractional CO2 laser + NB-UVB + topical clobetasol propionate 0.05% cream vs. NB-UVB = topical clobetasol propionate 0.05% cream | 27 | 2.5 | 23.1% of vitiligo lesions in the laser group achieved >50% pigmentation vs 3.9% of lesions in the non-laser group. The mean patient satisfaction score was 5.71 in the laser group vs. 3.48 in the non-laser group | The most common adverse effect was pain, which was more common in the laser group. The second most common was transient edema, occuring in the laser group |
| Shin et al | 2012 | CO2 laser therapy + NB-UVB vs. NB-UVB alone | 10 | 5 | CO2 laser therapy followed by NB-UVB was more efficacious in treating NSV than NB-UVB alone based on semi-quantitative and subjective | None reported |
| Cellular transplantation | ||||||
| Budania et al | 2012 | Noncultured epidermal cell suspension (NCES) vs. suction blister epidermal grafting | 41 | 4 | Repigmentation was excellent (90–100%) in 71% of lesions in the NCES group and 27% of lesions in the suction blister epidermal group (p=0.002); DLQI scores were significantly reduced in both | None reported |
| Ghosh et al | 2012 | Noncultured melanocyte keratinocyte transplantation (MKT) using poly (DL-lactic acid, PLA) film as a vector | 22 | 9 | Greater than 70% repigmentation was achieved in 45% of patients vs. 4.5% of controls (p=0.002) | None reported |
| Quezada et al | 2011 | Dermabrasion + epidermal and melanocyte sandpaper transfer vs. dermabrasion | 11 | 3 | No difference between sandpaper transfer + dermabrasion vs. dermabrasion alone; 6-87% repigmentation (n=9) occurred in the transfer | None reported |
| Sahni et al | 2011 | Noncultured melanocyte transplantation with cells suspended in patients’ serum vs. cells suspended in normal saline | 25 | 4 | Repigmentation results were excellent (>90%) and very good to excellent (>75%) in 44.4% and 66.7% of lesions, respectively, in Group A (control group) and 88.8% and 94.4% of lesions, respectively, in Group B | Halo phenomenon, infection, scarring, hyperpigmentation |
| Singh et al | 2013 | NCES | 30 | 4 | Repigmentation was considered “excellent” (90–100% repigmentation) in 83% of lesions and at least”good”( >75%) in 92% of lesions | None reported |
| Combination medical and surgical treatments | ||||||
| Linthorst Homan et al | 2012 | Epidermal punch grafting + NB-UVB vs. epidermal punch grafting + MEL laser therapy | 14 | 3 | No statistically significant repigmentation difference was observed after 3 months; while a 71.4% lower cumulative dose was achieved with MEL, patients were significantly more satisfied with NB-UVB and preferred it over MEL | None reported |
| Li et al | 2015 | CO2 laser + topical betamethasone + nbUVB vs. CO2 laser + nbUVB | 25 | 6 | 44% of patients in the treatment wing achieved >50% repigmentation at 6 months, compared with 8% of the control group | Pain, erythema, edema, burning sensation |
NB-UVB, Narrowband UVB; NCES, Non-cultured epidermal cell suspension; MKT, Non-cultured melanocyte keratinocyte transplanatation; NSV, Non-segmental Vitiligo; DLQI, Dermatology Life Quality Index; SBEG, Suction Blister Epidermal Grafting; MEL, Meditec Excimer Laser
CO2 fractional laser. Fractional CO2 lasers, originally developed for tissue rejuvenation and scar remodeling, have also been evaluated in the treatment of vitiligo.51 In a 10-patient, randomized, intra-patient controlled trial, fractional CO2 laser therapy followed by NB-UVB was more efficacious in treating NSV than NB-UVB alone, based on semi-quantitative and subjective assessments at five months follow-up.52 Recently, Vachiramon et al53 have shown in a prospective, randomized trial comparing NB-UVB therapy, clobetasol, and fractional CO2 laser therapy with NB-UVB and clobetasol alone that adding fractional CO2 laser treatment to above conventional therapies improves repigmentation rate as well as patient satisfaction. A similar study comparing NB-UVB alone to NB-UVB with fractional CO2 laser therapy to CO2 laser with topical steroid is currently recruiting (NCT02290717).10
Surgical transplantation. A variety of cellular transplantation techniques have been investigated in vitiligo, including needling, melanocyte keratinocyte transplantations, split-thickness grafts, autologous punch, and suction blister grafts. Surgical techniques are traditionally most effective in SV, in which lesions tend to be stable and focal.
One of the least invasive surgical options is needling. Needling involves the selective relocation of melanocytes from vitiligo lesion margins into the centrally depigmented area to serve as reservoirs for melanogenesis. Two small studies (n=4–12) assessing the efficacy of needling have reported 10- to 100-percent (mean = 61.36%) repigmentation at four months.54,55 A Phase 2/3 RCT comparing needling with and without topical corticosteroids is currently recruiting (NCT02191748).10
A recent systematic review of surgical treatments for vitiligo assessed various techniques of grafting and cellular transplantation and found that split-thickness and suction blister skin grafting consistently yielded 80- to 90-percent repigmentation rates.56 Suction blister grafting involves iatrogenic separation of the epidermis from the dermis and subsequent harvest and transplantation of the viable epidermis to a similarly treated recipient site. It has been found to have high success and low complication rates. A retrospective study of 28 patients and 129 grafts reported 87-percent graft survival and repigmentation in 68 percent of patients.57 Subjects less than 20 years old experienced the highest graft survival (100%), while those older than 40 years experienced the lowest rates (75–78%). Recipient sites on the neck and face also tended to experience superior outcomes, while those on the hands and feet experienced the least repigmentation.
Another technique, termed non-cultured epidermal cell suspension (NCES) or melanocyte keratinocyte transplant (MKTP), involves obtaining an autologous skin graft and blood sample, suspending the cells from the dermo-epidermal junction in the patient’s plasma, and transferring the solution to a receipient site, which has been mechanically or thermally dermabraded. Other techniques have also included injecting the cell suspension into iatrogenic blisters at the recipient site.58 A clinical trial assessing the efficacy of this method showed repigmentation in all 10 patients in the study (>76% [n=4], 51–75% [n=2], 26–50% [n=2], 0–25% [n=2]).59 Randomized studies have shown that NCES is significantly more effective than suction blister grafting at inducing repigmentation and increasing patient quality of life.60 NCES also yields comparable objective results and higher patient satisfaction than non-cultured hair follicle outer root sheath cell suspensions, with comparable safety.61 Furthermore, this technique has been shown to yield good-to-excellent (50–100%) repigmentation in greater than 90 percent of patients with leucotrichia at 9 to 12 months, obviating the need for hair transplantation.62
MKTP has also been shown to be more effective than dermabrasion alone, and results in an average VASI decrease of 45 percent (95% CI: 26–64%).63,64 Quezada et al65 conducted a small study of 11 patients to evaluate the safety and efficacy of dermabrasion and grafting for unresponsive vitiligo. They compared simple dermbarasion to dermabrasion of the recipient site combined with the use of the “sandpaper method,” in which sandpaper was used to obtain the melanocytes and epidermal cells used for grafting. They concluded that although early repigmentation was more prominent in the sandpaper group, there was no significant difference between the two groups by three months.65 A prospective, randomized, placebo-controlled, multicenter trial (n=22) found that MKTP using poly (DL-lactic acid, PLA) film as a vector for transplanted cells yielded greater than 70-percent repigmentation in 45 percent of patients vs. 4.5 percent of controls (p=0.002).66 A related cellular transplantation method, ReCell®, which involves spraying a cell suspension derived from a superficial skin graft pre-treated with UVA onto laser-dermabraded lesions, has also shown impressive results.67 Future directions include comparing combinations of different dermabrasion techniques (CO2 laser vs. mechanical) and dressing methods (collagen dressing vs. Vaseline®-impregnated gauze) at the MKTP recipient site (NCT02038257).10
Epidermal punch grafting, which entails transplantation of small, round plugs of normal skin and subcutaneous fat to similarly prepared recipient sites, has also resulted in successful repigmentation well beyond graft borders, particularly when accompanied by phototherapy. A retrospective, 30-patient case series, including more than 600 grafts, reported 87-percent graft survival with at least partial repigmentation at all surviving graft sites at a minimum of 10-weeks follow-up.68 Similar to the trends observed with suction blister grafting, patients younger than 20 years old and those with neck and trunk recipient sites experienced the highest mean repigmentation rates (61, 65, and 63%, respectively), while subjects over 60 years old and those with acral and joint involvement experienced the lowest repigmentation rates (38 and 46%, respectively).
Although surgical therapies can be effective for vitiligo, particularly segmental and focal vitiligo, certain limitations preclude this modality from being more commonly used. In addition to being costly and time consuming, special training, staff, and equipment are needed in order to perform the procedures. The size of the lesions must also be considered with grafting procedures necessitating small-to-medium sized lesions while cellular transplantation is typically limited to a size of 250cm2 or less.56 Additionally, patients must have stable vitiligo in order to be considered ideal surgical candidates. Criteria for stable vitiligo generally include the following: 1) no change in the size of lesion(s) for a period of at least six months, 2) no new lesions should appear during that time, 3) lack of koebnerization, and 4) no history of keloids or hypertrophic scars.56
COMBINATION MEDICAL AND SURGICAL TREATMENTS
A number of promising therapeutic combinations utilizing medical and surgical modalities have been investigated in the treatment of vitiligo (Table 2). Saldanha et al69 demonstrated in a small study (n=11) that punch grafting and topical mometasone yielded superior repigmentation to punch grafting alone. Similarly, a study of nine patients with stable SV treated with two weeks of prednisolone 20mg by mouth daily followed by epidermal blister grafting yielded excellent results (>90% repigmentation) in all patients at 1 to 2 months follow-up, complicated only by mild donor site hyperpigmentation.70
Other investigators have combined skin grafting with phototherapy. Multiple studies have found MEL therapy comparable to NB-UVB following skin grafting, with variable, and, at times, modest results, seemingly dependent on grafting technique.71,72 A study of 40 stable vitiligo patients treated with ultra-thin split-thickness grafts followed by NB-UVB, yielded >90-percent repigmentation in 83 percent of subjects, with good-to-excellent cosmetic results in 90 percent of recipient sites.73 A similar study of cultured melanocyte transplantation with NB-UVB compared with NB-UVB alone resulted in 90- to 100-percent repigmentation in 80.5 percent of patients in the treatment arm, compared with 43.6 percent in the control group.74 Perigraft depigmentation was seen in 15 percent, and five percent experienced hypertrophic scarring. A randomized study is currently comparing epidermal transplantation using the ReCell® method with or without adjuvant NB-UVB therapy (NCT00615355).10
In a more involved combination therapy investigation, El Hoseny et al75 treated 14 patients with stable NSV of the extremities with 0.3mg/kg oral prednisolone followed by CO2 laser resurfacing of the recipient site, subsequent epidermal skin grafting, and topical 0.1% betamethasone.75 This combination halted progression in 87.7 percent and yielded good-to-excellent (80–100%) repigmentation in 70.4 percent of patients.
CONCLUSION
Over the last decade, significant progress has been made in understanding the complex and multifactorial pathogenesis of vitiligo. It is now theorized that intrinsic metabolic, neuronal, and/or biochemical cutaneous perturbations trigger autoimmune melanocytic destruction, to which patients may be predisposed by certain genetic mutations and polymorphisms. How different pathogenic mechanisms give rise to vitiligo subtypes remains to be further elucidated. While at present there is no cure for vitiligo, a variety of advances in medical and surgical treatments as well as combinations of the two working synergistically have shown the ability to improve patients’ disease state and quality of life. A number of clinical trials are underway, and the future is looking promising for patients afflicted with this traditionally stigmatizing and challenging condition.
Footnotes
Disclosure:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. Epub 2015 Jan 15. [DOI] [PubMed] [Google Scholar]
- 2.Ezzedine K, Lim HW, Suzuki T, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25(3):E1–E13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159–64. doi: 10.1001/jamadermatol.2013.927. [DOI] [PubMed] [Google Scholar]
- 4.Dell’anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19(5):406–411. doi: 10.1111/j.1600-0749.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 5.van den Boorn JG, Picavet DI, van Swieten PF, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131:1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 6.Garg BJ, Saraswat A, Bhatia A, Katare OP. Topical treatment in vitiligo and the potential uses of new drug delivery systems. Indian J Dermatol Venereol Leprol. 2010;76:231–238. doi: 10.4103/0378-6323.62961. [DOI] [PubMed] [Google Scholar]
- 7.Ho N, Pope E, Weinstein M, et al. A double-blind, randomized, placebo-controlled trial of topical tacrolimus 0.1% vs. clobetasol propionate 0.05% in childhood vitiligo. Br J Dermatol. 2011;165:626–632. doi: 10.1111/j.1365-2133.2011.10351.x. [DOI] [PubMed] [Google Scholar]
- 8.Köse O, Arca E, Kurumlu Z. Mometasone cream versus pimecrolimus cream for the treatment of childhood localized vitiligo. J Dermatolog Treat. 2010;21:133–139. doi: 10.3109/09546630903266761. [DOI] [PubMed] [Google Scholar]
- 9.Udompataikul M, Boonsupthip P, Siriwattanagate R. Effectiveness of 0.1% topical tacrolimus in adult and children patients with vitiligo. J Dermatol. 2011;38:536–40. doi: 10.1111/j.1346-8138.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Bethesda, MD: Clinicaltrials.gov; [Google Scholar]
- 11.Kanwar AJ, Mahajan R, Parsad D. Low-dose oral mini-pulse dexamethasone therapy in progressive unstable vitiligo. J Cutan Med Surg. 2013;17:259–268. doi: 10.2310/7750.2013.12053. [DOI] [PubMed] [Google Scholar]
- 12.Lee D-Y, Kim C-R, Lee J-H, Yang J-M. Recent onset vitiligo treated with systemic corticosteroid and topical tacrolimus: need for early treatment in vitiligo. J Dermatol. 2010;37:1057–1059. doi: 10.1111/j.1346-8138.2010.00929.x. [DOI] [PubMed] [Google Scholar]
- 13.Song X, Xu A, Pan W, et al. Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med. 2008;22:9–16. [PubMed] [Google Scholar]
- 14.Parsad D, Kanwar A. Oral minocycline in the treatment of vitiligo—a preliminary study. Dermatol Ther. 2010;23:305–307. doi: 10.1111/j.1529-8019.2010.01328.x. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Kanwar AJ, Parsad D, Mahajan R. Randomized controlled study to evaluate the effectiveness of dexamethasone oral minipulse therapy versus oral minocycline in patients with active vitiligo vulgaris. Indian J Dermatol Venereol Leprol. 2014;80:29–35. doi: 10.4103/0378-6323.125479. [DOI] [PubMed] [Google Scholar]
- 16.Feily A, Baktash D, Mohebbipour A, Feily A. Potential advantages of simvastatin as a novel anti-vitiligo arsenal. Eur Rev Med Pharmacol Sci. 2013;17:1982–1983. [PubMed] [Google Scholar]
- 17.Namazi MR. Statins: novel additions to the dermatologic arsenal? Exp Dermatol. 2004;13:337–339. doi: 10.1111/j.0906-6705.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 18.Noël M, Gagné C, Bergeron J, et al. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004;3:7. doi: 10.1186/1476-511X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal P, Rashighi M, Essien KI, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135:1080–1088. doi: 10.1038/jid.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandra A, Pai S, Shenoi SD. Unstable vitiligo responding to methotrexate. Indian J Dermatol Venereol Leprol. 1998;64(6):309. [PubMed] [Google Scholar]
- 21.Alghamdi K, Khurrum H. Methotrexate for the treatment of generalized vitiligo. Saudi Pharm J. 2013;21(4):423–424. doi: 10.1016/j.jsps.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh H, Kumaran MS, Bains A, Parsad D. A randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatology. 2015;231(3):286–290. doi: 10.1159/000433424. [DOI] [PubMed] [Google Scholar]
- 23.Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110–1112. doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]
- 24.Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J Am Acad Dermatol. 2016;74(2):370–371. doi: 10.1016/j.jaad.2015.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anbar TS, El-Sawy AE, Attia SK, et al. Effect of PUVA therapy on melanocytes and keratinocytes in non-segmental vitiligo: histopathological, immuno-histochemical and ultrastructural study. Photodermatol Photoimmunol Photomed. 2012;28:17–25. doi: 10.1111/j.1600-0781.2011.00631.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Khandpur S, Sharma VK, Ramam M. Comparison of efficacy and side-effect profile of oral PUVA vs. oral PUVA sol in the treatment of vitiligo: a 36-week prospective study. J Eur Acad Dermatol Venereol. 2013;27:1344–1351. doi: 10.1111/jdv.12002. [DOI] [PubMed] [Google Scholar]
- 27.El Mofty M, Bosseila M, Mashaly HM, et al. Broadband ultraviolet A vs. psoralen ultraviolet A in the treatment of vitiligo: a randomized controlled trial. Clin Exp Dermatol. 2013;38:830–835. doi: 10.1111/ced.12099. [DOI] [PubMed] [Google Scholar]
- 28.El-Mofty M, Mostafa W, Youssef R, et al. BB-UVA vs. NB-UVB in the treatment of vitiligo: a randomized controlled clinical study (single blinded) Photodermatol Photoimmunol Photomed. 2013;29:239–246. doi: 10.1111/phpp.12061. [DOI] [PubMed] [Google Scholar]
- 29.Bansal S, Sahoo B, Garg V. Psoralen-narrowband UVB phototherapy for the treatment of vitiligo in comparison to narrowband UVB alone. Photodermatol Photoimmunol Photomed. 2013;29:311–317. doi: 10.1111/phpp.12072. [DOI] [PubMed] [Google Scholar]
- 30.El-Zawahry BM, Bassiouny DA, Sobhi RM, et al. A comparative study on efficacy of UVA1 vs. narrow-band UVB phototherapy in the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2012;28:84–90. doi: 10.1111/j.1600-0781.2011.00643.x. [DOI] [PubMed] [Google Scholar]
- 31.Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs. narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012;51:1107–1115. doi: 10.1111/j.1365-4632.2011.05454.x. [DOI] [PubMed] [Google Scholar]
- 32.Siadat AH, Zeinali N, Iraji F, et al. Narrow-band ultraviolet B versus oral minocycline in treatment of unstable vitiligo: a prospective comparative trial. Dermatol Res Pract. 2014;(2014):240856. doi: 10.1155/2014/240856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Duff F, Fontas E, Giacchero D, et al. 308-nm excimer lamp vs. 308-nm excimer laser for treating vitiligo: a randomized study. Br J Dermatol. 2010;163:188–92. doi: 10.1111/j.1365-2133.2010.09778.x. [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Li K, Fu J, et al. Comparison of the 308-nm excimer laser with the 308-nm excimer lamp in the treatment of vitiligo—a randomized bilateral comparison study. Photodermatol Photoimmunol Photomed. 2013;29:27–33. doi: 10.1111/phpp.12015. [DOI] [PubMed] [Google Scholar]
- 35.Do JE, Shin JY, Kim D-Y, et al. The effect of 308nm excimer laser on segmental vitiligo: a retrospective study of 80 patients with segmental vitiligo. Photodermatol Photoimmunol Photomed. 2011;27:147–51. doi: 10.1111/j.1600-0781.2011.00587.x. [DOI] [PubMed] [Google Scholar]
- 36.Verhaeghe E, Lodewick E, van Geel N, Lambert J. Intrapatient comparison of 308-nm monochromatic excimer light and localized narrow-band UVB phototherapy in the treatment of vitiligo: a randomized controlled trial. Dermatology. 2011;223:343–348. doi: 10.1159/000335272. (Basel, Switzerland) [DOI] [PubMed] [Google Scholar]
- 37.Baldo A, Lodi G, Di Caterino P, Monfrecola G. Vitiligo, NB-UVB and tacrolimus: our experience in Naples. G Ital Dermatol Venereol. 2014;149:123–130. [PubMed] [Google Scholar]
- 38.Majid I. Does topical tacrolimus ointment enhance the efficacy of narrowband ultraviolet B therapy in vitiligo? A left-right comparison study. Photodermatol Photoimmunol Photomed. 2010;26:230–234. doi: 10.1111/j.1600-0781.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 39.Nordal EJ, Guleng GE, Rönnevig JR. Treatment of vitiligo with narrowband-UVB (TL01) combined with tacrolimus ointment (0.1%) vs. placebo ointment, a randomized right/left double-blind comparative study. J Eur Acad Dermatol Venereol. 2011;25:1440–1443. doi: 10.1111/j.1468-3083.2011.04002.x. [DOI] [PubMed] [Google Scholar]
- 40.Akdeniz N, Yavuz IH, Gunes Bilgili S, et al. Comparison of efficacy of narrow band UVB therapies with UVB alone, in combination with calcipotriol, and with betamethasone and calcipotriol in vitiligo. J Dermatolog Treat. 2014;25:196–199. doi: 10.3109/09546634.2013.777381. [DOI] [PubMed] [Google Scholar]
- 41.Nisticò S, Chiricozzi A, Saraceno R, et al. Vitiligo treatment with monochromatic excimer light and tacrolimus: results of an open randomized controlled study. Photomed Laser Surg. 2012;30:26–30. doi: 10.1089/pho.2011.3029. [DOI] [PubMed] [Google Scholar]
- 42.Hui-Lan Y, Xiao-Yan H, Jian-Yong F, Zong-Rong L. Combination of 308-nm excimer laser with topical pimecrolimus for the treatment of childhood vitiligo. Pediatr Dermatol. 2009;26:354–356. doi: 10.1111/j.1525-1470.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 43.Jang YH, Jung S-E, Shin J, Kang HY. Triple combination of systemic corticosteroids, excimer laser, and topical tacrolimus in the treatment of recently developed localized vitiligo. Ann Dermatol. 2015;27:104–107. doi: 10.5021/ad.2015.27.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grimes PE, Hamzavi I, Lebwohl M, et al. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149:68–73. doi: 10.1001/2013.jamadermatol.386. [DOI] [PubMed] [Google Scholar]
- 45.Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol. 2015;151:42–50. doi: 10.1001/jamadermatol.2014.1875. [DOI] [PubMed] [Google Scholar]
- 46.Passeron T. Indications and limitations of afamelanotide for treating vitiligo. JAMA Dermatol. 2015;151:349–50. doi: 10.1001/jamadermatol.2014.4848. [DOI] [PubMed] [Google Scholar]
- 47.Anbar TS, El-Ammawi TS, Abdel-Rahman AT, Hanna MR. The effect of latanoprost on vitiligo: a preliminary comparative study. Int J Dermatol. 2015;54(5):587–593. doi: 10.1111/ijd.12631. [DOI] [PubMed] [Google Scholar]
- 48.Petrou I. Research uncovers new, innovative treatments for conditions In patients with skin of color. Dermatology Times. 2013. Web.
- 49.Noh S, Kim TG, Oh SH. Three cases of vitiligo showing response to application of latanoprost. Korean J Dermatol. 2010;48:350–353. [Google Scholar]
- 50.Bayoumi W, Fontas E, Sillard L, et al. Effect of a preceding laser dermabrasion on the outcome of combined therapy with narrowband ultraviolet B and potent topical steroids for treating nonsegmental vitiligo in resistant localizations. Br J Dermatol. 2012;166:208–211. doi: 10.1111/j.1365-2133.2011.10564.x. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Wu Y, Li L, et al. Triple combination treatment with fractional CO2 laser plus topical betamethasone solution and narrowband ultraviolet B for refractory vitiligo: a prospective, randomized half-body, comparative study. Dermatol Ther. 2015;28(3):131–134. doi: 10.1111/dth.12202. [DOI] [PubMed] [Google Scholar]
- 52.Shin J, Lee JS, Hann S-K, Oh SH. Combination treatment by 10 600 nm ablative fractional carbon dioxide laser and narrowband ultraviolet B in refractory nonsegmental vitiligo: a prospective, randomized half-body comparative study. Br J Dermatol. 2012;166:658–661. doi: 10.1111/j.1365-2133.2011.10723.x. [DOI] [PubMed] [Google Scholar]
- 53.Vachiramon V, Chaiyabutr C, Rattanaumpawan P, Kanokrungsee S. Effects of a preceding fractional carbon dioxide laser on the outcome of combined local narrowband ultraviolet B and topical steroids in patients with vitiligo in difficult-to-treat areas. Lasers Surg Med. 2016;48(2):197–202. doi: 10.1002/lsm.22389. [DOI] [PubMed] [Google Scholar]
- 54.Sharquie KE, Noaimi AA, Al-Mudaris HA. Melanocytes transplantation in patients with vitiligo using needling micrografting technique. J Drugs Dermatol. 2013;12:e74–e78. [PubMed] [Google Scholar]
- 55.Wassef C, Lombardi A, Khokher S, Rao BK. Vitiligo surgical, laser, and alternative therapies: a review and case series. J Drugs Dermatol. 2013;12:685–691. [PubMed] [Google Scholar]
- 56.Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol. 2013;169(Suppl 3):57–66. doi: 10.1111/bjd.12532. [DOI] [PubMed] [Google Scholar]
- 57.Gou D, Currimbhoy S, Pandya AG. Suction blister grafting for vitiligo: efficacy and clinical predictive factors. Dermatol Surg. 2015;41(5):633–639. doi: 10.1097/DSS.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 58.Sahni K, Parsad D, Kanwar AJ, Mehta SD. Autologous noncultured melanocyte transplantation for stable vitiligo: can suspending autologous melanocytes in the patients’ own serum improve repigmentation and patient satisfaction? Dermatol Surg. 2011;37:176–182. doi: 10.1111/j.1524-4725.2010.01847.x. [DOI] [PubMed] [Google Scholar]
- 59.Khodadadi L, Shafieyan S, Sotoudeh M, et al. Intraepidermal injection of dissociated epidermal cell suspension improves vitiligo. Arch Dermatol Res. 2010;302:593–599. doi: 10.1007/s00403-010-1034-7. [DOI] [PubMed] [Google Scholar]
- 60.Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol. 2012;167:1295–1301. doi: 10.1111/bjd.12007. [DOI] [PubMed] [Google Scholar]
- 61.Singh C, Parsad D, Kanwar AJ, et al. Comparison between autologous noncultured extracted hair follicle outer root sheath cell suspension and autologous noncultured epidermal cell suspension in the treatment of stable vitiligo: a randomized study. Br J Dermatol. 2013;169:287–293. doi: 10.1111/bjd.12325. [DOI] [PubMed] [Google Scholar]
- 62.Gan EY, van Geel N, Goh BK. Repigmentation of leucotrichia in vitiligo with noncultured cellular grafting. Br J Dermatol. 2012;166(1):196–199. doi: 10.1111/j.1365-2133.2011.10540.x. [DOI] [PubMed] [Google Scholar]
- 63.Huggins RH, Henderson MD, Mulekar SV, et al. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: the experience of an academic medical center in the United States. J Am Acad Dermatol. 2012;66:785–793. doi: 10.1016/j.jaad.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Toossi P, Shahidi-Dadras M, Mahmoudi Rad M, Fesharaki RJ. Non-cultured melanocyte-keratinocyte transplantation for the treatment of vitiligo: a clinical trial in an Iranian population. J Eur Acad Dermatol Venereol. 2011;25:1182–1186. doi: 10.1111/j.1468-3083.2010.03946.x. [DOI] [PubMed] [Google Scholar]
- 65.Quezada N, Machado Filho CA, De La Sotta P, Uribe P. Melanocytes and keratinocytes transfer using sandpaper technique combined with dermabrasion for stable vitiligo. Dermatol Surg. 2011;37:192–198. doi: 10.1111/j.1524-4725.2010.01856.x. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh D, Kuchroo P, Viswanathan C, et al. Efficacy and safety of autologous cultured melanocytes delivered on poly (DL-lactic acid) film: a prospective, open-label, randomized, multicenter study. Dermatol Surg. 2012;38:1981–1990. doi: 10.1111/dsu.12000. [DOI] [PubMed] [Google Scholar]
- 67.Cervelli V, De Angelis B, Balzani A, et al. Treatment of stable vitiligo by ReCell system. Acta Dermatovenerol Croat. 2009;17:273–278. [PubMed] [Google Scholar]
- 68.Feetham HJ, Chan JL, Pandya AG. Characterization of clinical response in patients with vitiligo undergoing autologous epidermal punch grafting. Dermatol Surg. 2012;38(1):14–19. doi: 10.1111/j.1524-4725.2011.02171.x. [DOI] [PubMed] [Google Scholar]
- 69.Saldanha KDD, Machado Filho CDAS, Paschoal FM. Action of topical mometasone on the pigmented halos of micrografting in patients with vitiligo. An Bras Dermatol. 2012;87:685–690. doi: 10.1590/s0365-05962012000500002. [DOI] [PubMed] [Google Scholar]
- 70.Lee D-Y, Lee K-J, Choi S-C, Lee J-H. Segmental vitiligo treated by the combination of epidermal grafting and systemic corticosteroids. Dermatol Surg. 2010;36:575–576. doi: 10.1111/j.1524-4725.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 71.Linthorst Homan MW, Spuls PI, Nieuweboer-Krobotova L, et al. A randomized comparison of excimer laser versus narrow-band ultraviolet B phototherapy after punch grafting in stable vitiligo patients. J Eur Acad Dermatol Venereol. 2012;26:690–695. doi: 10.1111/j.1468-3083.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 72.Sheth VM, Currimbhoy SD, Feetham HJ, et al. Efficacy of narrowband ultraviolet B versus excimer radiation in repigmenting vitiligo after minigrafting on the distal arms. J Am Acad Dermatol. 2012;67:318–320. doi: 10.1016/j.jaad.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 73.Majid I, Imran S. Ultrathin split-thickness skin grafting followed by narrowband UVB therapy for stable vitiligo: an effective and cosmetically satisfying treatment option. Indian J Dermatol Venereol Leprol. 2012;78(2):159–164. doi: 10.4103/0378-6323.93632. [DOI] [PubMed] [Google Scholar]
- 74.Zhang DM, Hong WS, Fu LF, et al. A randomized controlled study of the effects of different modalities of narrow-band ultraviolet B therapy on the outcome of cultured autologous melanocytes transplantation in treating vitiligo. Dermatol Surg. 2014;40(4):420–426. doi: 10.1111/dsu.12444. [DOI] [PubMed] [Google Scholar]
- 75.El Hoseny SM. Treatment of upper-and lower-extremity vitiligo with epidermal grafts after CO2 laser resurfacing with systemic and topical steroids. Aesthetic Plast Surg. 2010;34:157–166. doi: 10.1007/s00266-009-9376-2. [DOI] [PubMed] [Google Scholar]