Abstract
Background: Few hyaluronic acid fillers have been developed for superficial injection. Objective: To compare the diffusion and integration properties of cohesive polydensified matrix and Vycross® technology hyaluronic acid fillers with lidocaine following injection into the superficial reticular dermis. Methods and materials: Two subjects received two injections each of cohesive polydensified matrix and Vycross® hyaluronic acid (0.2mL/site) in the superficial reticular dermis of the buttock under ultrasound control. Biopsies were obtained at Days 0, 15, and/or 90. Ultrasound and histologic analyses were performed, plus a series of simple rheological tests. Results: Day 0 ultrasound images showed cohesive polydensified matrix hyaluronic acid homogeneous with the surrounding dermis. Vycross® hyaluronic acid showed more heterogeneity and some leakage into the hypodermis. Day 15 and Day 90 images were similar to Day 0. Histologic examination of biopsy tissue showed cohesive polydensified matrix hyaluronic acid homogeneously distributed among collagen fibrils with no visible particles. Vycross® hyaluronic acid appeared as variable-sized pools with a particulate appearance. Neither gel was associated with an inflammatory reaction. Laboratory tests showed cohesive polydensified matrix hyaluronic acid to have greater cohesivity and resistance to traction forces than Vycross®. Conclusion: cohesive polydensified matrix gel with lidocaine is homogeneously distributed following injection in the superficial reticular dermis and may be particularly suited for aesthetic indications requiring superficial injection.
THE DEPTH OF FILLER INJECTION is a key factor in obtaining an effective aesthetic result and is determined by relating the characteristics of the filler material to the type of correction required. The correction of fine lines and wrinkles requires superficial injection, but careful filler selection is necessary to avoid visible or palpable product and the risk of the Tyndall effect. Bovine collagen was the first filler to be used in this manner and was indicated for injection in the superficial reticular dermis.1,2 Some investigators interpreted this as injection in the superficial papillary dermis,3 which would imply an injection at a mean depth of 151 to 349μm, a real technical feat.4
The mean epidermal thickness is 150μm.4 Just below is the dermis, which can be divided into the following two layers: the papillary dermis with a mean thickness of 200μm and the reticular dermis. The thickness of the latter varies from 300μm to 4mm depending on the area of the body. The reticular dermis can subsequently be divided into the reticular superficial, mid, and deep dermis, each layer having a depth of 0.10 to 1.33mm. A number of studies have demonstrated that injection in the superficial and mid-reticular dermis is possible.5–8 In clinical practice, this is accomplished with good injection technique, a correct angle of needle penetration, a short length of needle implantation, and injection with the bevel up.
Among the hyaluronic acid (HA) gels for aesthetic use, only a few have been specifically developed with the properties suitable for injection in the superficial and the mid-reticular dermis including cohesive polydensified matrix 22.5mg/mL (CPM®) and Vycross® 15 mg/mL.9,10 These use different crosslinking technologies to stabilize the gel and create the specific rheological properties suitable for superficial injection.
CPM and Vycross gels use 1,4-butanediol diglycidyl ether (BDDE) as the crosslinking agent, but CPM differs from all other HA gels in the addition of another crosslinking step which stretches the matrix obtained in the first part of the process and adds more HA strands to continue the crosslinking process, without the addition of further BDDE. The result is a gel matrix that combines high levels of crosslinked HA with lighter levels of crosslinked HA in the same product. The lessdensely crosslinked zones allow the gel to penetrate the smallest intercellular spaces of the extracellular matrix, while the cohesivity of the matrix allows the gel to stay intact.7 An analogy would be honey percolating through the holes of a sponge. This specific polydensification of the matrix has been scientifically confirmed recently by sophisticated technologies.11
Vycross technology mixes high amounts of low molecular weight HA fibers (for crosslinking efficiency) with a smaller amount of high molecular weight HA fibers (for product cohesivity).12 The low molecular weight fibers produce a tighter network during the crosslinking process. As a result, less BDDE is used and the crosslinking is described as more efficient. The aim of this study was to compare the suitability of CPM and Vycross gels for superficial injection by examining how they diffuse when injected in the superficial reticular dermis and how they are integrated in the dermis.
METHODS
Gels examined. The two gels examined were Belotero® Balance (Anteis S.A., Geneva, Switzerland, a wholly owned subsidiary of Merz Pharmaceuticals GmbH) produced with CPM technology and Juvéderm Volbella® (Allergan, Pringy, France) produced with Vycross technology. The products are both approved in Europe, and Belotero Balance has also received United States Food and Drug Administration (FDA) approval. Both gels contain lidocaine, which is introduced during the crosslinking process by the manufacturers. Results for CPM gel were also compared with previous studies that have examined the ultrasound and histologic behavior of CPM gel without lidocaine.13
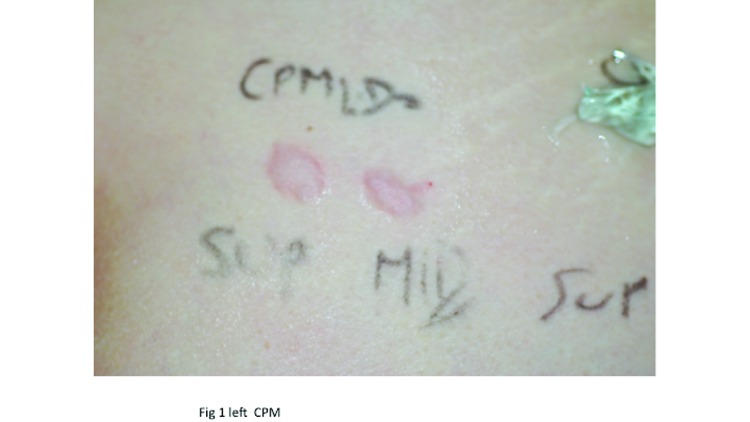
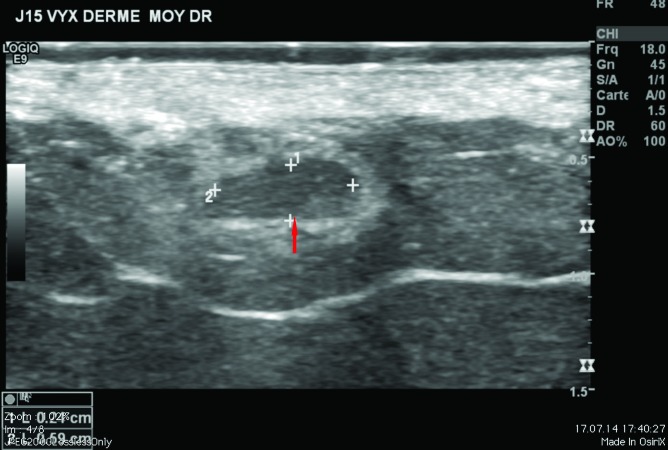
Ultrasound measurements of injected hyaluronic acid. The study was conducted in accordance with the ethical principles that had their origin in the Declaration of Helsinki. Two subjects participated in the study and both consented to have two injections of each HA gel (0.2mL/site) placed in the superficial reticular dermis of the buttocks, one product on either side. Both CPM and Vycross gels were injected using 30½ G (0.3 x 13mm) sharp needles under ultrasound control (Figure 1). Investigators used a General Electric logiQ E9® ultrasound instrument with a Hockey Stick L8 18i® probe (GE Healthcare, Little Chalfont, United Kingdom) set at 17MHz. To improve the visual image, an interface SonarAid GeistlichPharma® transducer (Wolhusen, Switzerland) lot 100353 was placed between the injected area and the ultrasound probe. All photographs were taken with a Nikon® digital camera D 40 X, lens AF Micro Nikkor 60mm 1:2.8D.
Figure 1.
Left: CPM superficial and mid-dermis injections. Right: Vycross superficial and mid-dermis injections.
Histologic analysis. For each product, two injections were performed approximately 2cm apart (Figure 1), one to obtain a biopsy following injection at Day 0 (after a 3-minute period of tissue stabilization) and the other for a biopsy at Day 15 and/or Day 90. Biopsies were obtained with a 4mm round punch, under local anesthesia with lidocaine 1% without epinephrine. Lidocaine was injected around the papule to avoid any interference with the implanted gel.5,7,8 Biopsy specimens were fixed in formalin and immediately sent to Viollier laboratory for preparation and staining with hematoxillin eosin, Alcian blue, and/or colloidal iron, followed by examination under a light microscope (Zeiss Axioskop 40) equipped with a camera (Olympus SC100).
Rheological tests. In addition to ultrasound and histologic examination, the two gels were also subjected to a series of simple rheological tests to provide further information on their tissue integration properties.
Resistance to stretch test. Without any manipulation or preparation, 0.2mL of each gel was pushed through the tip of a syringe into a Petri dish. The gels were then pinched with an Adson’s plier and stretched to obtain a thread as long as possible. A photo was taken of the gel at maximum stretch and the length noted using a measuring tape. For each gel, the test was performed a minimum of three times.
Cohesivity test. Similar to our previous study,14 0.2mL of each test gel was placed into 0.6mL of colored saline solution (dilution 1:3) by simple pressure on the syringe and the results observed and photographed. Two drops of ethanol 70% were then added with an Omnican® syringe and the recipient gently rotated, after which further photographs were taken.
Microscopic examination. The two gels were observed under a light microscope by spreading 0.1mL of each gel on a glass slide with a classic spatula used for blood examination. A solution of 0.069% toluidine blue was then placed on the gels for 30 seconds, after which the slides were rinsed twice with double distilled water. Adhesion to the slide during rinsing was examined. The slide was then covered and observed under a light microscope and photographed.
RESULTS
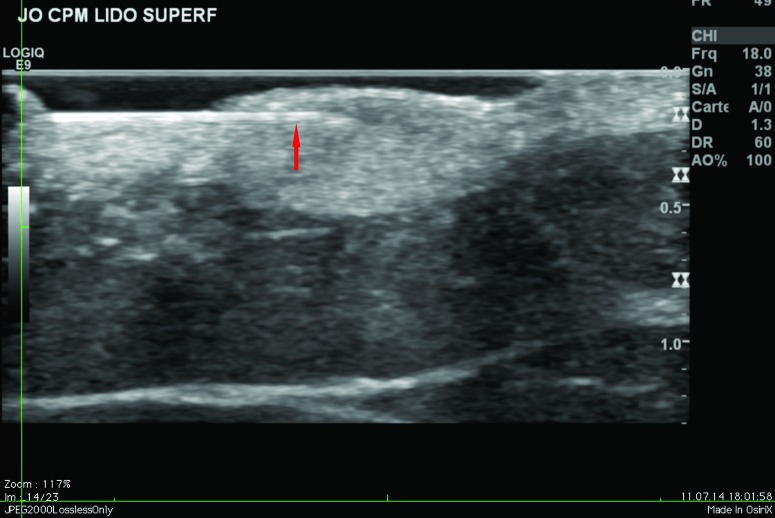
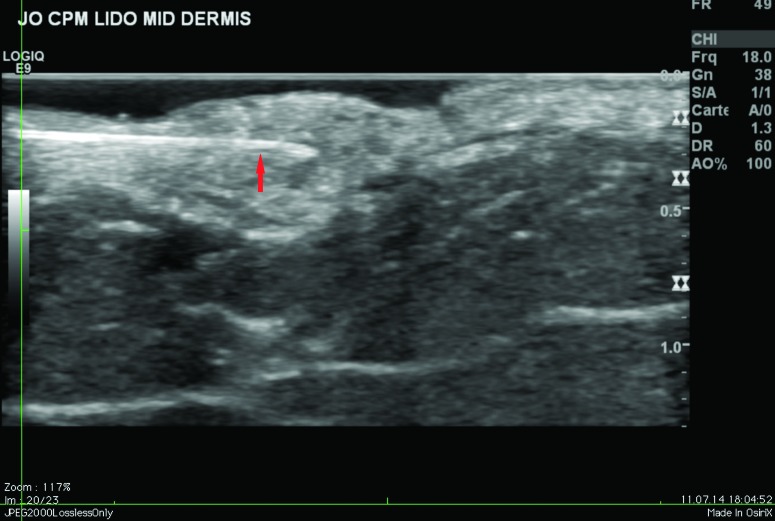
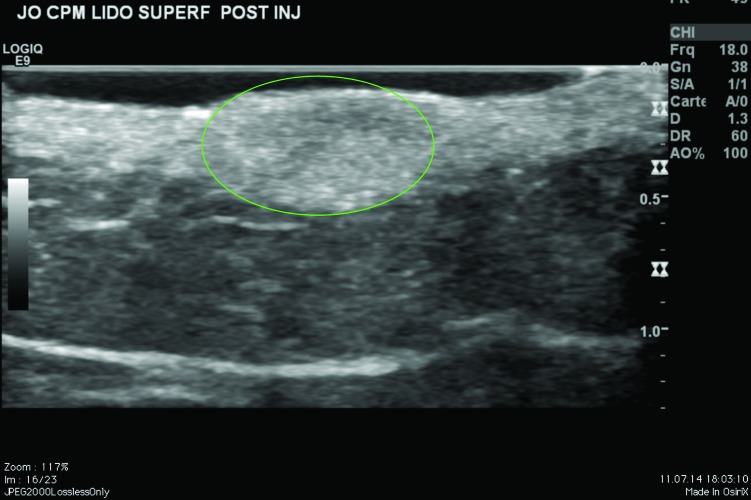
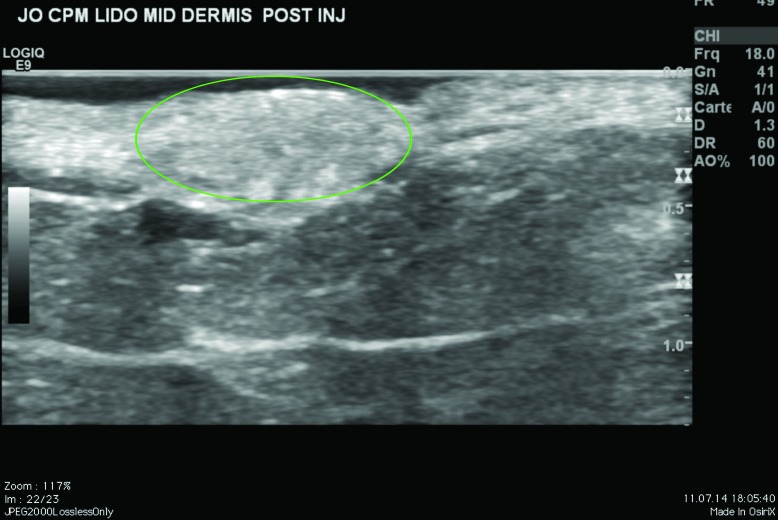
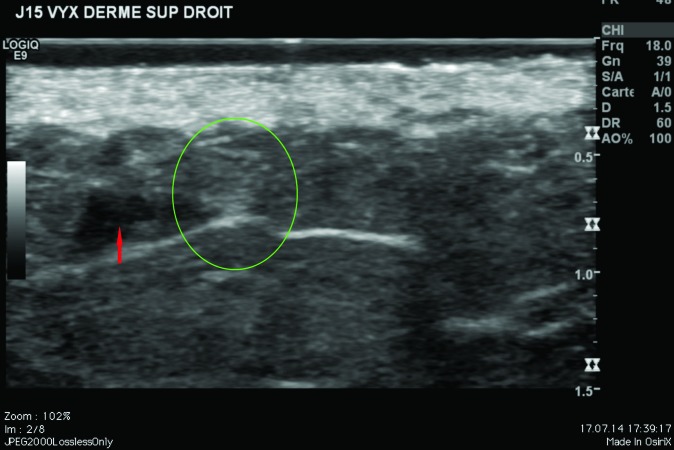
Ultrasound findings. After injection in the superficial reticular dermis of the buttocks, ultrasound findings at Day 0 showed CPM gel as an oblong, homogeneous papule, with an iso-echogenic aspect, compared with the non-injected surrounding dermis (Figure 2). There was no shadow around the border of the papule, indicating that there were no particles inside the gel to obstruct the ultrasound. There was also no leakage of product into the hypodermis on injection. Ultrasound observations for CPM gel with lidocaine at Day 15 were similar to those at Day 0. One subject was unavailable for ultrasound observations at Day 90, but ultrasound findings for CPM gel from the other subject were almost identical to those observed on Day 15.
Figure 2.
CPM ultrasound findings in the superficial and mid-reticular dermis at Day 0. Above left, superficial reticular dermis; above right, mid-reticular dermis, before withdrawal of the needle (red arrow); below left, superficial reticular dermis; below right, mid-reticular dermis after withdrawal of the needle showing perfect isoechogenicity between the dermis and the implant. Green circle = CPM implant.
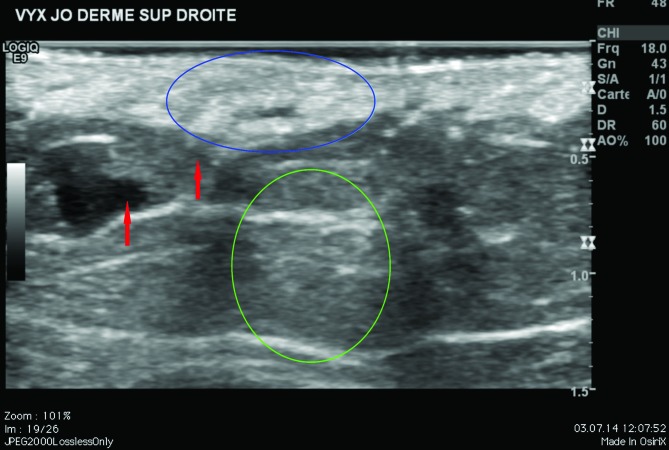
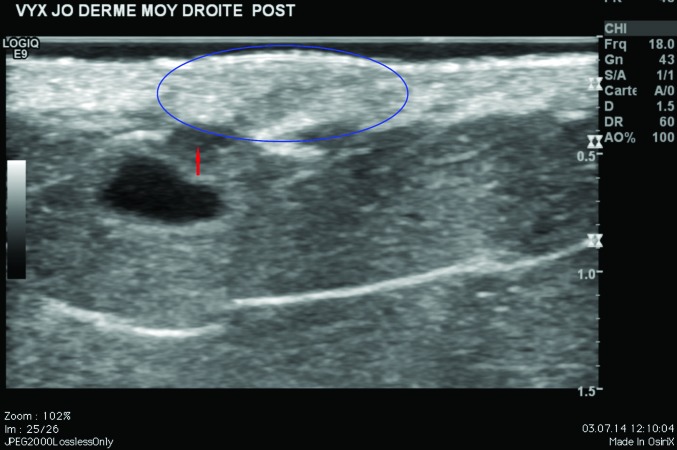
Ultrasound findings at Day 0 showed Vycross gel as a mixture of iso-and hypo-echogenic pools (Figure 3). In some areas, the gel was homogeneous and in others heterogeneous compared with the non-injected surrounding dermis. Ultrasound imaging revealed a leak of product into the hypodermis during the injection for both subjects. At Day 15 and Day 90, the ultrasound appearance was similar with a slight variation in the echogenicity and homogeneity. The leak into the hypodermis remained visible.
Figure 3.
Vycross ultrasound findings in the superficial and mid-reticular dermis at Day 0 and Day 15. Above left, Day 0 superficial reticular dermis; above right, Day 0 mid-reticular dermis. Below left, Day 15 superficial reticular dermis; below right, Day 15 mid-reticular dermis. Green circle = posterior ultrasound reinforcement. Blue circle = Vycross implant. Red arrow = leak in the hypodermis.
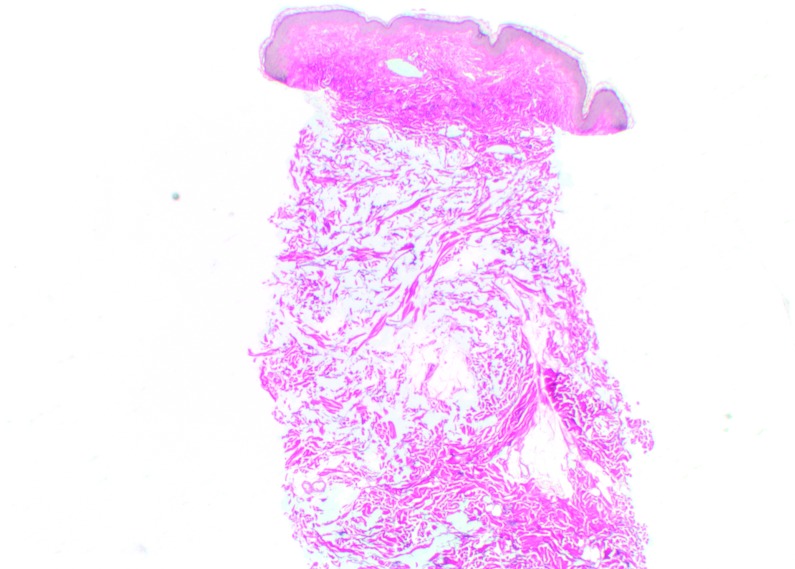
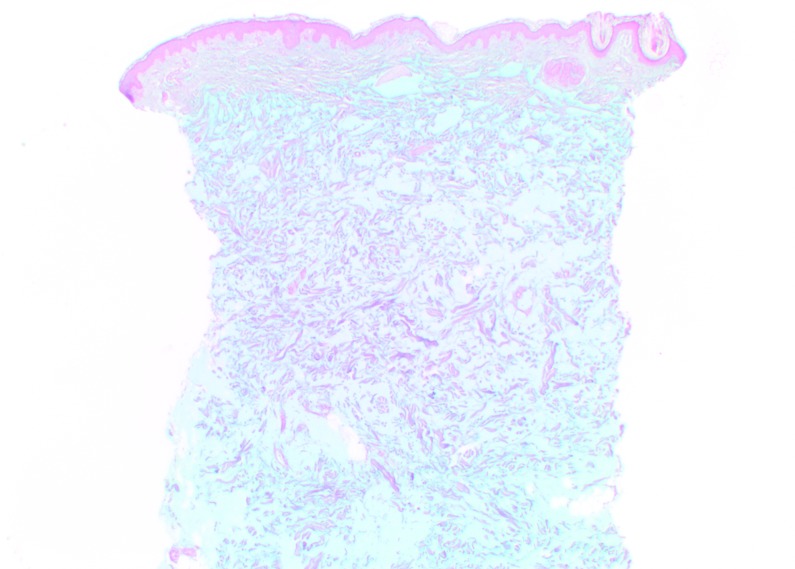
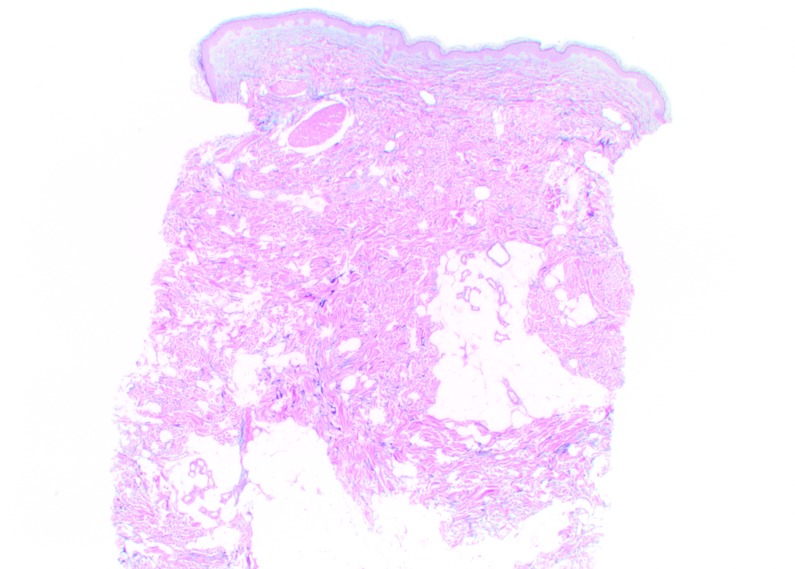
Histologic findings. At Day 0, CPM gel appeared homogeneously distributed and integrated among the collagen fibers, particularly in the mid-reticular dermis (Figure 4). Rare deposits were visible in the superficial and deep reticular dermis. Histologic analysis revealed no inflammatory reaction and no product in the hypodermis. When analyzed by a histopathologist (D.S.), the biopsy specimens of CPM gel with lidocaine were judged to be superimposable to those of previous specimens of CPM gel without lidocaine. Results at Day 15 for CPM gel with lidocaine were the same as those at Day 0. No inflammatory reactions were observed at any time during follow-up.
Figure 4.
CPM histologic findings in the superficial and mid-reticular dermis at Day 0 (above left and right images) and Day 15 (bottom left and right images). Magnification x12.5.
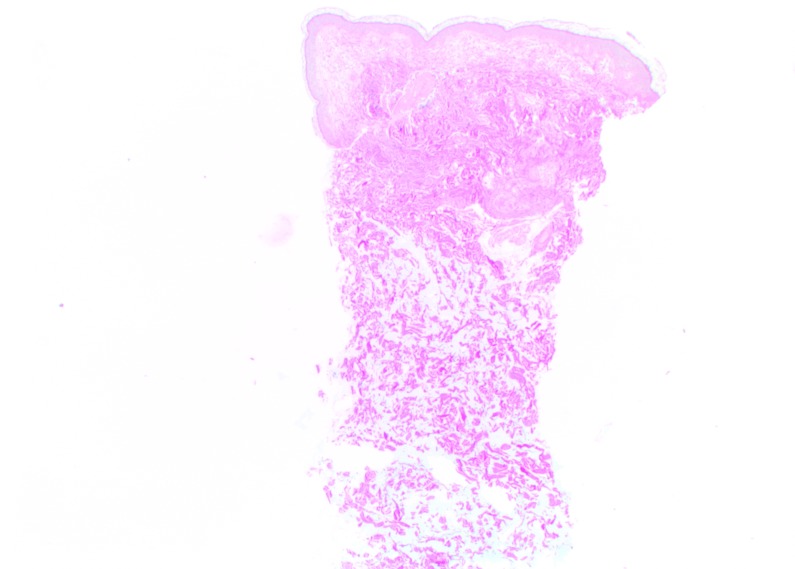
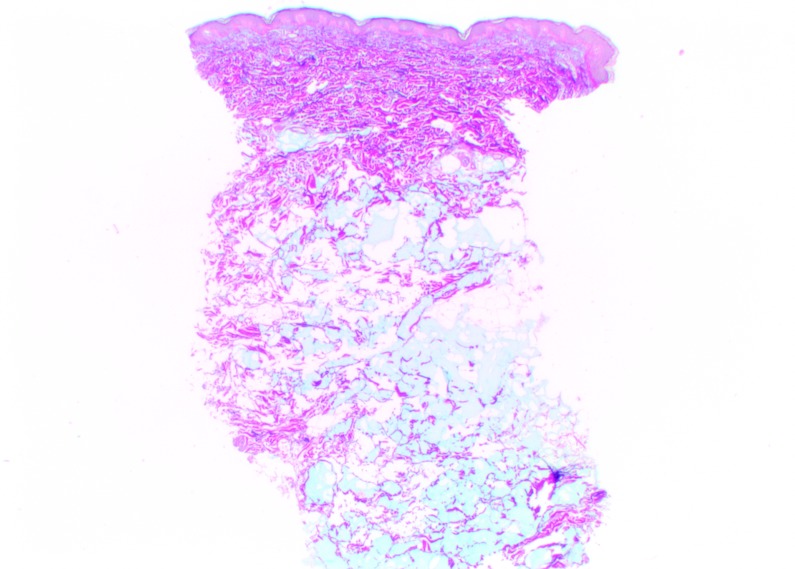
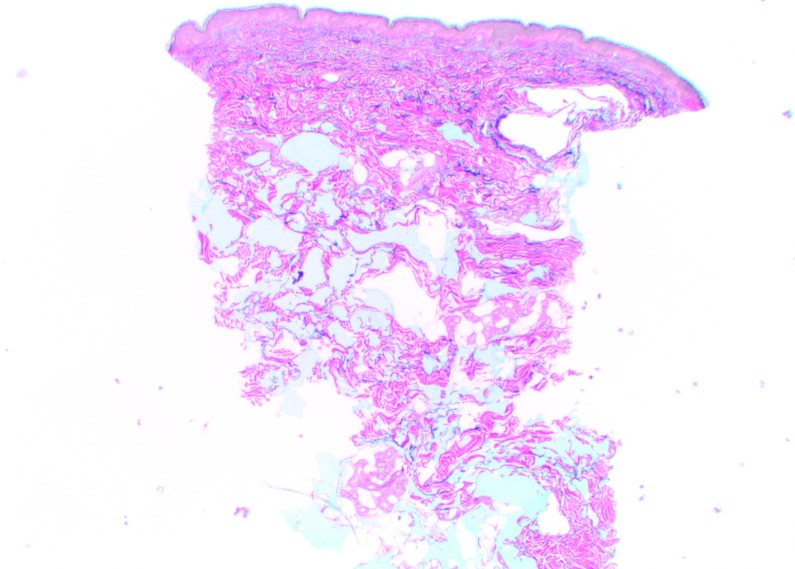
At Day 0, Vycross gel was visible as pools of very variable dimensions throughout the depth of the dermis, but mostly in the superficial and mid-dermis (Figure 5). Results were similar at Day 90, but with a visible leak into the superficial hypodermis. No inflammatory reactions were observed at any time during follow-up.
Figure 5.
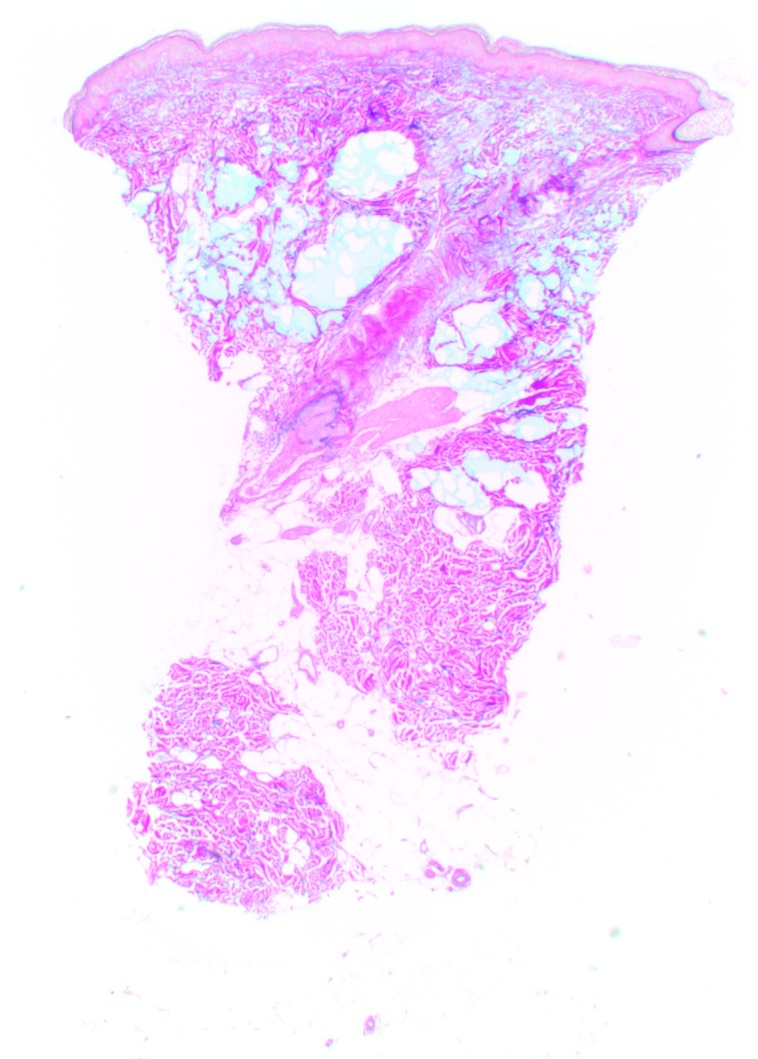
Vycross histologic findings in the superficial and mid-reticular dermis at Day 0 (above left and right images) and Day 15 (bottom left and right images). Magnification x12.5.
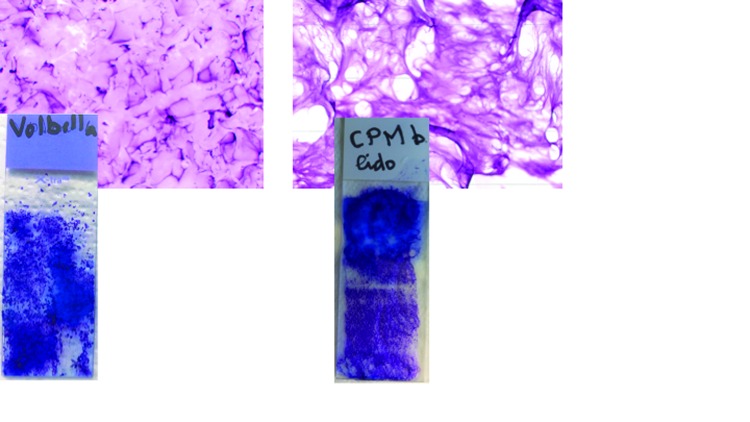
Rheological examinations. Resistance to stretch test. CPM gel with lidocaine could be stretched to a distance of 3 to 5cm. Vycross gel could not be drawn to a distance greater than 1cm without breaking (Figure 6).
Figure 6.


Results of cohesivity and resistance to stretch tests for CPM (left), and Vycross (right). The arrows in the CPM image show the gel visible as a single continuous strand. In the Vycross image, the arrows point to dispersed areas of gel illustrating its more particulate nature.
Cohesivity test. In contact with saline solution, CPM gel with lidocaine remained as a single continuous strand, even after the addition of ethanol. The gel is described as cohesive. Within seconds of contact with saline solution, Vycross gel dispersed into numerous rod shapes (Figure 6). The addition of ethanol increased this process. These results suggest that Vycross gel can be described as partially cohesive and are in line with results published elsewhere.15
Microscopic examination of the pure gels in the laboratory. A difference in adhesion was noted when preparing the slides for examination, particularly when spreading the gels. CPM gel with lidocaine was very adherent to the glass slide. It appeared as a cloud shape on the slide with some areas more colored than others. This may correspond to the polydensified nature of the gel, but could also be the result of the superimposition of several coats of gel with a light coloration. Under microscopic examination, no particles were observed, in agreement with the non-particulate nature of CPM gel (Figure 7).
Figure 7.
Above, microscopic examination of CPM and Vycross gels, magnification x25. Vycross appeared particulate in nature, while no particles were observed with CPM. Below, macroscopic examination of the gels after spreading. Vycross was poorly adherent to the glass slide, while CPM gel with lidocaine was very adherent.
Vycross gel had a high viscosity and resistance to spreading. It was poorly adhesive to the glass slide during spreading (Figure 7), but was adherent during rinsing with double distilled water. On microscopic examination, it appeared as extremely fine, closely packed beans, suggesting a particulate nature similar to NASHA gels.
DISCUSSION
This study used ultrasound imaging to demonstrate that both CPM and Vycross gels can be injected in the superficial reticular dermis, but with differences in tissue distribution. The results complement those of previous studies, which conducted ultrasound and histologic examination of three HA gels, including CPM gel without lidocaine, following injection in the superficial reticular dermis.5,13 Neither CPM nor Vycross gel with lidocaine were available at the time of these studies, which showed that HA gels integrate in the dermis in predictable patterns.5,13
In the current study, ultrasound imaging showed CPM gel with lidocaine as a homogeneous papule with the same density as the surrounding tissue and even integration. There was no cone of shadow, which is observed with some particulate HA gels and is thought to be a result of the particles creating an obstacle for the ultrasound and causing a shadow.13 The ultrasound findings from this study of CPM gel with lidocaine were consistent with those from a previous study which examined injection of CPM gel without lidocaine in the superficial reticular dermis.13 The addition of lidocaine by the manufacturer therefore has no effect on the tissue distribution of CPM gel. Tissue biopsies from the injection sites were examined histologically and confirmed the ultrasound results at Day 0 and Day 15. Previous studies have also shown that CPM gel without lidocaine diffuses in a similar, harmonious manner throughout the depth of the dermis.6–8
On ultrasound imaging, Vycross gel was visible as variable-sized pools throughout the depth of the dermis with varying levels of echogenicity. A leak of product into the hypodermis was observed and was confirmed by histologic analysis, which could mean that the product diffuses from the site of injection. There was no cone of shadow with Vycross although a posterior reinforcement was seen. This could be a consequence of the ultrasound passing through a very liquid (hypoechoic) substance relative to the surrounding tissue, or a result of tissue compression around the papule by injection of the gel itself, thereby creating a “pseudocapsule,” denser and more echogenic than the surrounding tissue.
The treatment of fine lines and wrinkles requires a product that can be placed superficially with no risk of visibility, palpability, or diffusion. The consistency of collagen-based products made them popular treatments for this purpose, but their short longevity (8 to 12 weeks) and requirement for skin testing limited their clinical use. Two HA gels currently marketed for injection in the superficial or mid-reticular dermis are CPM (Belotero Balance) and Vycross (Juvéderm Volbella). The authors’ ultrasound and histologic results show that there are differences in the tissue distribution of these gels within the dermis. The simple tests performed in their laboratory provide further information on how the gels integrate in the dermis and can be replicated by anyone in private practice. CPM gel remained totally cohesive when mixed with a colored saline solution, whereas Vycross gel dispersed into numerous rod shapes, suggesting partial cohesivity. A more sophisticated and standardized version of this assay has recently been developed and used to test the cohesivity of a number of HA products including CPM gel.15 A gel that disperses into separate pools and diffuses into deeper planes will likely require larger volumes of product to correct the defect. In the current study, the cohesivity of CPM gel was also demonstrated by its high resistance to stretch (3.5–5cm) compared with a lower resistance for Vycross gel (1cm). Similar results have been observed in previous studies for CPM gel without lidocaine.14
Cohesivity is one of a number of properties that determine a gel’s depth of injection and aesthetic indications. The CPM gel in the current study has high cohesivity, which maintains gel integrity, along with low elasticity (G prime) and a high tan delta (balance of elasticity versus viscosity).16 Together, these properties contribute to its soft, flowing qualities and its seamless dermal integration allowing CPM gel to interweave among the collagen bundles without breaking up into separate pools. The homogeneous tissue distribution and lack of particulate matter also reduce the risk of visible and palpable material following superficial injection and are thought to explain why the Tyndall effect does not occur with CPM gel.17,18 In contrast, the bluish discoloration has been reported when other HA fillers (e.g., NASHA) are injected too superficially in the dermis.19 Delayed-onset nodules and granuloma formation have been reported with HA products and may be influenced by particle size and surface properties of the injected product.20–23
The results of this study were obtained from the buttock area and were based on a small number of volunteer subjects. In addition, the gels were injected under ultrasound control and so their placement in the superficial reticular dermis could be confirmed. In clinical practice, injection in this plane is achieved by the angle of injection and injector experience using the blanching technique. The blanching effect (similar to that formerly observed with collagen injections) remains for only a few minutes and is proof that the product has been injected as superficially as possible.8 Furthermore, the technique requires only a small volume of product per wrinkle, allowing many areas to be treated with one syringe. The results of this ultrasound and histologic analysis illustrate the homogenous tissue integration and cohesive nature of CPM gel with lidocaine, and comparisons with other HA gels in similarly designed studies13 suggest that it has the optimal properties for injection in the superficial reticular dermis.
ACKNOWLEDGMENT
The authors would like to thank the Institut MedImage, Geneva, and the Laboratory of Histopathology Viollier, Geneva, Switzerland, for their collaboration and for the use of their facilities. They would also like to thank the manufacturers of the products used for the answers to their questions. The authors wish to acknowledge the contribution of Jenny Grice for assistance with providing a final version of this manuscript. Editorial assistance was funded by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
Footnotes
Disclosure:Editorial assistance was funded by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany. Dr. Micheels serves as a clinical investigator for Merz and was compensated for his work on this study. None of the other authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
REFERENCES
- 1.Klein A. Implantation techniques for injectable collagen: two and one half years of personal clinical experience. J Am Acad Dermatol. 1983;9:224–228. doi: 10.1016/s0190-9622(83)70133-7. [DOI] [PubMed] [Google Scholar]
- 2.Klein A. Indications and implantation techniques for various formulations of injectable collagen. J Dermatol Surg Oncol. 1988;14(Suppl 1):27–30. [Google Scholar]
- 3.Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging. 2007;2:369–376. doi: 10.2147/cia.s1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Della Volpe C, Andrac L, Casanova D, et al. La diversité de la peau: etude histologique de 140 résidus cutanés, adaptée à la chirurgie plastique. Ann Chir Plast Esthét. 2012;57:423–449. doi: 10.1016/j.anplas.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono- and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–643. doi: 10.1111/j.1524-4725.2010.01852.x. [DOI] [PubMed] [Google Scholar]
- 6.Micheels P, Besse S, Sarazin D, et al. Quantifying depth of injection of hyaluronic acid in the dermis: data from clinical, laboratory, and ultrasound settings. J Drugs Dermatol. 2016;15:611–618. [PubMed] [Google Scholar]
- 7.Tran CH, Carraux P, Micheels P, et al. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228:47–54. doi: 10.1159/000354384. [DOI] [PubMed] [Google Scholar]
- 8.Micheels P, Sarazin D, Besse S, et al. A blanching technique for intradermal injection of the hyaluronic acid Belotero. Plast Reconstr Surg. 2013;132(4) Suppl 2:69S–76S. doi: 10.1097/PRS.0b013e31829a02fb. [DOI] [PubMed] [Google Scholar]
- 9. Belotero® Balance Lidocaine instructions for use. Merz Aesthetics, Frankfurt am Main, Germany, IMD1-2 Lidocaine IfU, EU, ver.3.0, 13.04.2015.
- 10. Juvéderm® Volbella™ with Lidocaine [package insert]. Pringy, France: Allergan, Inc; 2015.
- 11.Flynn TC, Thomson D, Hyun S-H, Howell D. Ultrastructural analysis of 3 hyaluronic acid soft tissue fillers using scanning electron microscopy. Dermatol Surg. 2015;41:S143–S152. doi: 10.1097/01.DSS.0000452647.14389.a7. [DOI] [PubMed] [Google Scholar]
- 12.Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5) suppl:139S–148S. doi: 10.1097/PRS.0000000000001734. [DOI] [PubMed] [Google Scholar]
- 13.Micheels P, Besse S, Flynn TC, et al. Superficial dermal injection of hyaluronic acid soft tissue fillers: comparative ultrasound study. Dermatol Surg. 2012;38(7 Pt 2):1162–1169. doi: 10.1111/j.1524-4725.2012.02471.x. [DOI] [PubMed] [Google Scholar]
- 14.Micheels P, Sarazin D, Tran C, Salomon D. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15:600–606. [PubMed] [Google Scholar]
- 15.Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. doi: 10.1097/PRS.0000000000001638. [DOI] [PubMed] [Google Scholar]
- 16.Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4) Suppl 2:5S–21S. doi: 10.1097/PRS.0b013e31829d1d40. [DOI] [PubMed] [Google Scholar]
- 17.Hevia O, Cohen BH, Howell DJ. Safety and efficacy of a cohesive polydensified matrix hyaluronic acid for the correction of infraorbital hollow: an observational study with results at 40 weeks. J Drugs Dermatol. 2014;13:1030–1036. [PubMed] [Google Scholar]
- 18.Huber-Vorländer J, Kürten M. Correction of tear trough deformity with a cohesive polydensified matrix hyaluronic acid: a case series. Clin Cosmet Investig Dermatol. 2015;8:307–312. doi: 10.2147/CCID.S84117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morley AM, Malhotra R. Use of hyaluronic acid filler for tear-trough rejuvenation as an alternative to lower eyelid surgery. Ophthal Plast Reconstr Surg. 2011;27:69–73. doi: 10.1097/IOP.0b013e3181b80f93. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232–239. doi: 10.5999/aps.2015.42.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beleznay K, Carruthers JD, Carruthers A, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939. doi: 10.1097/DSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 22.Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42:31–37. doi: 10.1097/DSS.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 23.Goodman GJ. An interesting reaction to a high- and low-molecular weight combination hyaluronic acid. Dermatol Surg. 2015;41(Suppl 1):S164–S166. doi: 10.1097/DSS.0000000000000257. [DOI] [PubMed] [Google Scholar]