Abstract
Mycobacterium avium subsp. hominissuis (Mah) represents a health concern for humans and to a lesser extent for pigs, but its zoonotic potential remains elusive. Using multispacer sequence typing (MST) we previously identified 49 different genotypes of Mah among Belgian clinical and porcine isolates, with 5 MSTs shared by both hosts. Using experimental intranasal infection of BALB/c mice, we compared the virulence and immunogenicity of porcine and clinical human isolates with shared genotype or with a genotype only found in humans or pigs. Bacterial replication was monitored for 20 weeks in lungs, spleen and liver and mycobacteria specific spleen cell IFN-γ, IL-10 and IL-17 production as well as serum antibody responses were analyzed. Isolates varied in virulence, with human and porcine isolates sharing MST22 genotype showing a thousand fold higher bacterial replication in lungs and more dissemination to spleen and liver than the human and porcine MST91 isolates. Virulent MST22 type was also associated with progressive suppression of IFN-γ and IL-17 responses, and increased IL-10 production. Whole genome sequencing of the two virulent isolates with MST22 genotype and two avirulent isolates of genotype MST91 and comparison with two well-studied M. avium subsp. hominissuis reference strains i.e. Mah 104 and Mah TH135, identified in the two MST22 isolates nine specific virulence factors of the mammalian cell entry family, that were identical with Mah 104 strain. Despite the obvious limitations of the mouse model, a striking link of virulence and identity at the genome level of porcine and human isolates with the same multisequence type, for which no correlation of place of residence (humans) or farm of origin (pigs) was observed, seems to point to the existence in the environment of certain genotypes of Mah which may be more infectious both for humans and pigs than other genotypes.
Introduction
Among non-tuberculous mycobacteria (NTM), bacteria of the Mycobacterium avium complex (MAC) are the most frequently isolated from patients [1,2]. The M. avium species is divided into four subspecies: M. avium subsp. avium (Maa), M. avium subsp. silvaticum (Mas), M. avium subsp. paratuberculosis (Map) and M. avium subsp. hominissuis (Mah) [3,4]. Recently, a phylogenetic study showed that Mah represents diverse groups of organisms from which two distinct groups, Map and Maa/Mas, have evolved independently [5]. These four subspecies of M. avium are genetically close but they differ widely in their host range and pathogenicity. Indeed, Map is responsible for an intestinal illness in ruminants known as Johne’s disease [6] and could be implicated in human Crohn’s disease [7]. Maa and Mas mainly infect birds causing a tuberculosis-like disease, whereas Mah is a frequent agent of human and pig mycobacterioses [8–10]. As indicated by its name, M. avium subsp. hominissuis is the most frequently recovered M. avium subspecies in swine, although Maa can also be found. Although both subspecies can infect pigs, Agdestein et al. observed more extensive shedding of Mah than of Maa, which might explain the higher incidence of infection caused by the former subspecies [9,11].
In humans, Mah is the causative pathogen of two main types of disease: disseminated disease in immunocompromised hosts, such as AIDS patients and pulmonary disease in individuals without systemic immunosuppression [12]. Moreover, an association between Mah and human lymphadenitis has been described by Despierres et al. [13]. In a retrospective study of 67 MAC-infected patients diagnosed between July 1996 and March 2011, Mah was isolated from 24 out of 25 lymphadenitis patients, who were significantly younger than 42 non-lymphadenitis patients. Also, cervical topography found in 76.5% of lymphadenitis patients was significantly more frequent in non-immunocompromised patients (p = 0.04) [14]. In the French study, multispacer sequence typing (MST) of Mah isolates revealed an enormous genetic variability with 15 genotypes in 29 non-lymphadenitis isolates (molecular diversity, 0.622) versus 11 genotypes in 24 lymphadenitis isolates (molecular diversity, 0.578) [13]. Using the same multispacer sequence typing method as described by Despierres et al., we previously identified 49 different genotypes of Belgian Mah among pig isolates collected during 2012–2013 (11 MST types) and among clinical human isolates collected during 2011–2012 (43 MST types), and we observed that only 5 of these MST genotypes were shared by both hosts [15]. MST12 and MST22 genotypes were frequently found both in human and in pigs, whereas MST15, the second most frequently observed type in humans, was not detected in pigs. In the Despierres et al. study, 12/67 clinical isolates were typed as MST13 whereas in our study only 2/92 clinical isolates had that genotype [13]. In contrast, in France only 1/67 isolates had the MST22 genotype (isolated from a 40 years old male patient with pulmonary disease) whereas in our study MST22 genotype was found in 5/92 clinical isolates (and in 7/54 porcine isolates). Finally, MST12 genotype was not detected in the French study, whereas in Belgium this was really the preponderant genotype (16/92 human and 28/54 porcine isolates). In order to determine the evolution of M. avium infecting pigs over time, we recently performed a subspecies determination and genotyping on two older panels of porcine isolates (collected in 1967–1968 and 1992–1996). Unlike the omnipresence of Mah reported among the 2012–2013 isolates, a significant presence of both Maa and Mah, was observed in these older panels. Multispacer sequence types (MST) changed over time, although MST12 was already the predominant genotype in the older Mah panels as well (Soetaert et al., submitted for publication).
As Mah represents an increasing public health concern given its pathogenicity for humans, a detailed comparison of human clinical isolates and swine isolates could contribute to establish or exclude any epidemiological link between both hosts. Here we report on a comparison of bacterial replication and immunogenicity of Mah isolates with defined MST type in an experimental mouse model.
Since Mah has been recovered from the environment, more particularly in sawdust and peat [16], an intranasal infection was used to mimic airway exposure. We have previously shown that the BALB/c mouse strain shows the same susceptibility to M. avium subsp. paratuberculosis infection as the mutant C57BL/6 bg/bg mouse strain (considered to be the best mouse model for Mycobacterium avium complex disease [17]) and can be used for assessing mycobacterial virulence by monitoring bacterial replication in spleen, lungs and liver, as well as for immunogenicity studies [18].
BALB/c mice were infected by the intranasal route with four human and four porcine Mah isolates, with a shared MST genotype or with an MST genotype only found in humans or pigs. Bacterial replication was monitored for 20 weeks in lungs, spleen and liver and mycobacteria specific spleen cell IFN-γ, IL-10 and IL-17 production as well as serum antibody responses were analyzed.
For a porcine and human isolate with shared MST22 genotype and for a porcine and human isolate with shared MST91 genotype, we previously described the full genome sequencing [19], and in this paper we compared these sequences to those of two well-studied M. avium subsp. hominissuis reference strains i.e. Mah 104 [20] and Mah TH135 [21].
Materials and methods
Ethics statement
Clinical isolates of Mah were used with the approval of the ethical committee of Hôpital Erasme (ULB) (reference P2014/028). The study protocol was approved by the Ethical Committee of the Centrum voor Onderzoek in Diergeneeskunde en Agrochemie-Pasteur Instituut van Brussel-Wetenschappelijk Instituut voor Volksgezondheid regulations (CODA-PIB-WIV, permit number: 060202–02, President Dr. Els Goossens).
Clinical and porcine isolates
Table 1 shows the list of the eight Mah isolates used in this study. The four clinical human isolates of Mah were isolated from patients diagnosed by the Tuberculosis & Mycobacteria National Reference Laboratory of the Scientific Institute of Public Health (WIV-ISP) in Belgium in 2012 and the last trimester of 2011. Porcine Mah isolates were isolated from submandibular lymph nodes with visible lesions (identified after veterinary inspection in 2012–2013 by the UREAR-ULg) by the Belgian veterinary centre CODA-CERVA. The different porcine Mah isolates originated from different farms, and farm location had no relation with the selected human isolates [15].
Table 1. List of the porcine and human M. avium subsp. hominissuis isolates used in this study.
| Human clinical Mah isolates | |||||
| Isolate name | MST code | Ref. name in [19] | Age | Sex | Symptoms |
| Hu12 | 12 | 12_009 | Unknown | Female | Unknown |
| Hu15 | 15 | 12MY0204 | 4 years | Female | Lymphadenitis |
| Hu22 | 22 | 12_062 | 40 years | Male | HIV neg, disseminated |
| Hu91 | 91 | 12_067 | 40 years | Male | AIDS, disseminated |
| Porcine Mah isolates | |||||
| Isolate name | MST code | Ref. name in [19] | Symptoms | ||
| Po12 | 12 | LYM108 | Mandibular lymphadenitis | ||
| Po22 | 22 | LYM122 | Mandibular lymphadenitis | ||
| Po91 | 91 | LYM86 | Mandibular lymphadenitis | ||
| Po103 | 103 | LYM119 | Mandibular lymphadenitis | ||
Mah identification and MST typing
M. avium species identification was realized as a routine activity of the reference laboratory, by sequencing of the gene coding for the 16SrRNA, as previously described [22]. All the Mah isolates were identified at the subspecies level by sequencing of the PCR-amplified rpoB gene and by IS1245/IS901 analysis. Genotyping of the Mah isolates was performed by Multispacer Sequence Typing MST. The PCR amplification of the spacers MST2, MST4, MST15 and MST16 was realized as described by Cayrou et al. [23]. Purification of the amplicons and sequencing was realized as described above, with the same forward and reverse primers as used for the different MST PCRs [23]. The MST type was determined by consultation of the MST database of the “Université de la Méditerranée” accessible via the following link: http://ifr48.timone.univ-mrs.fr/mst/mycobacterium_avium/
In total 49 different genotypes of Mah were identified among pigs (11 MST types among 54 isolates) and humans (43 MST types among 92 isolates), with only 5 MST types shared by both hosts [15]. As we have previously indicated, no correlation was observed between MST type and place of residence or the farm of origin for human and porcine isolates respectively [15].
Mah cultivation
The Mah isolates were grown in plastic Roux flasks in liquid Middlebrook 7H9 medium (BD, Franklin Lakes, NJ, USA), containing 10% Oleic Acid-Albumin-Dextrose-Catalase, for 14 days at 39°C under static aerobic conditions in horizontal position to maximize oxygen supply. Bacteria were harvested by centrifugation, washed three times with PBS and then frozen at -80°C in Middlebrook 7H9 medium supplemented with 20% glycerol until use. After one freeze/thaw cycle and 2 min sonication, stocks were enumerated by plating serial dilutions on Middlebrook 7H11 (BD, Franklin Lakes, NJ, USA) supplemented with 10% OADC. The list of the eight Mah isolates used for infection is given in Table 1.
Whole genome sequencing
Whole genome sequencing was performed on the human and porcine MST22 isolates and on the human and porcine MST91 isolates, with an ILLUMINA Miseq 2X150 bp and a quality analysis was realized using FastQC v0.11.5. Total genome sequence length and number of protein coding sequences were previously reported and whole sequences have been deposited at the European Nucleotide Archive [19]. For the sake of clarity, the 12_062, 12_067, LYM122 and LYM086 isolates have been renamed here as Hu22, Hu91, Po22 and Po91 respectively.
Mice
Female BALB/c mice aged 6–8 weeks were bred and kept (5 animals per standard cage) at the WIV-ISP experimental animal facilities (Site Ukkel), complying with the Belgian legislation that transposes European Directive 2009/41/EC, repealing Directive 90/219/EC (EC, 2009). All efforts were made to minimize animal suffering. Anaesthesia was performed using isoflurane.
Experimental infection
Mice were infected by the intranasal route with 107 CFU of Mah. Infection dose was verified by plating serial dilutions on solid Middlebrook 7H11 agar supplemented with 10% OADC. After blood sampling, animals (n = 5/group at each time point) were sacrificed by cervical dislocation. Two infection runs were realized, one run with the eight isolates and mice sacrificed at week 1, 5, 10 and 20 weeks post-infection and a second run with seven of the eight isolates (Hu15 isolate missing), and with time-point measures at 5, 9 and 20 weeks post-infection. Non-infected mice were used as negative control. Spleen, lungs and liver of individual animals were removed aseptically. Bacteria were enumerated by plating serial threefold organ dilutions in duplicate on solid Middlebrook 7H11 agar supplemented with 10% OADC. Petri dishes were incubated for 14 days at 39°C and number of colonies were counted visually.
Cytokine production
Spleens were collected at week 5 and week 20 post-infection and homogenized using a loosely fitting Dounce homogenizer. Leucocytes (4 x 106 cells/mL as measured in a Coulter Counter) were cultivated at 37°C in a humidified CO2 incubator in round-bottom microwell plates in a volume of 200 μL RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 5 x 10−5 M 2-mercaptoethanol, 1% penicillin-streptomycin and 10% fetal calf serum (FCS). Spleen cells of five mice per group were analysed individually. Cells were stimulated with Culture Filtrate (CF) of Map [24] or with purified recombinant E. coli-expressed Map proteins at a final concentration of 5 μg/mL [24,25]. Culture supernatants were harvested after 72h. Supernatants were stored frozen at -20°C until analysis.
Cytokine ELISA
IFN-γ, IL-10 and IL-17A were quantified in 72h culture supernatants of spleen cell cultures using a sandwich enzyme-linked immunosorbent assay (ELISA). IFN-γ was detected using coating antibody R4-6A2 and biotinylated detection antibody XMG1.2 (BD Pharmingen, Franklin Lakes, NJ, USA). Plates were revealed with O-phenylenediamine dihydrochloride substrate (OPD; Sigma-Aldrich, St. Louis, MO, USA); the reaction was stopped with 1 M H2SO4, and the optical density was read at 490 nm. IL-10 and IL-17A were detected with commercial ELISA kits (eBioscience, San Diego, CA, USA).
Characteristics of 35 recombinant Map proteins
The list of 35 Map proteins is given in Table 2. Antigens 1–18 were previously identified by proteomic and immunoproteomic analysis of standard Map biofilm culture filtrate of bacteria grown as a surface pellicle on synthetic Sauton medium [26]. Antigens 19–24 were identified using an in silico analysis of Map genome [27]. Finally, antigens 25–35 were identified by proteomic analysis of in Sauton culture filtrate of Map submitted to different stress conditions (hypoxia, acidic pH, nutrient starvation or non-toxic NO) as described for M. tuberculosis by Voskuil et al. [28]. In total, seven proteins were found to be up-regulated under stress conditions (highlighted in grey) (Roupie et al., manuscript in preparation). The 35 proteins were produced as recombinant histidine-tagged proteins in E. coli and purified by affinity chromatography on immobilized nickel-chelate (Ni-NTA) columns.
Table 2. List of 35 recombinant M. avium subsp. paratuberculosis antigens.
| Nr. | Antigen | Description | E value | Ident. | Accession | Myco |
|---|---|---|---|---|---|---|
| 1 | MAP0139c | transcriptional regulator, PadR family protein [Mycobacterium avium 104] | 8,00E-144 | 99% | ABK65696.1 | MAA104 |
| 2 | MAP0586c | transglycosylase SLT domain protein [Mycobacterium avium 104] | 0 | 99% | ABK65241.1 | MAA104 |
| 3 | MAP0907 | morphine 6-dehydrogenase [Mycobacterium avium 104] | 0 | 98% | ABK68334.1 | MAA104 |
| MAP0907 | oxidoreductase [Mycobacterium avium subsp. hominissuis TH135] | 1,00E-52 | 41% | BAN32966.1 | MAH TH135 | |
| 4 | MAP1438c | alpha/beta hydrolase fold domain protein [Mycobacterium avium 104] | 0 | 99% | ABK65668.1 | MAA104 |
| MAP1438c | alpha/beta hydrolase [Mycobacterium avium subsp. hominissuis TH135] | 2,00E-24 | 40% | BAN33440.1 | MAH TH135 | |
| 5 | MAP1562c | conserved hypothetical protein [Mycobacterium avium 104] | 1,00E-89 | 100% | ABK66813.1 | MAA104 |
| 6 | MAP1693c-FL | peptidyl-prolyl cis-trans isomerase, fkbp-type domain protein [Mycobacterium avium 104] | 1,00E-125 | 98% | ABK64725.1 | MAA104 |
| MAP1693c-FL | peptidyl-prolyl cis-trans isomerase domain-containing protein [Mycobacterium avium subsp. hominissuis TH135] | 4,00E-113 | 100% | BAN31629.1 | MAH TH135 | |
| 7 | MAP2411 | pyridoxamine 5-phosphate oxidase [Mycobacterium avium subsp. hominissuis 10–4249] | 9,00E-101 | 100% | ETB30907.1 | MAH |
| 8 | MAP2677c | glyoxalase family protein [Mycobacterium avium 104] | 8,00E-92 | 98% | ABK69112.1 | MAA104 |
| 9 | MAP2746 | hypothetical protein MAH_1788 [Mycobacterium avium subsp. hominissuis TH135] | 4,00E-119 | 98% | BAN30862.1 | MAH TH135 |
| MAP2746 | conserved hypothetical protein [Mycobacterium avium 104] | 2,00E-109 | 99% | ABK64828.1 | MAA104 | |
| 10 | MAP-CF036-Ag3 | integral membrane protein YrbE1A [Mycobacterium avium subsp. hominissuis TH135] | 3,4 | 20% | BAN33502.1 | MAH TH135 |
| MAP-CF036-Ag3 | conserved hypothetical protein [Mycobacterium avium 104] | 3,4 | 20% | ABK66937.1 | MAA104 | |
| 11 | MAP3385 | methyltransferase [Mycobacterium avium subsp. hominissuis TH135] | 8,00E-65 | 64% | BAN32924.1 | MAH TH135 |
| MAP3385 | hypothetical protein MAH_3962 [Mycobacterium avium subsp. hominissuis TH135] | 2,00E-64 | 44% | BAN33036.1 | MAH TH135 | |
| MAP3385 | methyltransferase, putative, family protein [Mycobacterium avium 104] | 5,00E-32 | 40% | ABK67089.1 | MAA104 | |
| 12 | MAP3486 | lactate 2-monooxygenase [Mycobacterium avium 104] | 0 | 99% | ABK65670.1 | MAA104 |
| MAP3486 | LldD2 protein [Mycobacterium avium subsp. hominissuis TH135] | 4,00E-35 | 32% | BAN31520.1 | MAH TH135 | |
| 13 | MAP3547c | hypothetical protein MAV3388_23345 [Mycobacterium avium subsp. hominissuis 3388] | 0 | 99% | KDO92449.1 | MAH |
| MAP3547c | conserved hypothetical protein [Mycobacterium avium 104] | 2,00E-17 | 26% | ABK65078.1 | MAA104 | |
| 14 | MAP3680c | Formate dehydrogenase [Mycobacterium avium subsp. hominissuis TH135] | 0 | 99% | BAN33415.1 | MAH TH135 |
| MAP3680c | formate dehydrogenase [Mycobacterium avium 104] | 0 | 99% | ABK68013.1 | MAA104 | |
| 15 | MAP3731c | O-antigen export system ATP-binding protein RfbB [Mycobacterium avium 104] | 0,13 | 21% | ABK65477.1 | MAA104 |
| MAP3731c | O-antigen export system ATP-binding protein RfbB [Mycobacterium avium subsp. hominissuis TH135] | 0,16 | 21% | BAN29326.1 | MAH TH135 | |
| 16 | MAP3804 | glycosyl hydrolases family protein 16 [Mycobacterium avium 104] | 0 | 100% | ABK67366.1 | MAA104 |
| MAP3804 | glycosyl hydrolases family protein 16 [Mycobacterium avium subsp. hominissuis TH135] | 7,00E-174 | 100% | BAN33331.1 | MAH TH135 | |
| 17 | MAP4096 | nitroreductase family protein [Mycobacterium avium 104] | 6,00E-115 | 99% | ABK64565.1 | MAA104 |
| MAP4096 | nitroreductase [Mycobacterium avium subsp. hominissuis TH135] | 2,00E-04 | 23% | BAN29848.1 | MAH TH135 | |
| 18 | MAP4308c | fructose-bisphosphate aldolase class-I [Mycobacterium avium 104] | 0 | 99% | ABK68800.1 | MAA104 |
| 19 | Ag5 | hypothetical protein MAPs_16490 [Mycobacterium avium subsp. paratuberculosis S397] | 2,00E-166 | 100% | EGO37081.1 | MAP |
| 20 | Ag6 | MULTISPECIES: ATP-dependent helicase [Mycobacterium avium complex (MAC) | 0,047 | 39% | WP_038536272.1 | MAC |
| 21 | MAP1637c | M. avium UbD family decarboxylase | 0,00E+00 | 99% | WP_016706304.1 | M. avium |
| 22 | MAP0388 | hypothetical protein MAP_0388 [Mycobacterium avium subsp. paratuberculosis K-10] | 0 | 99% | AAS02705.1 | MAP |
| 23 | MAP3743-Ag9 | hypothetical protein MAP_3743 [Mycobacterium avium subsp. paratuberculosis K-10] | 0 | 99% | AAS06293.1 | MAP |
| 24 | MAP3744m-Ag10 | thiazolinyl imide reductase [Mycobacterium avium subsp. paratuberculosis 10–4404] | 0 | 99% | ETB06990.1 | MAP |
| 24 | MAP3744m-Ag10 | thiazolinyl imide reductase [Mycobacterium avium] | 0 | 99% | WP_016705779.1 | M. avium |
| 25 | MAP0217 | antigen 85-C [Mycobacterium avium 104] | 7,00E-180 | 99% | ABK64781.1 | MAA104 |
| MAP0217 | antigen 85-C protein [Mycobacterium avium subsp. hominissuis TH135] | 3,00E-179 | 99% | BAN29303.1 | MAH TH135 | |
| 26 | MAP0353 | glycerol kinase [Mycobacterium avium 104] | 0 | 99% | ABK67587.1 | MAA104 |
| 27 | MAP1166 | RecName: Full = Triosephosphate isomerase; Short = TIM; Short = TPI; AltName: Full = Triose-phosphate isomerase | 0 | 99% | A0QHY3.1 | MAA104 |
| 28 | MAP1362 | arginine biosynthesis bifunctional protein ArgJ [Mycobacterium avium 104] | 9,00E-145 | 99% | ABK65024.1 | MAA104 |
| 29 | MAP1803 | dienelactone hydrolase [Mycobacterium avium subsp. hominissuis TH135] | 4,00E-166 | 99% | BAN31765.1 | MAH TH135 |
| MAP1803 | dienelactone hydrolase family protein [Mycobacterium avium 104] | 2,00E-143 | 99% | ABK69268.1 | MAA104 | |
| 30 | MAP2800 | enoyl-CoA hydratase [Mycobacterium avium 104] | 0 | 99% | ABK64508.1 | MAA104 |
| MAP2800 | enoyl-CoA hydratase [Mycobacterium avium subsp. hominissuis TH135] | 2,00E-24 | 30% | BAN30137.1 | MAH TH135 | |
| 31 | MAP2864c | dihydrodipicolinate synthase [Mycobacterium avium 104] | 0 | 99% | ABK66322.1 | MAA104 |
| 32 | MAP3413 | aldehyde dehydrogenase (NAD) family protein [Mycobacterium avium 104] | 0 | 99% | ABK69290.1 | MAA104 |
| MAP3413 | P-cumic aldehyde dehydrogenase [Mycobacterium avium subsp. hominissuis TH135] | 3,00E-41 | 27% | BAN31590.1 | MAH TH135 | |
| 33 | MAP3634 | ErfK/YbiS/YcfS/YnhG family protein [Mycobacterium avium 104]Lipoprotein lprQ | 0 | 100% | ABK64614.1 | MAA104 |
| MAP3634 | hypothetical protein MAH_1464 [Mycobacterium avium subsp. hominissuis TH135] | 6,00E-48 | 41% | BAN30538.1 | MAH TH135 | |
| 34 | MAP3668c | phosphotriesterase-like protein [Mycobacterium avium subsp. hominissuis TH135] | 0 | 99% | BAN33426.1 | MAH TH135 |
| MAP3668c | phosphotriesterase-like protein [Mycobacterium avium 104] | 0 | 99% | ABK64738.1 | MAA104 | |
| 35 | MAP2541c | Malate dehydrogenase | 0 | 99% | A0QCI6.1 | MAA104 |
Ags 1–18: Map antigens identified by proteomic and immunoproteomic analysis of Map culture filtrate [26]; Ags 19–24: Map antigens identified by in silico predictions [27]; Ags 25–35: Map antigens identified in Map cultures submitted to stress conditions: nutrient starvation, hypoxia or acidic pH (Roupie et al., manuscript in preparation). Proteins actually upregulated in stress conditions are highlighted in grey. Putative function and results of BlastP comparison (E value and % protein identity) with Mah104 and Mah TH135 genome.
Antibody responses
Pooled sera of five mice infected by the porcine MST12 isolate were collected at week 20 post-infection and analyzed for mycobacteria-specific IgG antibodies against whole Map culture filtrate by ELISA and against the 35 recombinant Map proteins (100 ng/well in borate buffer) using a dot-blot coupled to Western blot using pooled sera/group diluted 1:50, peroxidase-labelled rat anti-mouse IgG (LO-MK-1, purchased at Experimental Immunology Unit, Université Catholique de Louvain, Brussels, Belgium) diluted 1:500 and α-chloronaphtol (Sigma-Aldrich, St. Louis, MO, USA) in H2O2 for revelation. Reaction was stopped by washing in tap water. As the dot-blot assay required serum volumes in the order of 0.5 mL, sera were pooled. Non-infected mice were used as negative control.
Statistical analysis
GraphPad Prism software (San Diego, CA, USA) was used to perform statistical analysis. One-way ANOVA (with Tukey’s post-t-tests) and Student t tests were performed to demonstrate statistical differences. For all tests p-values < 0.05 were considered significant.
Results
Bacterial replication of 8 Mah isolates in intranasally infected BALB/c mice
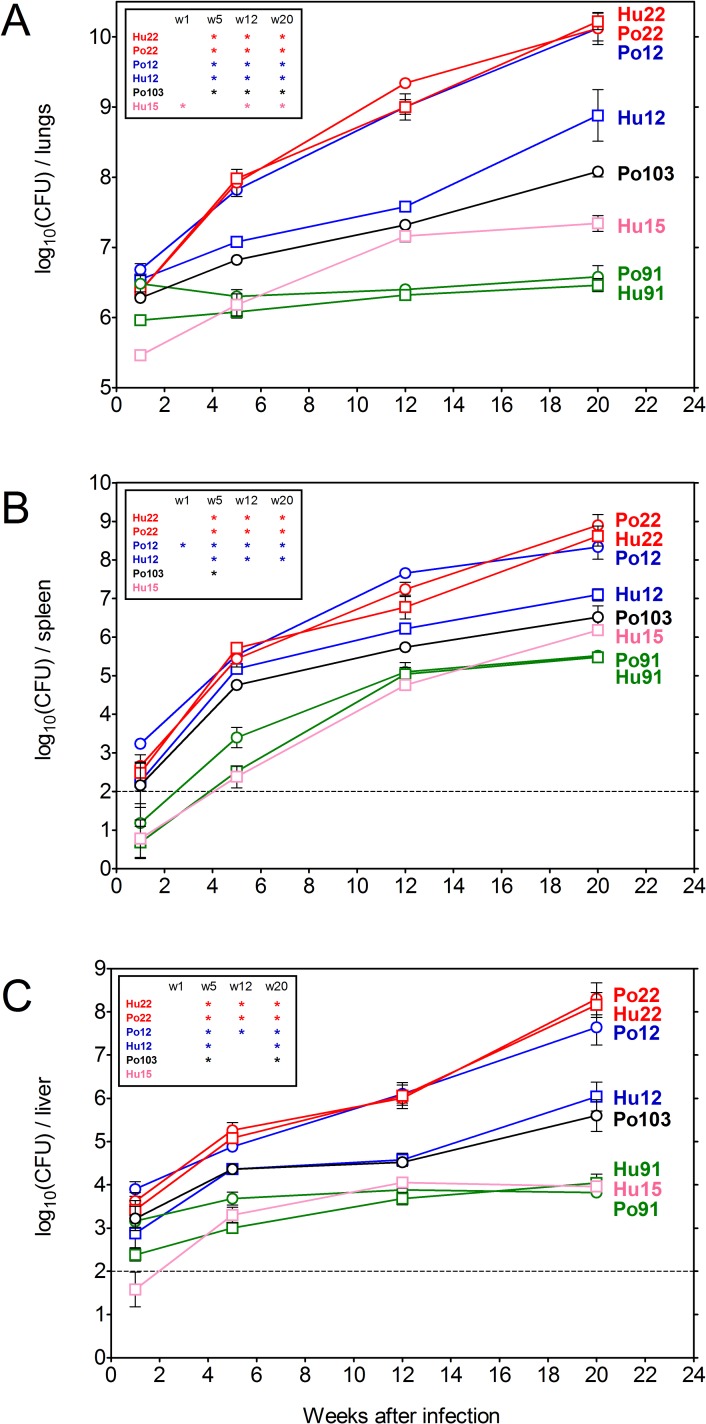
BALB/c mice were infected by the intranasal route with 107 CFU of the eight Mah isolates listed in Table 1. Virulence of the strains was examined, by enumeration of the bacteria in lungs, spleen and liver at week 1, 5, 12 and 20, as previously reported for testing Map virulence [18]. Pronounced differences were observed in bacterial replication of the eight isolates and their MST type seemed to correlate with this variation for four of them (Fig 1). Thus, the human and porcine isolates with shared MST22 (in red) showed a more than 1,000 fold increase in bacterial numbers in lungs (6.5 log10 at week 1 to 10 log10 at week 20) and a dramatic dissemination to spleen (2 log10 at week 1 to 8 log10 at week 20) and liver (3.5 log10 at week 1 to 8 log10 at week 20), whereas the human and porcine isolates with shared MST91 (in green) showed no replication in lungs (stable CFU counts at 6.5 log10 throughout the entire period) or liver (stable CFU counts at 3 log10) and minor dissemination to spleen (1.0 log10 to 3 log10). For the two MST12 isolates, the link with bacterial replication was less clear as the porcine MST12 isolate replicated to the same extent as the two MST22 isolates, whereas the human MST12 isolate showed intermediate virulence. Porcine MST103 finally also showed an intermediate replication and human MST15 isolate finally replicated to a minor extent in the lungs, but did not disseminate to liver or spleen.
Fig 1.
Bacterial replication in lungs (A), spleen (B) and liver (C) of BALB/c mice infected with one of the eight Mah isolates, as measured at 1, 5, 12 and 20 weeks post-infection. Data represent mean CFU (log10)/organ ± SEM of five animals tested per group. Isolate IDs represent the host (Hu: human; Po: swine) followed by the respective multispacer sequence type (MST). Statistical significance is depicted for each strain as compared to both Po91 and Hu91 (*: p<0.05).
Another infection run was realized with seven of these eight Mah strains. The results of this run (lacking the Hu15 isolate, and with time-point measures at 5, 9 and 20 weeks post-infection) are shown in S1 Fig. A similar variation in bacterial replication, associated with a thousand fold increase in bacterial numbers in lungs of mice infected with the two MST22 isolates and the porcine MST12 isolate and a stable number of bacteria in the lungs of mice infected with the two MST91 isolates was observed.
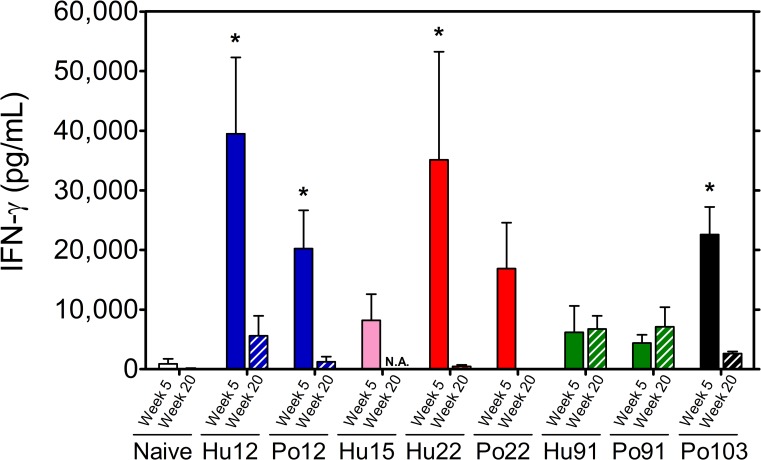
Cytokine production
At five weeks post-infection, strong IFN-γ production was detected in response to M. avium culture filtrate (and PPD, data not shown) in mice infected with MST12 and MST22 isolates (ranging between 20,000 and 40,000 pg/mL), whereas IFN-γ levels were about tenfold lower in mice infected with MST91 isolates (Fig 2). At week 20 p.i., IFN-γ responses induced by M. avium CF were completely abrogated in mice infected with the human and porcine MST22 and the porcine MST12 isolates, whereas IFN-γ responses in mice infected with both Mah MST91 isolates were sustained at levels even slightly, although not significantly higher than at the early time point (Figs 2 and 3A).
Fig 2. Spleen cell IFN-γ production in response to M. avium culture filtrate (5 μg/mL) in BALB/c mice infected for 5 weeks (filled bars) or 20 weeks (striped bars) with the different Mah isolates.
Results represent mean IFN-γ levels (pg/mL) ± SEM of 3–5 mice tested individually. Statistical significance is depicted for each human or porcine strain as compared to respectively Hu91 or Po91 (*: p<0.05). N.A.: not available.
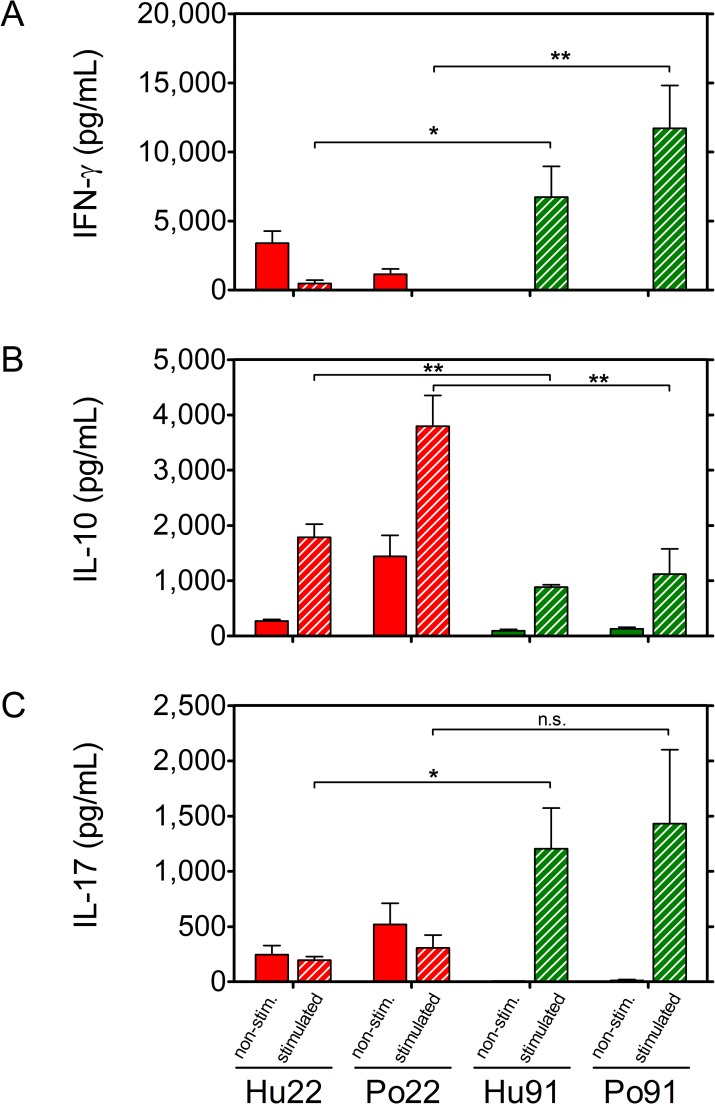
Fig 3.
IFN-γ (A), IL-10 (B) and IL-17 (C) production in 72h spleen cell culture supernatant of mice infected for 20 weeks with human and porcine MST22 (red) and human or porcine MST91 (green) Mah isolates. Cells were unstimulated (filled bars) or stimulated with culture filtrate (striped bars). Results represent mean cytokine levels (pg/mL) ± SEM of 5 mice tested individually in each group. Statistical significance is depicted for MST22 strains as compared to MST91 strains (*: p<0.05; **: p<0.005; n.s.: non-significant).
In order to find out, whether the suppressed IFN-γ response at 20 weeks post-infection in spleen of MST22 infected mice was the result of a shift from Th1 to Th2, Th17 or Treg helper T cell phenotype, we measured the production of IL-4, IL-10, IL-17 and IL-1β by ELISA in week 5 and week 20 spleen cell culture supernatants of mice infected with the two MST22 and the two MST91 isolates. At week 5, only weak IL-10 levels were detected in culture supernatants of the four groups (data not shown), but at week 20 a significantly higher IL-10 production was measured in supernatants of MST22 than of MST91 infected mice (p<0.005) (Fig 3B), suggesting that the abrogated IFN-γ response in the MST22 infected mice may have been the result of an activation of IL-10 producing regulatory macrophages and/or T cells. IL-17 could be detected only in week 20 culture supernatants (Fig 3C). In MST22 infected mice, levels of IL-17 were comparable in supernatants from non-stimulated or CF-A stimulated cells, whereas in MST91 infected mice, stimulation with CF-A induced increased IL-17 production as compared to non-stimulated cells. IL-4 levels were below detection level at the two time points for all four Mah groups, and very weak IL-1β levels were detected that were not different between the two time points and the four Mah groups (data not shown).
IFN-γ production and IgG antibodies in response to 35 recombinant, purified Map antigens in Mah infected BALB/c mice
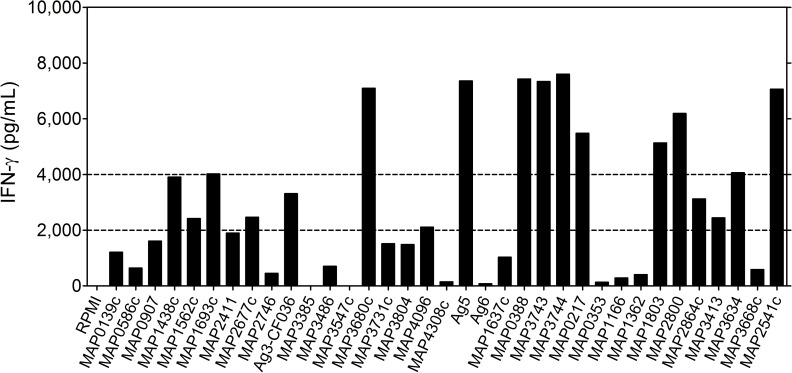
A series of 35 M. avium subsp. paratuberculosis protein sequences were compared for identity with proteins in Mah 104 and Mah TH135, using BlastP (Table 2). Except for the six in silico predicted antigens (antigens 19–24 in Table 2), predicted identity between the Map and Mah proteins was in the order of 99% for twenty-six of the twenty-nine other proteins (Table 2). Three MAP proteins, i.e. Ag 3, MAP3385 and MAP3731, showed a lower percentage of identity with Mah of 20%, 64% and 21% respectively. In view of these strong identities and as cloned and purified Mah products were not available, we tested the purified Map proteins in an IFN-γ release assay (Fig 4). Nine of the thirty-five antigens induced strong IFN-γ responses (> 4,000 pg/mL) at week 5 p.i. in spleen cell cultures from mice infected with virulent porcine MST12: MAP3680c a formate dehydrogenase, MAP0217 (Ag85C) a member of the highly conserved immunodominant Ag85 family, which functions as mycolyl-transferase involved in the coupling of trehalose to the arabinogalactan of the mycobacterial cell wall [29], MAP1803 belonging to the dienelactone hydrolase family protein, MAP2800 predicted to be an enoyl-CoA hydratase and MAP2541 finally a malate dehydrogenase. Somewhat surprisingly, the hypothetical proteins Ag5, MAP0388, MAP3743 and the predicted thiazolinyl imide reductase MAP3744, all four previously selected in silico (and for which a new blast analysis confirmed the absence of homology with other M. avium subspecies) also stimulated a strong IFN-γ production in spleen cells of mice infected with the MST12 Mah isolate.
Fig 4. IFN-γ response of BALB/c mice infected intranasally with porcine MST12 isolate (Po12).
Mice (n = 5) were sacrificed 5 weeks post-infection and pooled spleen cells were stimulated for 72h with each of the 35 Map antigens (5 μg/mL). IFN-γ levels are expressed in pg/mL.
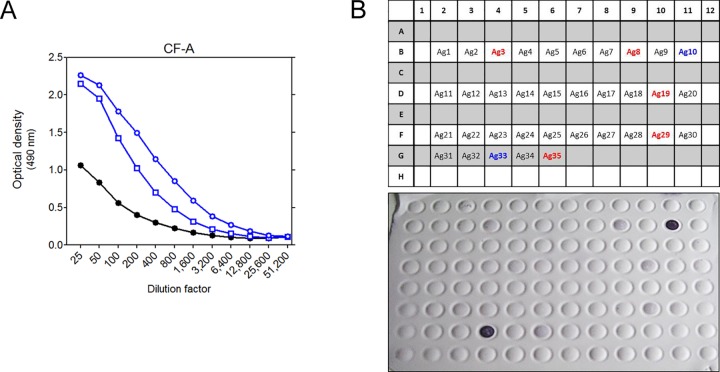
Serial dilutions of pooled sera of BALB/c mice infected for 20 weeks with porcine Po12 and human Hu12 or sera from non-infected mice were tested against M. avium CF by ELISA (Fig 5A). Strong CF-A specific antibodies were detected in sera from Mah Po12 and Hu12 infected mice. Pooled sera from Mah Po12 infected mice were also tested against the 35 Map antigens in a dot-blot assay (Fig 5B). Two proteins were strongly recognized (Nr.10/CF-036 and Nr.33/MAP3634, homologous to lprQ lipoprotein) and five proteins (Nr.3/MAP0907, a predicted morphine 6-dehydrogenase, Nr.8/MAP2677c of the glyoxalase family protein, Nr.19/Ag5, Nr.29/MAP1803 and Nr.35/MAP2541c) were recognized but more weakly in the dot-blot. These results are consistent with other dot-blot experiments in which MAP3634 was also very strongly recognized by sera from M. avium subsp. avium infected mice (data not shown), suggesting this antigen had a potential for serodiagnosis.
Fig 5.
A: IgG antibodies against M. avium culture filtrate in serial dilutions of pooled sera of BALB/c mice infected for 20 weeks with porcine (blue circles) or human (blue squares) Mah MST12 isolate (pooled sera from naïve mice represented by black dots). B: Plate organization and Western blot image of immunoblot of week 20 pooled Mah Po12 serum tested against the 35 Map antigens. Numbers of the two antigens giving the strongest signals are indicated in blue, numbers of the five antigens giving weaker signals are indicated in red.
Comparison of sequences of MST22 and MST91 isolates with sequences of two Mah reference strains
As differences in MST type seemed to be reflected (at least to some extent) by differences in virulence in BALB/c mice, whole genome sequencing of the two MST22 and the two MST91 isolates was performed, sequences have been deposited at the European Nucleotide Archive and genome statistics of the four isolates was reported [19]. MST22 rather than MST12 isolates were chosen for sequencing because of the limited clinical information for the human MST12 isolate.
Next, the genome sequences of MST22 and MST91 isolates were compared with two M. avium subsp. hominissuis reference strains, i.e. Mah 104 and Mah TH135, isolated from an AIDS patient with disseminated disease and a HIV-negative patient with pulmonary disease respectively. In a very interesting study, the genome of these two reference strains was compared by Uchiya et al. and described to encode for a series of specific virulence factors [21]. Here, we analyzed whether the sequences of these virulence factors were also present in the genome of the MST22 and MST91 isolates (Table 3). One gene sequence from the Mah TH135 reference strain, namely MAH_1657, and ten other sequences from the Mah 104 reference strain were found to be present at 100% identity in both MST22 isolates (Hu22 and Po22). For the MST91 isolates, only two gene sequences (MAV_0117 and MAV_0953) were found to be identical with the sequence of the genes reported from the reference strain Mah 104. Interestingly, the nine gene sequences present in the two virulent isolates of genotype 22 and coding for specific virulence factors of the mammalian cell entry (Mce family) are indeed absent in the two avirulent isolates of genotype 91. Among these nine genes, five were localized by Uchiya et al. in a SR21 strain-specific region identified in the Mah 104 reference strain [21]. Besides the Mce genes, two genes identical to PPE proteins were found in MST22 isolates: MAH_1657 (TH135) sharing 100% identity and MAV _1347 (104) 99% identical with M. avium subsp. avium. Genomes of the two MST91 isolates had an identical copy MAV_0117, encoding a PE protein, 99% identical with M. avium subsp. avium. Finally, BlastN analysis did not reveal any sequence homology in our four Belgian isolates with the plasmid pMAH135 (data not shown), which was reported to be associated with progression to pulmonary disease in Japan [30].
Table 3. Genes present at 100% identity in the sequenced Mah strains.
| Genes identified by [21] in reference strain Mah TH135 | |||||
| Gene sequence name | Predicted protein | Presence in Hu22 | Presence in Po22 | Presence in Hu91 | Presence in Po91 |
| MAH_1657 | PPE | X | X | ||
| Genes identified by [21] in reference strain Mah 104 | |||||
| Gene sequence name | Predicted protein | Presence in Hu22 | Presence in Po22 | Presence in Hu91 | Presence in Po91 |
| MAV_RS00575/MAV_0117 | PE | X | X | ||
| MAV_RS04540/MAV_0948 | Mce | X | X | ||
| MAV_RS04550/MAV_0950 | Mce | X | X | ||
| MAV_RS04555/MAV_0951 | Mce | X | X | ||
| MAV_RS04565/MAV_0953 | Mce | X | X | ||
| MAV_RS06460/MAV_1347 | PPE | X | X | ||
| MAV_RS24220/MAV_5047 | SR-21 Mce | X | X | ||
| MAV_RS24225/MAV_5048 | SR-21 Mce | X | X | ||
| MAV_RS24230/MAV_5049 | SR-21 Mce | X | X | ||
| MAV_RS24240/MAV_5051 | SR-21 Mce | X | X | ||
| MAV_RS24245/MAV_5052 | SR-21 Mce | X | X | ||
Discussion
A worldwide increase in the prevalence of human nontuberculous mycobacterial (NTM) infections has been observed since 2000 [31]. NTM infections are thought to be triggered essentially by exposure to environmental NTM that reside in soil and water. In recent years it has become clear that members of the Mycobacterium avium complex such as the subspecies M. avium subsp. hominissuis (Mah) cause a problem in immunocompetent adults (mostly respiratory tract infections) and children (lymphadenitis) as well as in immunocompromised AIDS patients [32,33]. Interestingly, advancement in sequence-based identification and genotyping has demonstrated a remarkable genetic diversity of the Mah subspecies [2].
On the basis of multisequence typing, we selected four swine and four human Mah isolates for a detailed analysis of in vivo virulence and confirmed a strong variation between the different isolates. Monitoring bacterial replication in the lungs over a period of 20 weeks, a human and porcine isolate with MST22 type were found to be highly virulent, whereas a human and porcine isolate with MST91 type were found to be fully avirulent. Mycobacteria-specific IFN-γ responses were highest in mice infected with the virulent isolates early during infection, but at the late stage, these responses were markedly suppressed and in parallel IL-10 responses were increased. These findings are reminiscent of the report of Roque et al. who compared the susceptibility of BALB/c and C57BL/6 mice to intravenous infection with M. avium subsp. avium strain 2447 and showed that the higher susceptibility of BALB/c than of C57BL/6 mice was related to higher IL-10 responses in the former strain [34]. In contrast, mice infected with Mah MST91 isolates, showed more modest but sustained IFN-γ levels throughout the entire study period, and at the late time point significant mycobacteria-specific IL-17 production could be measured, reflecting the presence of a possibly protective Th17 memory T cell population in mice infected with these Mah isolates of which the multiplication was controlled. Also in NTM patients with nodular bronchiectasis, it was suggested that susceptibility to pulmonary disease may reflect low IL-17 and high IL-10 responses, rather than a Th1 deficiency [35].
Immunodiagnosis of M. avium infection is hampered by the lack of specific antigens. Avian and bovine tuberculins (which are purified protein derivatives of autoclaved bacteria) are the reagents commercially available to assess exposure to Map in cell-mediated immune assays such as intradermal skin tests and IFN-γ release assays, but these assays are hampered by the presence in PPDs of many cross-reactive antigens. The use of M. avium specific antigens may help to overcome this specificity issue if a sufficient sensitivity could be obtained. By screening a series of 35 antigens, many predicted to be specific for M. avium, we identified here some protein candidates that hold promise for the detection of early infection using IFN-γ release assays (CF-036 and MAP2541c) and for the serodiagnosis of multibacillary disease (MAP3634). The MAP3634 antigen (new name MAP_RS18645) has a predicted function of a lipoprotein-anchoring transpeptidase, also known as lipoprotein LprQ. Interestingly, other lipoproteins such as the phosphate-binding protein PstS-1 of M. tuberculosis [36] and PstS-2 and PstS-3 of M. bovis BCG [37,38] are known to be powerful B cell antigens. A detailed analysis of the immunodiagnostic potential of these 35 proteins in cattle is in progress (Roupie et al., manuscript in preparation).
As stated by Winthrop et al., “studies of NTM pathogenesis and host immunity to NTM have been hampered by the lack of a robust animal model that can mimic human non-disseminated MAC pulmonary disease” [39]. Studies in non-human primates such as rhesus macaques could be a solution, but our results show that an intranasal infection of BALB/c mice with a properly selected avirulent strain such as the Mah MST91 could be a valuable alternative.
Uchiya et al. reported on genetic diversity in Mah strains that cause pulmonary and disseminated disease, and performed a comparative genome analysis of MahTH135, isolated from a HIV-negative patient with pulmonary disease [21] and Mah104, isolated from an adult AIDS patient in Southern California in 1983. The sequenced genome of M. avium strain 104 has been associated with disease in multiple patients in the western United States. Although M. avium is known for its genetic plasticity, this observation indicates that certain strains of the pathogen such as the Mah 104 isolate can be genotypically stable over extended periods of time [20]. In our study, the virulent human Mah MST22 strain was isolated from a 40 year old HIV-negative man with disseminated disease and the avirulent human Mah MST91 strain from a 40 year old AIDS patient with disseminated disease. Whole genome sequencing of these two human isolates was performed (in parallel with the MST22 and MST91 pig isolates) [19] and sequences were compared to the Mah TH135 and Mah 104 sequence, with respect to twenty-five candidate strain 104 specific genes and to twenty-two candidate strain TH135 specific genes, reported by Uchiya [21]. Porcine and human sequences aligned according to their MST type, and the two MST22 isolates had ten identical Mah strain 104 specific genes and one Mah TH135 gene, whereas the two MST91 isolates had only 2 genes identical with Mah strain 104 (Table 3). Among the nine putative Mce proteins identical in the MST22 isolates, five are localized in the strain-specific SR-21 region of the reference 104 strain, region with a sequence similarity of 70–75% with M. abscessus [21]. Although the precise mechanisms involving Mce proteins in virulence remain unclear, these proteins are thought to help mycobacteria to enter into macrophages, but they have also been implicated in uptake of cholesterol essential for long term survival in host cells.
Thus, genomic comparison indicated that the human and porcine MST22 isolates, were much more similar to the reference Mah 104 type than to the Mah TH135 type with respect to predicted virulence factors. Mah isolates exhibit geographic differences in genetic diversity, with isolates from Japan (such as Mah TH135) sharing a high degree of relatedness with Korean isolates, but not with isolates from Europe or the United States [2]. The strong genetic similarity of the human MST22 strain isolated in 2012 in Belgium (from an HIV-negative patient) with the Mah 104 strain (isolated in 1983 in the USA from an AIDS patient) seems to indicate that genetic differences are determined by the geographic origin rather than by the type of clinical symptoms. On the other hand, Moriyama et al. reported recently on an association between the presence of a pMAH135 plasmid in Mah isolates and the progression of pulmonary disease [30]. Interestingly, although a plasmid seems indeed to be involved for Japanese isolates, in our study, careful genome comparison failed to find any homology with the pMAH135 sequence in the four Belgian isolates.
In conclusion, our results show that intranasal infection of BALB/c mice can be used as a reliable experimental model to analyse variations in virulence of different Mah isolates. Very recently, Uchiya et al. performed a comparative genome analysis of 79 M. avium strains and on the basis of single nucleotide polymorphisms a phylogenetic tree was drawn [40]. The four Belgian Mah isolates were included in the study and they localized on the phylogenetic tree two by two according to their MST genotype, more specifically in the IIa subcluster [40]. Therefore, the striking link of virulence and identity at the genome level of porcine and human isolates with the same multisequence type, for which no correlation of place of residence (humans) or farm of origin (pigs) was observed, points to the presence in the environment of certain genotypes of Mah which may be more infectious for both humans and pigs than other genotypes.
Supporting information
Bacterial replication in lungs (A), spleen (B) and liver (C) of BALB/c mice infected with one of the seven Mah isolates, as measured at 2, 5, 9 and 20 weeks post-infection. Data represent mean CFU (log10)/organ ± SEM of five animals tested per group. Isolate IDs represent the host (Hu: human; Po: swine) followed by the respective multispacer sequence type (MST). Statistical significance is depicted for each strain as compared to both Po91 and Hu91 (*: p<0.05).
(TIF)
Acknowledgments
We are very grateful to Halo Cheickoito (Haute Ecole Francisco Ferrer) for help with the dot-blot assays.
Abbreviations
- CODA-CERVA
Centrum voor Onderzoek in Diergeneeskunde en Agrochemie- Onderzoek- Centre d’Etude et de Recherches Vétérinaires et Agrochimiques
- CODA-PIB-WIV
Centrum voor Onderzoek in Diergeneeskunde en Agrochemie-Pasteur Instituut van Brussel-Wetenschappelijk Instituut voor Volksgezondheid
- IFN-γ
interferon-gamma
- IL
interleukin
- IgG
Immunoglobulin
- MAC
Mycobacterium avium complex
- MST
multispacer sequence type
- Mce
mammalian cell entry
- ni-NTA
nickel- nitrilotriacetic acid
- NO
nitric oxide
- NTM
non tuberculous mycobacteria
- OADC
oleic acid-albumin-dextrose-catalase
- OPD
o-phenylenediamine
- PPD
purified protein derivative (of tuberculin)
- CF-A
culture filtrate of M. avium
- PPE
proline-proline-glutamic acid
- PE
proline-glutamic acid
- SR-21
Specific Region 21
- WIV-ISP
Wetenschappelijk Instituut voor Volksgezondheid-Institut de Santé Publique
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially financed by the Federal Public Service of Public Health, Safety of the Food Chain and Environment (Convention RT-07/6 PARATUB, 2008-2012 and RT12/5 LYMPHINDIC 1, 2012-2015). This work was also funded through a grant from the Walloon Region (J-JL and RW). The National Reference Centre for Tuberculosis & Mycobacteria is partially supported by the Belgian Ministry of Social Affairs through a fund within the Health Insurance System. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Genoscreen provided support in the form of salaries for author OC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Falkinham JO III (2015) Environmental sources of nontuberculous mycobacteria. Clin Chest Med 36: 35–41. S0272-5231(14)00106-3 [pii]; 10.1016/j.ccm.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa K, van Ingen J, Koh WJ, Wagner D, Salfinger M, Inagaki T et al. (2015) Genetic diversity of clinical Mycobacterium avium subsp. hominissuis and Mycobacterium intracellulare isolates causing pulmonary diseases recovered from different geographical regions. Infect Genet Evol 36: 250–255. S1567-1348(15)00405-0 [pii]; 10.1016/j.meegid.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 3.Turenne CY, Wallace R Jr., Behr MA (2007) Mycobacterium avium in the postgenomic era. Clin Microbiol Rev 20: 205–229. 20/2/205 [pii]. 10.1128/CMR.00036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mijs W, de HP, Rossau R, Van der Laan T, Rigouts L, Portaels F et al. (2002) Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and 'M. avium subsp. hominissuis' for the human/porcine type of M. avium. Int J Syst Evol Microbiol 52: 1505–1518. 10.1099/00207713-52-5-1505 [DOI] [PubMed] [Google Scholar]
- 5.Turenne CY, Collins DM, Alexander DC, Behr MA (2008) Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium are independently evolved pathogenic clones of a much broader group of M. avium organisms. J Bacteriol 190: 2479–2487. JB.01691-07 [pii]. 10.1128/JB.01691-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris NB, Barletta RG (2001) Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin Microbiol Rev 14: 489–512. 10.1128/CMR.14.3.489-512.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feller M, Huwiler K, Stephan R (2007) Mycobacterium avium subsp paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis 7: 607–613. 10.1016/S1473-3099(07)70211-6 [DOI] [PubMed] [Google Scholar]
- 8.Komijn RE, de Haas PE, Schneider MM, Eger T, Nieuwenhuijs JH, van den Hoek RJ et al. (1999) Prevalence of Mycobacterium avium in slaughter pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates. J Clin Microbiol 37: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agdestein A, Olsen I, Jorgensen A, Djonne B, Johansen TB (2014) Novel insights into transmission routes of Mycobacterium avium in pigs and possible implications for human health. Vet Res 45: 46 1297-9716-45-46 [pii]. 10.1186/1297-9716-45-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirkkonen T, Pakarinen J, Rintala E, Ali-Vehmas T, Marttila H, Peltoniemi OA et al. (2010) Comparison of variable-number tandem-repeat markers typing and IS1245 restriction fragment length polymorphism fingerprinting of Mycobacterium avium subsp. hominissuis from human and porcine origins. Acta Vet Scand 52: 21 1751-0147-52-21 [pii]. 10.1186/1751-0147-52-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agdestein A, Johansen TB, Kolbjornsen O, Jorgensen A, Djonne B, Olsen I (2012) A comparative study of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis in experimentally infected pigs. BMC Vet Res 8: 11 1746-6148-8-11 [pii]. 10.1186/1746-6148-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss CH, Glassroth J (2012) Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med 6: 597–612. 10.1586/ers.12.58 [DOI] [PubMed] [Google Scholar]
- 13.Despierres L, Cohen-Bacrie S, Richet H, Drancourt M (2012) Diversity of Mycobacterium avium subsp. hominissuis mycobacteria causing lymphadenitis, France. Eur J Clin Microbiol Infect Dis 31: 1373–1379. 10.1007/s10096-011-1452-2 [DOI] [PubMed] [Google Scholar]
- 14.Borsutzky S, Kretschmer K, Becker PD, Muhlradt PF, Kirschning CJ, Weiss S et al. (2005) The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol 174: 6308–6313. 174/10/6308 [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Vluggen C, Soetaert K, Duytschaever L, Denoel J, Fauville-Dufaux M, Smeets F et al. (2016) Genotyping and strain distribution of Mycobacterium avium subspecies hominissuis isolated from humans and pigs in Belgium, 2011–2013. Euro Surveill 21: 30111 30111 [pii]. 10.2807/1560-7917.ES.2016.21.3.30111 [DOI] [PubMed] [Google Scholar]
- 16.Johansen TB, Agdestein A, Lium B, Jorgensen A, Djonne B (2014) Mycobacterium avium subsp. hominissuis infection in swine associated with peat used for bedding. Biomed Res Int 2014: 189649 10.1155/2014/189649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangadharam PR (1995) Beige mouse model for Mycobacterium avium complex disease. Antimicrob Agents Chemother 39: 1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roupie V, Rosseels V, Piersoel V, Zinniel DK, Barletta RG, Huygen K (2008) Genetic resistance of mice to Mycobacterium paratuberculosis is influenced by Slc11a1 at the early but not at the late stage of infection. Infect Immun 76: 2099–2105. IAI.01137-07 [pii]. 10.1128/IAI.01137-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruffaerts N, Vluggen C, Duytschaever L, Mathys V, Saegerman C, Chapeira O et al. (2016) Genome Sequences of Four Strains of Mycobacterium avium subsp. hominissuis, Isolated from Swine and Humans, Differing in Virulence in a Murine Intranasal Infection Model. Genome Announc 4. 4/3/e00533-16 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horan KL, Freeman R, Weigel K, Semret M, Pfaller S, Covert TC et al. (2006) Isolation of the genome sequence strain Mycobacterium avium 104 from multiple patients over a 17-year period. J Clin Microbiol 44: 783–789. 44/3/783 [pii]. 10.1128/JCM.44.3.783-789.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchiya K, Takahashi H, Yagi T, Moriyama M, Inagaki T, Ichikawa K et al. (2013) Comparative genome analysis of Mycobacterium avium revealed genetic diversity in strains that cause pulmonary and disseminated disease. PLoS One 8: e71831 PONE-D-13-12459 [pii]. 10.1371/journal.pone.0071831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M et al. (1993) Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol 31: 2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayrou C, Turenne C, Behr MA, Drancourt M (2010) Genotyping of Mycobacterium avium complex organisms using multispacer sequence typing. Microbiology 156: 687–694. mic.0.033522–0 [pii]; 10.1099/mic.0.033522-0 [DOI] [PubMed] [Google Scholar]
- 24.Roupie V, Leroy B, Rosseels V, Piersoel V, Noel-Georis I, Romano M et al. (2008) Immunogenicity and protective efficacy of DNA vaccines encoding MAP0586c and MAP4308c of Mycobacterium avium subsp. paratuberculosis secretome. Vaccine 26: 4783–4794. S0264-410X(08)00902-X [pii]; 10.1016/j.vaccine.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Roupie V, Viart S, Leroy B, Romano M, Trichiero N, Govaerts M et al. (2012) Immunogenicity of eight Mycobacterium avium subsp. paratuberculosis specific antigens in DNA vaccinated and MAP infected mice. Vet Immunol Immunopathol 145: 74–85. 10.1016/j.vetimm.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 26.Leroy B, Roupie V, Noël-Georis I, Rosseels V, Walravens K, Govaerts M et al. (2007) Antigen discovery: a postgenomic approach to paratuberculosis diagnosis. Proteomics 7: 1164–1176. 10.1002/pmic.200600988 [DOI] [PubMed] [Google Scholar]
- 27.Leroy B, Viart S, Trinchero N, Roupie V, Govaerts M, Letesson JJ et al. (2009) Use of Mycobacterium avium subsp. paratuberculosis specific coding sequences for serodiagnosis of bovine paratuberculosis. Vet Microbiol 135: 313–319. 10.1016/j.vetmic.2008.09.065 [DOI] [PubMed] [Google Scholar]
- 28.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR et al. (2003) Inhibition of Respiration by Nitric Oxide Induces a Mycobacterium tuberculosis Dormancy Program. J Exp Med 198: 705–713. 10.1084/jem.20030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huygen K (2014) The Immunodominant T-Cell Epitopes of the Mycolyl-Transferases of the Antigen 85 Complex of M. tuberculosis. Front Immunol 5: 321 10.3389/fimmu.2014.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriyama M, Ogawa K, Nakagawa T, Nikai T, Uchiya K (2016) Association between a pMAH 135 plasmid and the progression of pulmonary disease caused by Mycobacterium avium. Kekkaku 91: 9–15. [PubMed] [Google Scholar]
- 31.Prevots DR, Marras TK (2015) Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36: 13–34. S0272-5231(14)00105-1 [pii]; 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook JL (2010) Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br Med Bull 96: 45–59. ldq035 [pii]; 10.1093/bmb/ldq035 [DOI] [PubMed] [Google Scholar]
- 33.Slany M, Ulmann V, Slana I (2016) Avian Mycobacteriosis: Still Existing Threat to Humans. Biomed Res Int 2016: 4387461 10.1155/2016/4387461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roque S, Nobrega C, Appelberg R, Correia-Neves M (2007) IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J Immunol 178: 8028–8035. 178/12/8028 [pii]. [DOI] [PubMed] [Google Scholar]
- 35.Lim A, Allison C, Price P, Waterer G (2010) Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol 137: 296–302. S1521-6616(10)00677-7 [pii]; 10.1016/j.clim.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson RJ, Haslov K, Rappuoli R, Giovannoni F, Narayanan PR, Desai CR et al. (1997) Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. S -Myc 79 35: 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huygen K, Drowart A, Harboe M, ten BR, Cogniaux J, Van Vooren JP (1993) Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect Immun 61: 2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanghe A, Lefevre P, Denis O, D'Souza S, Braibant M, Lozes E et al. (1999) Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol 162: 1113–1119. [PubMed] [Google Scholar]
- 39.Winthrop K, Rivera A, Engelmann F, Rose S, Lewis A, Ku J et al. (2016) A Rhesus Macaque Model of Pulmonary Nontuberculous Mycobacterial Disease. Am J Respir Cell Mol Biol 54: 170–176. 10.1165/rcmb.2015-0256RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchiya KI, Tomida S, Nakagawa T, Asahi S, Nikai T, Ogawa K (2017) Comparative genome analyses of Mycobacterium avium reveal genomic features of its subspecies and strains that cause progression of pulmonary disease. Sci Rep 7: 39750 srep39750 [pii]; 10.1038/srep39750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial replication in lungs (A), spleen (B) and liver (C) of BALB/c mice infected with one of the seven Mah isolates, as measured at 2, 5, 9 and 20 weeks post-infection. Data represent mean CFU (log10)/organ ± SEM of five animals tested per group. Isolate IDs represent the host (Hu: human; Po: swine) followed by the respective multispacer sequence type (MST). Statistical significance is depicted for each strain as compared to both Po91 and Hu91 (*: p<0.05).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.