Abstract
Teledermatology is a rapidly expanding niche within telemedicine still in its infancy. It has become increasingly more feasible in recent years with the expansion of information and communication technologies. Here, the authors present the details of their collaboration and propose a model for constructing a teledermatology network. In the year 2014, the authors’ Dermatopathology institute in Torrance, California, partnered with Mbingo Baptist Hospital, a tertiary referral center in Cameroon, Africa. During this time, 145 cases were received. The authors present highlights of specific cases as well as the strengths and challenges encountered. They have found the use of the store and forward method to be an effective tool with an acceptable concordance rate. With basic bandwidth speeds and images taken with smart devices shared via email, providers are given the unique opportunity to provide specialty care and alleviate disease burden where dermatology and dermatopathology resources are scarce.
TELEMEDICINE WAS A TERM first coined in the 1970s by Thomas Bird, although its accelerated growth did not occur until recent years owing its success to the advent of the internet and now the wide ownership of personal smartphones and tablet devices.1 Some authors quote 40 years as the length of time for which telemedicine has been in existence although its rudimentary manifestations can be traced back to 1906, when Dutch physiologist, Willem Einthoven, recorded electric cardiac signals from patients at a distant hospital.2 With the readiness of modern information and information communication technologies (ICTs), providers such as ourselves have been given the distinct opportunity to expand access to care to underserved populations in remote areas and in lesser world countries.
The visual nature of dermatology has lent itself to the application of telemedicine and is now one of the leading operational and dynamic specialties within this field among others including teleradiology, telepathology, and telepsychology.3 Data from the American Telemedicine Association in 2012 indicates 38 active teledermatology programs in the United States, not including the authors’ program. The majority of them are associated with major universities.4
In 2014, the authors were given the unique opportunity to collaborate with physicians at Mbingo Baptist Hospital (MBH) in Cameroon, Africa in order to deliver dermatopathology consultations to an area in great need of specialty consultation. Mbingo Baptist Hospital serves as a tertiary referral center allowing for a wide diversity of challenging cases. Starting in January of 2014, the authors received their first set of cases in the form of glass slides. Soon thereafter, information was received as digital photos of clinical and histopathology. Their Dermatopathology Institute in Torrance, California, shared cases in consultation with dermatology residents to assist in triaging cases and to enhance the teaching curriculum. During this time, they successfully rendered diagnoses, which were then relayed back to physicians in Cameroon. Several barriers were encountered including time zone differences, limited bandwidth, technical resources, additional medical subspecialties, and available treatment options. The authors aim to provide a summary overview of the cases and to discuss their personal experiences with teledermatology, its challenges, and future directions.
DESIGN
January through December 2014, there were in total 145 cases transmitted to the authors’ Dermatopathology Institute from MBH. The data was collected and compiled into an online shared excel sheet. It was then broadly categorized by the type of information received: glass slides (GS), clinical photos (CP), histopathological photos (HP), or clinicohistopathological photos (CHP). In the early stages of the program, a batch of 11 glass slides, each with an associated clinical history was received. Shortly after, digital photos taken by smartphones and tablets were transmitted to the authors via email. Of these digital photos, 19 stand-alone clinical photos, 85 stand-alone histopathological digital photos, and 30 cases with both clinical and histopathological photos were received. Selected cases were shared with the dermatology residents of Western Health Sciences Osteopathic College of Medicine and Harbor-UCLA Medical Center.
OVERVIEW AND HIGHLIGHTS OF CASES RECEIVED
Within each of the four categories, a brief overview of the cases by disease type is provided. Several exemplary cases are presented in greater detail.
Glass slides. Among the slides, seven were dermatopathology. Of the dermatopathology slides, 3 of the 7 were neoplasms including invasive squamous cell carcinoma, Kaposi’s, and metastatic breast cancer. The other three dermatopathology slides included granuloma annulare, acute ulceration with bacterial colonization, and chronic ulceration with granulation tissue. The remaining four were general pathology.
Clinical photos. Workup revealed diverse pathologies both of the common variety as well as rare conditions, ranging from benign to neoplastic. The majority of cases were infectious, including a human papillomavirus (HPV)-related lesion, a viral exanthem, molluscum contagiosum, folliculitis barbae with keloids, and candidal infection. The next largest category included the papulosquamous and eczematous dermatoses: two cases of contact dermatitis and one case of eczema. Of the urticarial and erythematous lesions were photodermatitis and a drug-reactive rash. Two were diagnosed as disorders of keratinization: one case of icthyosis and one case of keratoderma with the differential including Mal de Meleda versus Papillon Lefevre syndrome. In addition there was one keloid, one case of vitiligo, two genodermatoses including neurofibromatosis and possible Melkersson-Rosenthal syndrome. One case yielded chronic ulceration secondary to possible infection, pyoderma gangrenosum, or a foreign body reaction. Lastly, there was one case of a left ulcerated thigh mass that appeared to be possible psoriasis, fungal, or seborrheic dermatitis with secondary impetiginization.
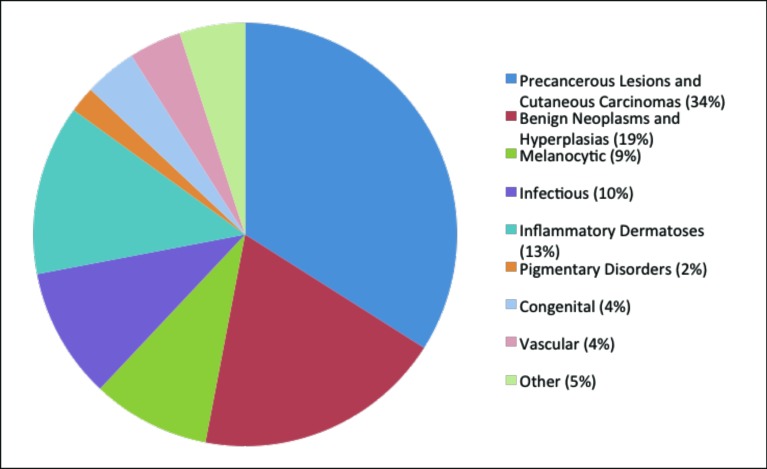
Histopathology. The largest category consisted of the precancerous and cancerous lesions (34%), followed by benign neoplasms and hyperplasias (19%), and lastly the melanocytic lesions (9%) (Figure 1). Fifteen of the 84 cases were Kaposi’s sarcoma. This finding is consistent with Cameroon being both a Kaposi’s sarcoma and human immunodeficiency virus (HIV) endemic area. Of 14 million inhabitants in Cameroon, 11.8 percent are currently living with HIV.5 Acquired immune deficiency syndrome (AIDS)-related Kaposi’s is the most common form and is also highly aggressive in its clinical course, making this disease a crucial target for teledermatology programs serving areas like these. Beyond these cases, several other malignant neoplasms, such as squamous cell carcinoma, basal cell carcinoma, dermatofibrosarcoma, and two cases of acral malignant melanoma, were identified. The remaining fell into a variety of categories and are enumerated in greater detail in Table 1.
Figure 1.
Categorical distribution of stand-alone histopathological digital photos
TABLE 1.
Summary of stand-alone histopathological digital photos
| DISEASE CATEGORY | DIAGNOSIS | NO. CASES, N |
|---|---|---|
| Precancerous lesions and cutaneous carcinomas | Kaposi’s sarcoma | 15 |
| Malignant spindle cell tumor | 3 | |
| Squamous cell carcinoma | 3 | |
| Basal cell carcinoma | 2 | |
| Large cell lymphoma | 2 | |
| Dermatofibrosarcoma | 1 | |
| Eccrine porocarcinoma | 1 | |
| Spindle cell sarcoma | 1 | |
| Verrucous carcinoma | 1 | |
| Benign neoplasms and hyperplasias | Dermatofibroma | 3 |
| Epidermal hyperplasia | 3 | |
| Angioleiomyoma | 1 | |
| Angiolipoma | 1 | |
| Benign spindle cell tumor | 1 | |
| Eccrine poroma | 1 | |
| Glomangioma | 1 | |
| Hamartoma | 1 | |
| Proliferating pilar tumor | 1 | |
| PEH with stasis dermatitis | 1 | |
| Seborrheic keratosis | 1 | |
| Scar with granulation tissue | 1 | |
| Melanocytic | Compound melanocytic nevus | 2 |
| Acral malignant melanoma | 2 | |
| Conjunctival nevus | 1 | |
| Intradermal melanocytic nevus | 1 | |
| Melanoma | 1 | |
| Nodular Hidradenoma | 1 | |
| infectious | Abscess | 4 |
| Draining osteomyelitic sinus | 1 | |
| Histoplasmosis | 1 | |
| Histoid lepromatous leprosy | 1 | |
| Mucormycosis | 1 | |
| Tapeworm larva | 1 | |
| inflammatory Dermatoses (vesiculobullous) | Lichenoid dermatitis | 3 |
| Chronic spongiotic dermatitis | 2 | |
| Bullous pemphigoid | 1 | |
| Caseating granulomatous dermatitis | 1 | |
| Grover’s disease | 1 | |
| Lichenoid mucositis | 1 | |
| Non-caseating granulomatous dermatitis | 1 | |
| Pemphigus foliaceus | 1 | |
| Pigmentary Disorders | Vitiligo | 1 |
| Post inflammatory hyperpigmentation | 1 | |
| Congenital | Epidermal nevus | 1 |
| Ichthyosis | ||
| Acrokeratosis verruciformis of Hopf | ||
| Vascular | Hemangioma | 2 |
| Arterio-venous malformation | 1 | |
| other | Panniculitis | 2 |
| Atypical mononuclear cell infiltrate | 1 | |
| Diverticulum with HPV cytopathic changes | 1 |
No.=number; PEH=pseudoepitheliomatous hyperplasia
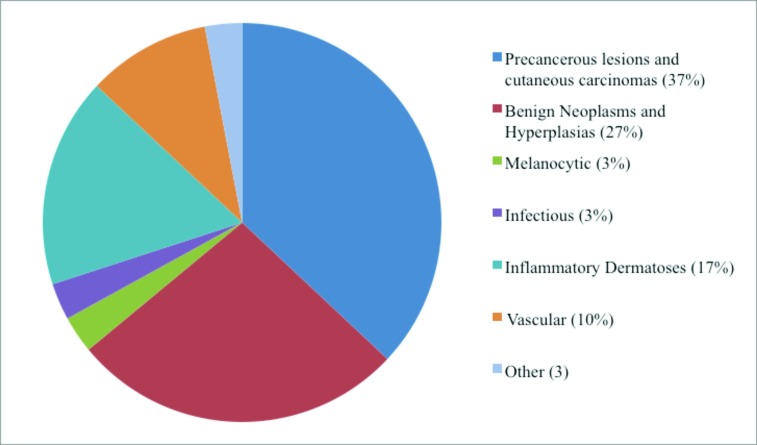
A summary of the cases with both clinical and histopathological photos (Table 2) and their categorical distributions (Figure 2) are provided below.
TABLE 2.
Summary of cases with clinical and histopathological photos
| DISEASE CATEGORY | DIAGNOSIS | NO. CASES, N |
|---|---|---|
| Precancerous lesions and cutaneous carcinomas | Kaposi’s sarcoma | 3 |
| Adenosquamous cell carcinoma | 1 | |
| Cytologically malignant neoplasm | 1 | |
| Collision tumor* | 1 | |
| Eccrine adenocarcinoma | 1 | |
| Eccrine porocarcinoma | 1 | |
| Poorly differentiated malignancy | 1 | |
| Syringomatous carcinoma | 1 | |
| Benign Neoplasms and Hyperplasias | Lichen simplex chronicus | 3 |
| Eccrine poroma | 2 | |
| Cranial fasciitis | 1 | |
| Dermatofibroma | 1 | |
| Verrucous vulgaris | 1 | |
| Melanocytic | Melanoma | 1 |
| Infectious | Elephantiasis | 1 |
| Inflammatory Dermatoses (vesiculobullous) | Chronic spongiotic dermatitis | 1 |
| Discoid lupus erythematosus | 1 | |
| Interface dermatitis | 1 | |
| Intraepidermal vesicular dermatitis dermatitis | 1 | |
| Lichen planus | 1 | |
| Vascular | Hemangioma | 2 |
| Nodular vasculitis | 1 | |
| Other | Atypical spindle cell proliferation | 1 |
Sarcomatoid squamous cell carcinoma and basal cell carcinoma
Figure 2.
Categorical distribution of clinical and histopathological photos
HIGHLIGHTS OF SPECIFIC CASES
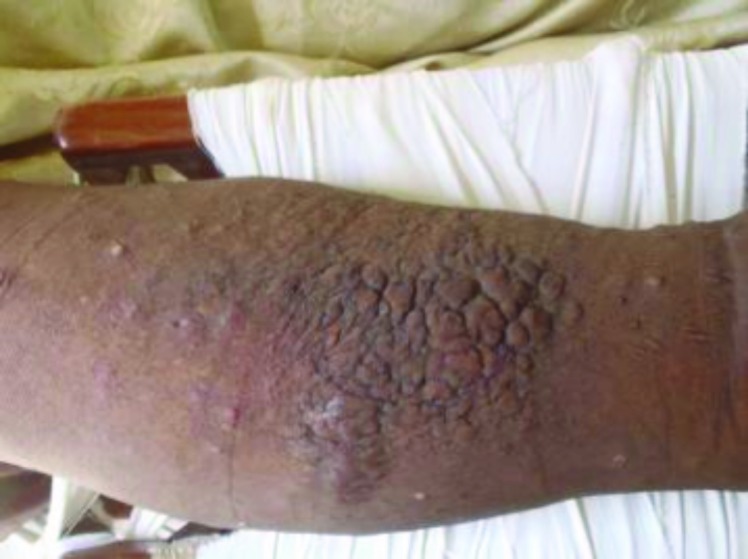
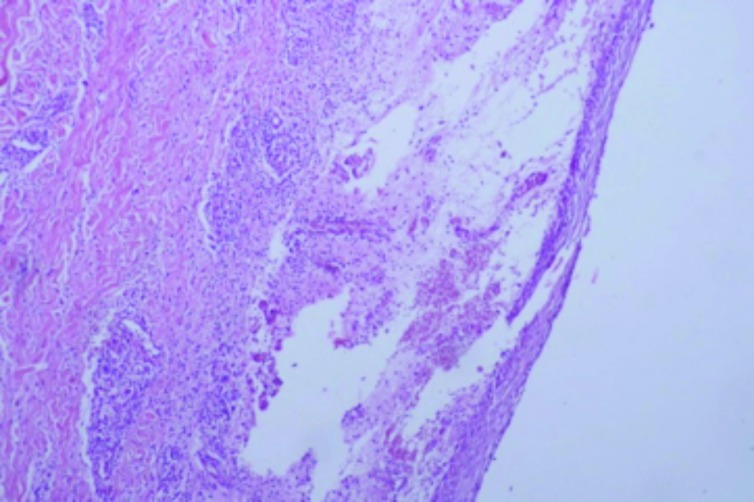
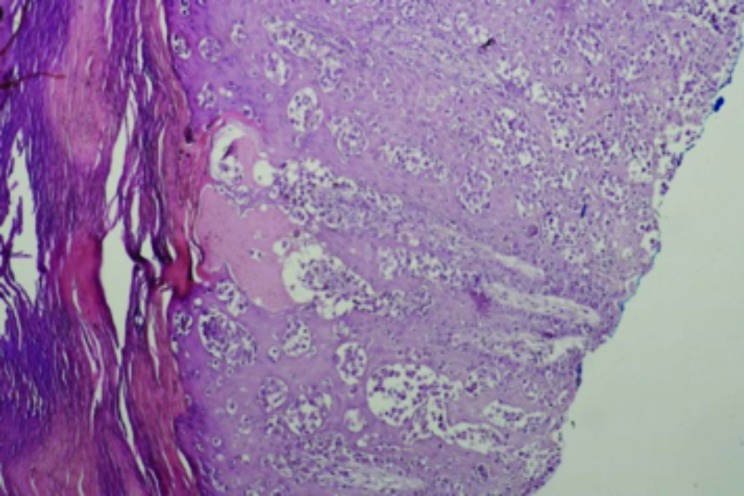
Case 1. This was a classic case of elephantiasis nostras verrucosa. A 43-year-old woman presented with a 12-year history of unilateral right leg swelling that was painful and itchy. Examination revealed nonpitting edema of the right leg with hyperkeratotic plaques and scattered pustules (Figure 3). A skin biopsy was performed consistent with the diagnosis of lymphatic filariasis (Figure 4). Among the causative parasitic worms, Wucheria bancrofti is the most common cause in Cameroon, closely followed by onchocerciasis. Both conditions can be effectively treated with diethylcarbamazine or ivermectin.
Figure 3.
Nonpitting edema of the right leg with hyperkeratotic plaques and scattered pustules.
Figure 4.
Chronic lymphedema and scarring (x10)
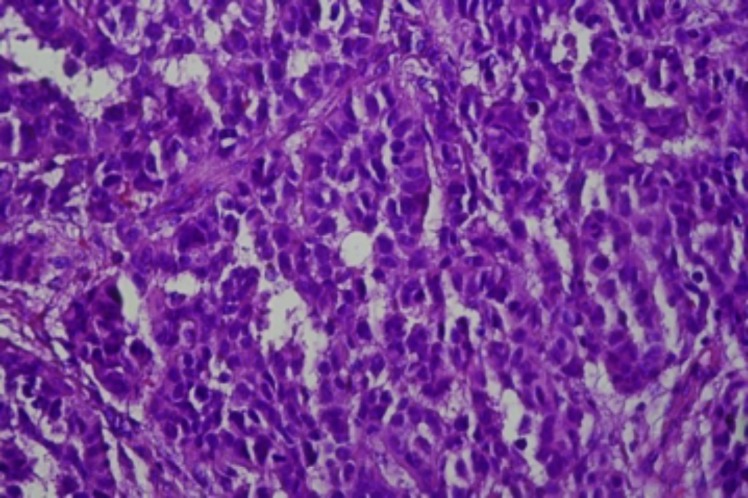
Case 2. This was an advanced case of acral malignant melanoma in a 70-year-old man, which was originally suspected to be basal cell carcinoma. He presented with a fungating mass on the right big toe for five months. There was associated hyperpigmentation and hardening of the skin of the right leg. Biopsy revealed an acral malignant melanoma, a common variant occurring among Africans (Figures 5 and 6). A complete amputation of the patient’s right toe was performed. No additional follow-up could be obtained after eight months.
Figure 5.
Acral skin biopsy with a proliferation of pleomorphic melanocytes showing considerable upward intraepithelial spread as well as extension to the biopsy margins (x10)
Figure 6.
Pleomorphic melanocytes (x40)
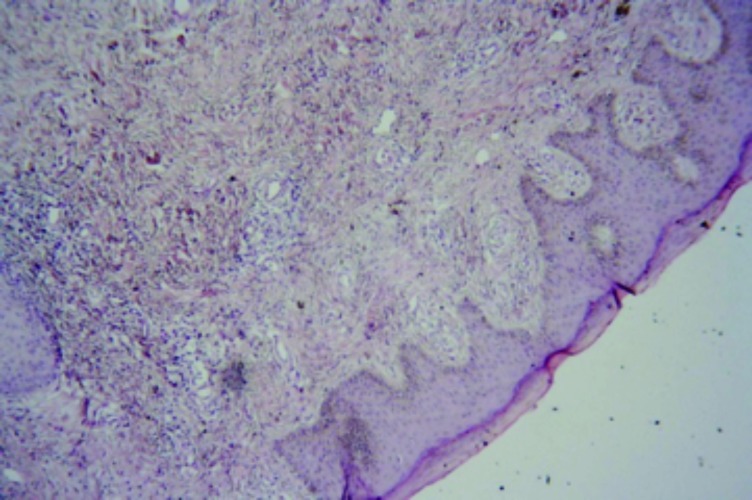
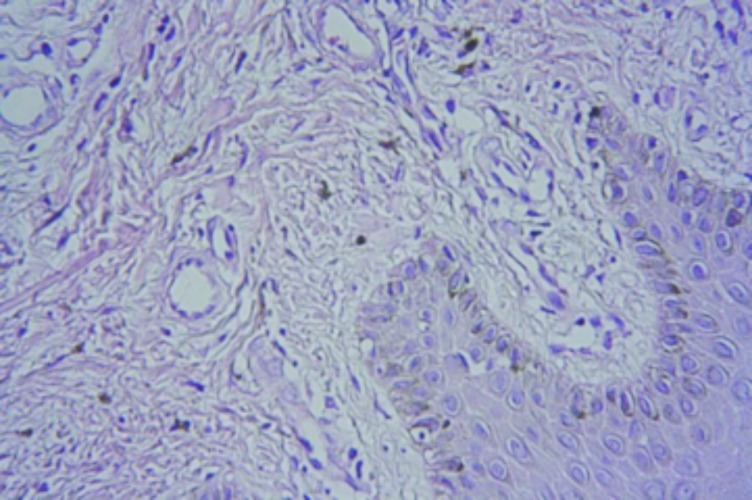
Case 3. This was a case suspicious for Kaposi’s sarcoma in a 43-year-old man. The patient had a history of swelling in the right leg. The histopathology was indefinite with features suggestive of a chronic lymphedema (Figures 7 and 8). However, there was an accompanying atypical vascular proliferation. In this setting of an HIV+ patient, early patch stage Kaposi’s sarcoma could not be excluded. The tissue paraffin block was sent to the author’s laboratory in the United States and additional immunohistochemical studies were performed with antibodies directed against HHV-8. This antibody was negative. An additional hematoxylin and eosin stain (H&E) section was also obtained. Thus, although the histopathology was still suspicious for Kaposi’s sarcoma, the HHV-8 antibody stain was negative and a definitive diagnosis of Kaposi’s sarcoma could not be established.
Figure 7.
Chronic lymphedema with a proliferation of capillary-sized vessels suspicious for Kaposi’s sarcoma. HHV-8 immunohistochemical antibody stains were negative (x10).
Figure 8.
Higher power micrograph (x40)
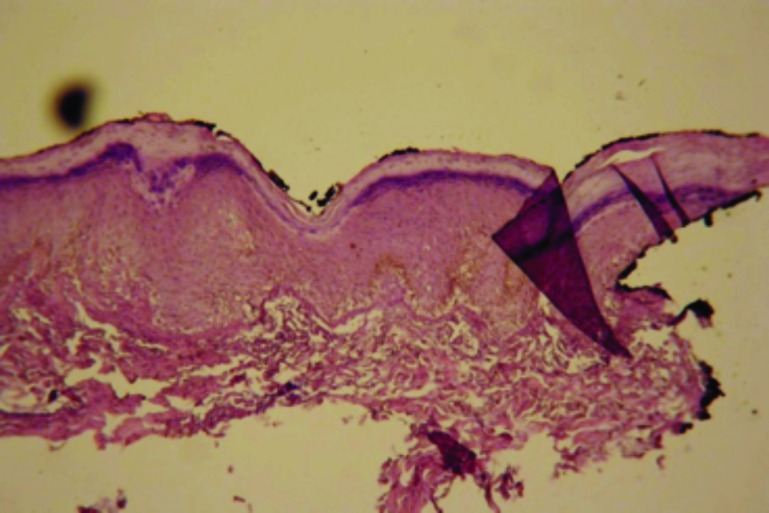
Case 4. This case of an 18-year-old woman illustrates the diversity of diseases and the importance of accurate clinicopathological correlation. The histopathology was interpreted as a cell-poor interface dermatitis (Figure 9). Additional clinical information was obtained.
Figure 9.
Cell-poor interface dermatitis with slight increase in dermal mucinosis (x20)
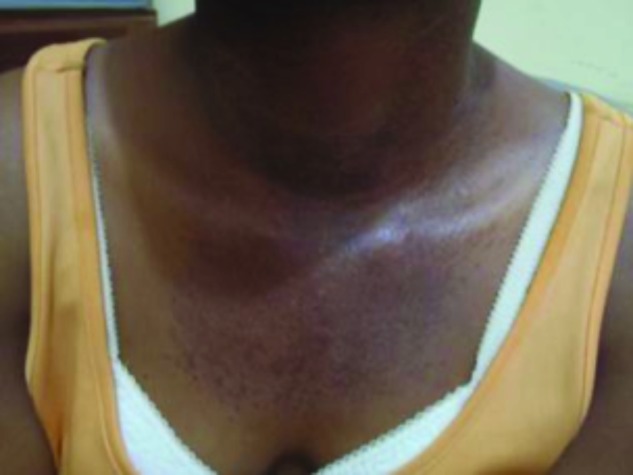
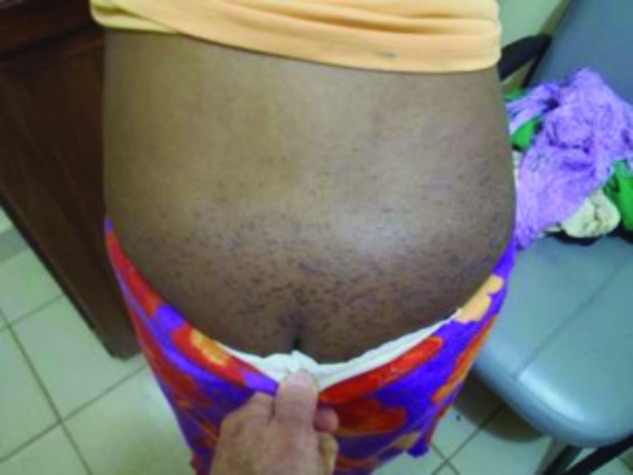
The lesions were mostly in the photodistributed areas of her face, chest, hands, and neck as well as her upper buttocks (Figures 10 and 11). The patient admitted to a six-month history of the skin lesions, difficulty raising the arms above the head, and difficulty running or climbing stairs. The final diagnosis was consistent with dermatomyositis.
Figure 10.
V sign rash. Erythema and scaling in a photodistributed region of the neck and upper chest
Figure 11.
Discrete and confluent macular erythema affecting the upper buttocks
DISCUSSION
In recent years, healthcare has seen an increase in the use of technology in innovative ways. Tablet devices offer both convenience and mobility and are being utilized in a number of practice settings. Such technological devices while helping to streamline and increase efficiency in areas with established healthcare networks, are also finding their way into the hands of providers in underserved areas. Africa is one such nation in which substandard healthcare is rampant and subspecialty care, such as dermatology and dermatopathology, are even more restricted. Several barriers have been cited by others including the scarcity of subspecialty training, poor access to specialists, and limited laboratory techniques, such as immunohistochemistry and special stains.6 There is much opportunity to be had in bringing teledermatological services to these areas. In collaborating with Mbingo physicians in Cameroon, Africa, the authors were able to provide consultation services to alleviate some of these barriers. Even so, they recognized possible areas of improvement. It is important to note that solutions to some of these limitations are beyond their capacity, such as that of bandwidth and laboratory capabilities.
The authors’ program utilizes the store and forward method. Digital images are captured by physicians in Mbingo on smartphones and are communicated to us via email. This method has several advantages. Providers are able to view the data and render diagnoses during regular business hours. In contrast, the video conferencing method is more involved, and occurs in real time between the provider and patient. Although more interactive, this method is demanding on both parties as issues of time zones and the higher cost of advanced equipment must be taken into consideration.7 The authors’ images are transmitted as JPEGs and the quality has been satisfactory for the purpose of diagnosis. The original photos were 500 to 700 kilobytes in size, which were then compressed via a digital photographic program to 200 to 300 kilobyte-sized images. This was in order to facilitate email transmission given the limited bandwidth. Should bandwidth capacities expand in the future, switching to a different compression format may be worthwhile. Studies show that while JPEGs are transferred using little data, image quality is subpar relative to other compression formats. JPEG2000 would require slightly more data, but image quality would improve significantly.8
The use of the store and forward, while sufficient and cost effective, has several disadvantages. In regard to the histopathological photos, on occasion the submitted photo may not include the relevant histopathology required to render an accurate diagnosis. In these cases, additional images are requested to gain a more representative view of the area of interest. In contrast, robotic dynamic systems and virtual slide systems (VSSs) have the advantage of decreasing sampling errors and increasing diagnostic accuracy, but are much more costly. A recently published paper utilized the novel idea of a tablet personal computer (PC).9 Images were transmitted to the PC via a live feed from a distant location. The submitting provider controlled the magnification while the consulting pathologist manipulated the touch screen in order to expand, contract, and focus in on relevant areas. This method was found to be cost effective while allowing for employees with minimal training to transmit slides. This real time set up has the advantage of better sampling and greater diagnostic accuracy, given the stipulation that a trained technician be present after hours given the time differences.
While image quality is sufficient, difficulties in the area of histopathology are much greater. Routine H&E, tissue Gram stain, stains for periodic acid-Schiff stain, gomori methenamine silver, and acid-fast bacilli are available on-site, but immunohistochemical stains utilizing monoclonal antibodies are not available. Shipping costs are extremely high and out-of-pocket costs are unsustainable if these stains are requested frequently. Perhaps the most disheartening aspect is the lack of adequate treatment options. Even with successful rendering of accurate diagnoses, lack of treatment has been an obstacle toward alleviating the burden of disease. In regard to Cameroon, antiretroviral therapy for Kaposi’s sarcoma has become progressively less costly in recent years. This has allowed providers to afford more complete care to patients. In this scenario, teledermatology proves to be an impactful and cost-effective alternative for diagnostic consultation.
The authors’ collaboration with Mbingo physicians is a small-scale, pilot, pro bono undertaking started in January 2014 and would need to be modified in order to accommodate a larger scale venture with higher volume and more providers. Current use of shared online excel sheets would be difficult to sustain. More sophisticated tools, such as telemedicine software, may be necessary. Telemed-ETH is software developed in Switzerland for use in Ethiopia that has seen some success and could be considered as a model.10 In the future, the authors hope to expand their network of physicians and physician extenders with Mbingo hospital serving as a central hub. By recruiting providers and supplying distant clinics with smart pads and smart phones, care can be extended geographically in order to encompass a larger population in need.
Concordance is another primary concern for teledermatology operations. It is defined as the diagnostic linearity between slides viewed in the traditional way via a light microscope and those diagnoses arrived at by some other method. In the authors’ setting, concordance is defined as the agreement between a diagnosis arrived via light microscopy versus images via the SAF method. Without sufficient data, we have yet to rigorously test the exact diagnostic accuracy of these virtual systems. Studies thus far show promising results with an acceptable level of concordance and diagnostic confidence between physicians using SAF methods as compared to live face-to-face consultations.11,12 In the tablet PC study, investigators found a 98.8-percent concordance rate. It is interesting to note that discrepancies were found between superficial squamous cell carcinoma versus inflamed seborrheic keratosis when comparing conventional light microscopy to that of teledermatopathology.13 The authors’ deferral rate, which is the rate at which a diagnosis could not be made without further information, was 2.8 percent, which was generally lower compared to other studies. The tablet PC study found a 7.5 percent deferral rate while Gabril et al reported a deferral rate of 7.7 percent in evaluating the diagnostic accuracy of telepathology.13–14
The authors hope to take advantage of the data and to utilize it in innovative and informative ways in the nearby future. By creating a teaching atlas of clinical dermatology and dermatopathology comprised of interesting and uncommon pathologies, the authors can continue to expand the knowledge of both their residents and medical students. Such exposure may aid and encourage our future providers in tackling not only dermatological disease in the states but globally. Furthermore, few studies have looked at the incidence of cutaneous diseases in Cameroon, Africa. With the authors’ expanding database, it would be possible to analyze the data not only to shed light on the rates of disease, but to characterize their clinical and histological features. It would also be of interest to investigate the concordance rate within their own operation. Lastly, with the authors’ goal of expanding the network of providers in Cameroon, a training manual will be created in order to aid in training physician and physician extenders in the fundamentals of dermatology and dermatopathology.
CONCLUSION
Teledermatology and teledermatopathology are new and notable fields of modernized medicine. These disciplines have flourished in the past few years owing their success to technological innovations. Providers are given the opportunity to broaden care to underserved and remote areas around the globe. The authors’ collaboration with Mbingo Baptist Hospital, a region where resources are limited, demonstrates the effectiveness of the use of smartphones to capture images, which can then be transmitted via the SAF method.
The success of teledermatology programs ultimately requires the support of policy makers, politicians, and the general population. The methods used must be tailored to accommodate the resources and needs of a specific area. With the help of more cost-effective technology, practical and sustainable business models, organized leadership, and increased awareness among officials and the community, teledermatology can thrive and expand toward alleviating the disease burden in marginalized societies.
Footnotes
Disclosure:The authors report no relevant conflicts of interest. The American Academy of Dermatology provided financial support for this research.
REFERENCES
- 1.Zundel KM. Telemedicine: history, applications, and impact on librarianship. Bull Med Libr Assoc. 1996;84(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- 2.Strehle EM, Shabde N. One hundred years of telemedicine: does this new technology have a place in paediatrics? Arch Dis Child. 2006;91(12):956–959. doi: 10.1136/adc.2006.099622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu S. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth 2009 (Global Observatory for eHealth Series, Volume 2) Healthcare Informatics Research. 2012;18(2):153–155. doi:10.4258/hir.2012.18.2.153. [Google Scholar]
- 4.Armstrong, et al. U.S. Teledermatology Survey 2012. [Accessed on December 2, 2014]. http://www.americantelemed.org/docs/default-source/member-groups/current-active-teledermatology-programs.pdf?sfvrsn=0
- 5.Josephine M, Issac E, George A, et al. Patterns of skin manifestations and their relationships with CD4 counts among HIV/AIDS patients in Cameroon. Int J Dermatol. 2006;45(3):280–284. doi: 10.1111/j.1365-4632.2004.02529.x. [DOI] [PubMed] [Google Scholar]
- 6.Gimbel DC, Legesse TB. Dermatopathology practice in ethiopia. Arch Pathol Lab Med. 2013;137(6):798–804. doi: 10.5858/arpa.2012-0041-RA. [DOI] [PubMed] [Google Scholar]
- 7.Fischer MK, Kayembe MK, Scheer AJ, et al. Establishing telepathology in Africa: lessons from Botswana. J Am Acad Dermatol. 2011;64(5):986–987. doi: 10.1016/j.jaad.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarneri F, Vaccaro M, Guarneri C. Digital image compression in dermatology: format comparison. Telemed J E Health. 2008;14(7):666–670. doi: 10.1089/tmj.2007.0119. [DOI] [PubMed] [Google Scholar]
- 9.Speiser JJ, Hughes I, Mehta V, et al. Mobile teledermatopathology: using a tablet PC as a novel and cost-efficient method to remotely diagnose dermatopathology cases. Am J Dermatopathol. 2014;36(1):54–57. doi: 10.1097/DAD.0b013e3182863186. [DOI] [PubMed] [Google Scholar]
- 10.Shiferaw F, Zolfo M. The role of information communication technology (ICT) towards universal health coverage: the first steps of a telemedicine project in Ethiopia. Glob Health Action. 2012;5:1–8. doi: 10.3402/gha.v5i0.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vañó-galvàn S, Hidalgo A, Aguayo-leiva I, et al. [Store-and-forward teledermatology: assessment of validity in a series of 2000 observations] Actas Dermosifiliogr. 2011;102(4):277–283. doi: 10.1016/j.ad.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Nami N, Massone C, Rubegni P, et al. Concordance and time estimation of store-and-forward mobile teledermatology compared to classical face-to-face consultation. Acta Derm Venereol. 2015;95(1):35–39. doi: 10.2340/00015555-1876. [DOI] [PubMed] [Google Scholar]
- 13.Speiser JJ, Hughes I, Mehta V, et al. Mobile teledermatopathology: using a tablet PC as a novel and cost-efficient method to remotely diagnose dermatopathology cases. Am J Dermatopathol. 2014;36(1):54–57. doi: 10.1097/DAD.0b013e3182863186. [DOI] [PubMed] [Google Scholar]
- 14.Gabril MY, Yousef GM. Informatics for practicing anatomical pathologists: marking a new era in pathology practice. Mod Pathol. 2010;23(3):349–358. doi: 10.1038/modpathol.2009.190. [DOI] [PubMed] [Google Scholar]