Abstract
The dorsal raphe nucleus (DRN) is embedded in the ventral part of the caudal periaqueductal gray (PAG). Electrical or chemical activation of neurons throughout this region produces antinociception. The objective of this manuscript is to determine whether the ventrolateral PAG and DRN are distinct antinociceptive systems. This hypothesis was tested by determining the antinociceptive potency of microinjecting morphine into each structure (Experiment 1), creating a map of effective microinjection sites that produce antinociception (Experiment 2), and comparing the development of antinociceptive tolerance to repeated microinjections of morphine into the ventrolateral PAG and DRN (Experiment 3). Morphine was more potent following cumulative injections (1.0, 2.2, 4.6, &10 μg/0.2 μl) into the ventrolateral PAG (D50 = 3.3 μg) compared to the lateral (4.3 μg) or medial DRN (5.8 μg). Antinociception occurred following 94% of the morphine injections into the ventrolateral PAG, whereas only 68.3% and 78.3% of the injections into the lateral and medial aspects of the DRN produced antinociception. Repeated microinjections of morphine into the ventrolateral PAG produced tolerance as indicated by a 528% difference in potency between morphine and saline pretreated rats. In contrast, relatively small changes in potency occurred following repeated microinjections of morphine into the lateral and medial aspects of the DRN (107% and 49%, respectively). These data indicate that the ventrolateral PAG and DRN are distinct antinociceptive structures. Antinociception is greater with injections into the ventrolateral PAG compared to the DRN, but this antinociception disappears rapidly because of the development of tolerance.
Keywords: Analgesia, Opioid, Periaqueductal gray, Pain modulation, Microinjection
Graphical Abstract
Microinjection of morphine produced greater antinociception and greater tolerance to this antinociception following injections into the ventrolateral periqueductal gray (PAG) compared to the lateral or medial aspects of the dorsal raphe nucleus (DRN). These data indicate that the ventrolateral PAG and adjacent DRN are distinct pain modulatory systems.
Introduction
Activation of the periaqueductal gray (PAG) produces antinociception in a wide range of animals including humans (Mayer, 1984). These antinociceptive effects vary depending on whether neurons in the ventrolateral or lateral/dorsal regions of the PAG are activated. Antinociception mediated by the ventrolateral PAG has a rapid onset (Yaksh et al., 1976), is susceptible to tolerance (Morgan & Liebeskind, 1987; Tortorici et al., 1999; Morgan et al., 2006), and is associated with defensive immobility (Fardin et al., 1984a; Morgan et al., 1998). In contrast, antinociception mediated by the lateral and dorsal regions of the PAG has a slower onset, is resistant to the development of tolerance, and is associated with defensive flight. Although differences between the lateral and ventrolateral PAG have been well characterized (Morgan, 1991), differences in antinociception between the ventrolateral PAG and adjacent dorsal raphe nucleus (DRN) are less clear.
The DRN is a midline group of serotonergic neurons located in the ventral caudal aspect of the PAG adjacent to the ventrolateral PAG column. The DRN contains opioid receptors (Yaksh et al., 1976; Mansour et al., 1995; Jolas & Aghajanian, 1997; Kalyuzhny & Wessendorf, 1997; Prado & Faganello, 2000; Ferreira & Menescal-de-Oliveira, 2014), and microinjection of the opioid morphine produces antinociception that seems indistinguishable from that mediated by the ventrolateral PAG (Yaksh et al., 1976; Prado & Faganello, 2000; Ferreira & Menescal-de-Oliveira, 2014). That is, antinociception occurs in the absence of overt behavioral reactions (Fardin et al., 1984a), and tolerance to the antinociceptive effects appears to occur with repeated microinjections of morphine into either site (Morgan et al., 2006; Ge et al., 2007). Unfortunately, these studies do not reveal whether the PAG and DRN are distinct antinociceptive systems or if opioids produce similar effects because they simply diffuse from one structure to the other.
The objective of the present study is to compare the antinociceptive effects of morphine microinjections into the DRN and ventrolateral PAG. Given the unique pharmacology and anatomy of these two structures (Hornung, 2003; Carrive & Morgan, 2012), it is hypothesized that the antinociception mediated by the DRN and ventrolateral PAG is distinct. The first experiment will compare the potency for morphine to produce antinociception when microinjected into the DRN and ventrolateral PAG. The second experiment will map the antinociceptive effects of microinjecting morphine at sites across the DRN and ventrolateral PAG. And, Experiment 3 will examine differences in susceptibility to tolerance to repeated microinjections of morphine into the DRN and ventrolateral PAG.
Materials and methods
Subjects
Male Sprague-Dawley rats (230–400 g; Harlan Laboratories, Livermore, California, USA) were anesthetized with pentobarbital (60 mg/kg, i.p) and implanted with a guide cannula (23 gauge, 9 mm long) aimed at either the ventrolateral PAG or DRN using stereotaxic techniques (AP: +1.7 mm, DV: −4.6 mm from lambda, and ML= +0.6 or 0.0 mm for ventrolateral PAG and DRN, respectively). The guide cannula was secured to two screws in the skull with dental cement. At the end of surgery a stylet was inserted to plug the guide cannula, and the rat was placed under a heat lamp until awake.
The rat was housed individually or in pairs following surgery in a temperature controlled room on a standard 12 hr light cycle (On at 0700). Food and water were available continuously except during testing. Rats were handled daily prior to testing. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Institutional Animal Care and Use Committee at Washington State University. The number of rats used were minimized by using a cumulative dosing procedure to generate dose response curves and culling raw data from previously conducted studies instead of testing new rats when possible. Suffering was kept to a minimum by measuring the threshold for nociception using the hot plate test.
Hot Plate Test
Nociception was assessed with the hot plate test. Rats were placed on a 52.5 °C hot plate and the latency to lick a hind paw was measured. The rat was removed from the hot plate if no response occurred within 50 s. These rats were assigned a hot plate latency of 50 s for all subsequent tests and no additional testing was conducted. The hot plate test was selected because it has been used extensively to assess antinociception mediated by the PAG, and it can be applied repeatedly with minimal suffering to the rat.
Microinjections
An 11 mm injection cannula (31 gauge) was inserted into the guide cannula one day prior to testing to habituate the rat to the procedure and reduce potential confounds from mechanical activation of neurons. Morphine sulfate (a gift form the National Institute on Drug Abuse) was administered one day later. The cannula extended 2 mm beyond the tip of the guide cannula. Morphine was administered in a volume of 0.2 or 0.4 μl at a rate of 0.1 μl/10 s while the rat was gently restrained by hand. The injection cannula remained in place for an additional 20 s to limit backflow of morphine through the guide cannula. The rat was returned to its home cage until testing.
Experiment 1: Morphine Potency
A cumulative dose microinjection procedure was used to generate antinociceptive dose response curves as described in our previous manuscript (Morgan et al., 2006). Third log doses of morphine sulfate (1.0, 2.2, 4.6, &10 μg/0.2 μl) were microinjected into the ventrolateral PAG and DRN. Morphine was injected in 20 min intervals, and nociception was assessed 15 min after each injection. Potency (D50), defined as the dose of half maximal antinociception, was calculated using GraphPad Prism (La Jolla, California, USA).
Experiment 2: Localization of Morphine Antinociception
The effect of morphine microinjections into the ventrolateral PAG and DRN were examined in 119 rats. In order to get a large enough sample size to map the ventrolateral PAG and DRN, data from four of our previous experiments (Bobeck et al., 2009; Bobeck et al., 2012; Bobeck et al., 2014; Morgan et al., 2014) in which morphine was injected as a control were reanalyzed based on injection site. The procedure was identical in all four experiments. Only rats injected with morphine in the absence of any other drugs were included.
Rats were tested one week following cannula implantation. Nociception was assessed using the hot plate test before and 30 min after microinjection of morphine (5 μg/0.4 μl). Rats were euthanized following completion of testing, and the brain was removed to identify the injection site. Only injection sites located in the ventrolateral PAG or DRN were included in data analysis.
Experiment 3: Morphine Tolerance
The magnitude of tolerance in the ventrolateral PAG and lateral and medial divisions of the DRN was assessed. Thirty-nine rats received repeated morphine injections and 57 rats received repeated saline injections as a control. A subset of these data (30 morphine and 35 saline rats) were compiled from the control groups in a previously published manuscript (Morgan et al., 2014). The overall sample size for each condition ranged from 8 – 25 rats with 3 – 8 rats in each condition prepared and tested specifically for this experiment. There was no significant difference between previously and currently tested rats for morphine tolerant (F(1,37) = 0.330, p = .57) or saline control rats (F(1,55) = 2.098, p = .15). All rats underwent the same procedure: Morphine (5 μg/0.4 μl) or saline (0.4 μl) was microinjected twice a day for 2 days. Tolerance was assessed on Day 3 using cumulative doses of morphine (1.0, 2.2, 4.6, &10 μg) administered at 20 min intervals. Nociception was assessed using the hot-plate test 15 min after each injection. Tolerance was assessed within the ventrolateral PAG and DRN by comparing the difference in morphine potency between rats pretreated with saline and morphine.
Histology
Following the last test, the rat was exposed to a lethal dose of the inhalation anesthetic Halothane. The brain was removed and placed in formalin (10%). At least two days later the PAG was sectioned coronally (100 μm sections) and placed on a slide. The slide was viewed at 10× magnification to identify the location of the injection site at the end of the cannula track (Paxinos & Watson, 2005).
Data Analysis
Antinociceptive dose response curves for morphine were plotted from raw hot plate data using non-linear regression (GraphPad Prism). The lower limit was set at the mean baseline hot plate latency and the upper limit was set at the cut-off value of 50 s. ANOVA was used to compare the antinociceptive effects between injection sites. Only rats with injections in or on the border of the ventrolateral PAG or DRN were included in the analysis.
Results
Experiment 1: Morphine potency
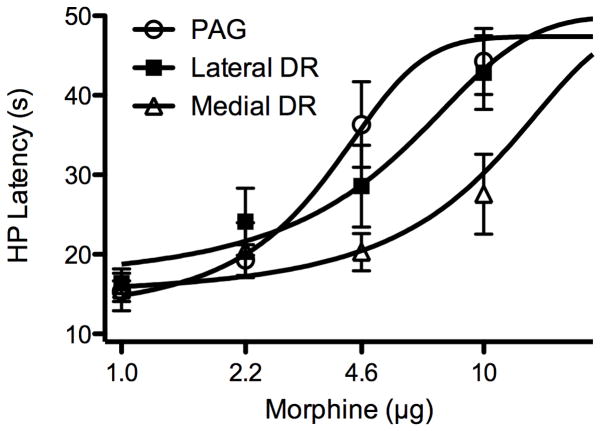
Morphine potency to produce antinociception was determined by administering cumulative doses (1.0, 2.2, 4.6, &10 μg/0.2 μl) into the ventrolateral PAG and DRN. Microinjection of morphine produced a dose-dependent increase in hot plate latency whether injected into the ventrolateral PAG or DRN (Figure 1). The antinociceptive potency varied depending on whether morphine was injected in the ventrolateral PAG, lateral DRN, or medial DRN (these three regions are identified by distinct shading in Figure 2A). Morphine potency to produce antinociception was greatest in the ventrolateral PAG (D50 = 3.3 μg) compared to injections in the lateral (D50 = 4.3 μg) and medial DRN (D50 = 5.8 μg) (Figure 1). A significant difference in the antinociception mediated by these regions was only evident at the two highest doses of morphine (F(2,22) = 4.922. p = 0.017).
Figure 1.
Morphine produces greater antinociception when microinjected into the ventrolateral PAG compared to the dorsal raphe nucleus (DRN). Antinociception to cumulative injections of third log doses of morphine (0.2 μl) was assessed using the hot plate (HP) test. Morphine potency was greatest following microinjections into the ventrolateral PAG (3.3 μg; n = 9) compared to the lateral (4.3 μg; n = 7) or medial DRN (5.8 μg; n = 9) (the shading in Figure 2A identifies the boundaries of these three regions). Antinociception was significantly greater following injections of the two highest doses of morphine into the ventrolateral PAG compared to the medial DRN (p < .05).
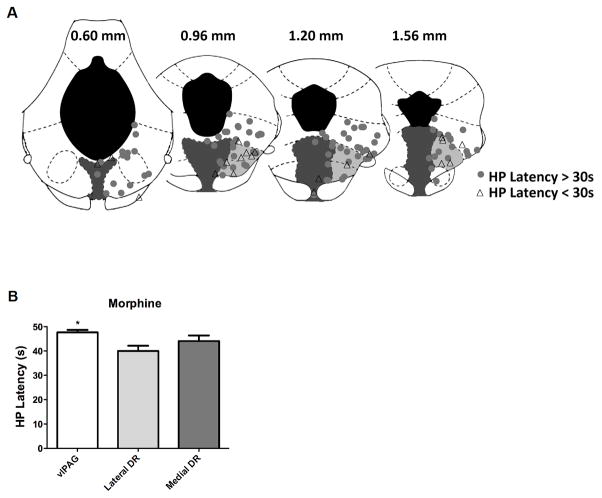
Figure 2.
Antinociception is greatest following microinjection of morphine into the ventrolateral PAG. A) Location of morphine injection sites on coronal planes through the PAG and DRN relative to the interaural line (Paxinos & Watson, 2005). Injections into the laterodorsal tegmental nucleus in the caudal PAG (0.60 mm section) were included as part of the ventrolateral PAG (white). The medial DRN (dark gray) is directly below the aqueduct and the lateral DRN (light gray) is lateral to the medial DRN and ventral to the ventrolateral PAG. Antinociception (circles) was defined as a hot plate latency greater than 30 s (triangles indicate a hot plate latency < 30 s). Almost all of the morphine injections into the ventrolateral PAG or medial aspect of the DRN produced antinociception compared to injections into the lateral aspect of the DRN. The location of these injection sites are representative of the injection sites in Experiments 1 & 2 except the distribution of injections into the core of the medial DRN were greater in Experiments 1 & 2. B) Mean hot plate latency was also significantly higher following morphine microinjections into the ventrolateral PAG (n = 55) and medial DRN (n = 23) compared to the lateral DRN (n = 41) (*F(2,116) = 5.954, p = .004).
Experiment 2: Localization of Morphine Antinociception
The objective of this experiment was to create a map of ventrolateral PAG and DRN injection sites that produce morphine antinociception. The antinociceptive effect of an acute microinjection of morphine (5 μg/0.4 μl) was examined at 119 sites. Most of the injections were in the ventrolateral PAG (n = 55) followed by the lateral DRN (41 injections) and medial DRN (n = 23).
Antinociception was evident with microinjections throughout the ventrolateral PAG and DRN. The location of these microinjections revealed that the ventrolateral PAG was the most effective site for morphine antinociception, and the lateral aspect of the DRN was the least effective region. Antinociception, defined as an increase in hot plate latency greater than 30 s, occurred at 94.5% of morphine injections into the ventrolateral PAG compared to 68.3% in the lateral DRN and 78.3% in the medial DRN (Figure 2A). Chi Square analysis revealed that injections of morphine into the ventrolateral PAG were significantly more likely to produce antinociception (X2 = 11.5, p = .003). This difference in probability corresponded to a small differences in mean antinociception (Figure 2B). Mean hot plate latency was greatest following microinjection of morphine into the ventrolateral PAG compared to the lateral or medial DRN (F(2,116) = 5.954, p = .004). In contrast, there was no difference in hot plate latency when posterior (Interaural sections 0.60 & 0.96 mm; n = 57) and anterior (Interaural sections 1.20 & 1.56; n = 62) injections were compared. Mean hot plate latencies (± SEM) at posterior and anterior microinjection sites were 44.0 ± 1.5 and 44.8 ± 1.5 s, respectively.
Experiment 3: Morphine Tolerance
The objective of this experiment was to determine whether the greater antinociceptive potency in the ventrolateral PAG compared to the DRN reported in Experiments 1 and 2 would lead to greater antinociceptive tolerance. This hypothesis was tested by examining morphine potency following two days of twice-daily microinjections of morphine or saline into the ventrolateral PAG or DRN.
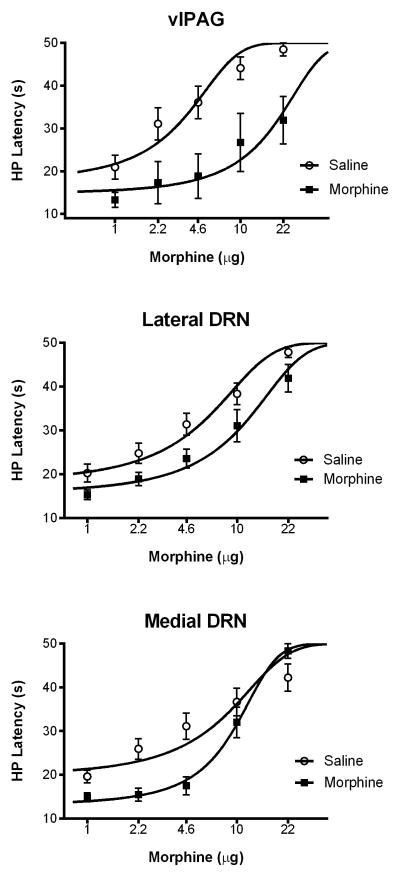
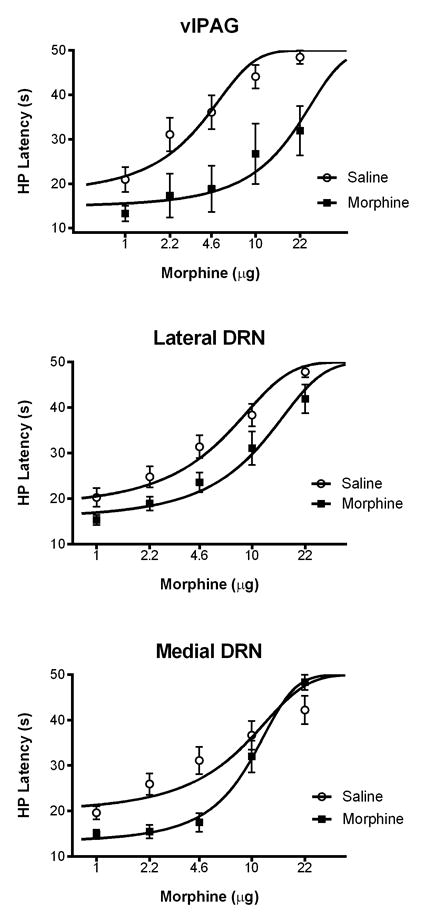
The antinociception produced by microinjection of morphine into the ventrolateral PAG or DRN was susceptible to tolerance with repeated administration. The decrease in morphine potency in rats pretreated with morphine as opposed to saline was statistically significant following injections into the ventrolateral PAG (F(1,134) = 36.22, p = .0001), lateral DRN (F(1,264) = 22.73, p = .0001), and medial DRN (F(1,162) = 5.158, p = .024) (Figure 3). However, the magnitude of tolerance measured as a decrease in morphine potency between saline and morphine pretreated rats was much greater with microinjections into the ventrolateral PAG compared to injections into the lateral or medial DRN (Table 1).
Figure 3.
Tolerance to morphine antinociception was greatest following microinjections into the ventrolateral PAG compared to the DRN. A significant rightward shift in the morphine dose response curve occurred in all three brain regions following repeated morphine microinjections. Changes in morphine potency were compared to saline pretreated rats and measured with the hot plate (HP) test. The magnitude of morphine tolerance was greatest with injections into the ventrolateral PAG (top) compared to the lateral (middle) or medial (bottom) DRN (see Table 1).
Table 1.
Comparison of change in morphine potency (D50) following repeated microinjections into the ventrolateral PAG, lateral, and medial DRN.
| Injection Site | Saline | Morphine | % Decrease in Potency |
|---|---|---|---|
| Ventrolateral PAG | 2.5 μg | 15.7 μg | 528% |
| Lateral DRN | 4.3 μg | 8.9 μg | 107% |
| Medial DRN | 6.3 μg | 9.4 μg | 49% |
Note: PAG = Periaqueductal Gray; DRN = Dorsal Raphe Nucleus
Discussion
The present data show that the potency for morphine antinociception and the development of antinociceptive tolerance is greater in the ventrolateral PAG compared to the DRN. The difference in antinociception is driven both by greater morphine potency and a greater probability that injection sites within the ventrolateral PAG will produce antinociception compared to injections in the DRN. The difference in morphine tolerance between these regions is consistent with this difference in antinociception. Repeated injections of morphine into the ventrolateral PAG produced a large decrease in morphine potency to produce antinociception compared to repeated injections into the DRN. Taken together, these data reveal clear differences in morphine antinociception mediated by the ventrolateral PAG and DRN.
Morphine was more potent in producing antinociception when injected into the ventrolateral PAG compared to the DRN whether a small (0.2 μl in Experiment 1) or moderate (0.4 μl in Experiments 2) volume was injected, or measured as a difference in potency (Experiment 1) or the number of sites producing antinociception (Experiment 2). Morphine potency was greater following cumulative injections of 0.4 μl (D50 = 2.5 μg for the PAG control group in the tolerance experiment) compared to 0.2 μl of morphine (D50 = 3.3 μg) as would be expected with greater spread of drug with a large volume, but the greater potency with microinjections into the ventrolateral PAG compared to the DRN was consistent regardless of injection volume. Although previous studies have reported “strong antinociception” following an injection of morphine into the DRN (Sharpe et al., 1974; Yaksh et al., 1976; Prado & Faganello, 2000), the present study is the first to assess antinociceptive potency following dose-response analysis. Our cumulative dosing procedure (Morgan et al., 2006) shows that morphine is more potent when injected into the ventrolateral PAG compared to the DRN.
Although microinjection of morphine into the DRN could be caused by diffusion into the ventrolateral PAG, our data indicate that the ventrolateral PAG and DRN are distinct antinociceptive systems. It is possible that diffusion of morphine from the DRN to the ventrolateral PAG contributes to morphine antinociception in that antinociceptive potency was lowest for injections into the medial DRN, the most distant site from the PAG, whether 0.2 μl (Experiment 1) or 0.4 μl (Control groups in Experiment 3) was injected. However, diffusion of morphine to the ventrolateral PAG does not account for all of the antinociceptive effects because the mapping experiment (Experiment 2) showed that the lateral DRN located between the ventrolateral PAG and medial DRN is the least likely to support morphine antinociception. If antinociception were caused by diffusion of morphine from the DRN to the ventrolateral PAG, then antinociception would be greater with injections into the adjacent lateral DRN than the more distally located medial DRN. The medial and lateral DRN also differ in that serotonergic neurons are located in the medial DRN and GABAergic neurons more laterally (Challis et al., 2013). Mu-opioid receptors are present in both the PAG and DRN (Kalyuzhny et al., 1996), and in both locations, opioids appear to produce antinociception by inhibiting GABAergic neurons (Jolas & Aghajanian, 1997; Vaughan et al., 1997; Tao & Auerbach, 2005). Analysis of the 119 injections also revealed that antinociception is independent of proximity to the cerebral aqueduct. Antinociception occurred whether the injection site was near or far from the aqueduct, a finding consistent with previous mapping studies using morphine microinjections (Yaksh et al., 1976) or electrical stimulation (Fardin et al., 1984b).
Proximity of the injection to the ventrolateral PAG corresponded to the magnitude of tolerance to the antinociceptive effect of repeated morphine microinjections. A large rightward shift in the morphine dose response curve was evident in rats receiving repeated morphine injections into the ventrolateral PAG. The magnitude of tolerance decreased in a step-wise manner as the injections moved from the ventrolateral PAG to the adjacent lateral DRN and then to the more distal medial DRN (Table 1). These data indicate that tolerance is caused by adaptations in the ventrolateral PAG. Recent studies indicate that both intracellular signaling adaptations (Morgan et al., 2014) and morphine activation of glia (Eidson & Murphy, 2013) contribute to this tolerance.
Although tolerance to morphine microinjections into the ventrolateral PAG have been reported numerous times previously (Jacquet & Lajtha, 1976; Siuciak & Advokat, 1987; Tortorici et al., 1999; Tortorici et al., 2001; Lane et al., 2004; Morgan et al., 2006; Bobeck et al., 2012), the present study shows that the magnitude of tolerance is much greater when injections are limited to the ventrolateral PAG as opposed to adjacent structures. Our previous research showing a lack of tolerance to repeated morphine microinjections or continuous electrical stimulation of the lateral PAG (Morgan & Liebeskind, 1987; Tortorici et al., 1999) puts a similar focus on the ventrolateral PAG as a key brain region contributing to tolerance to the antinociceptive effects of opioids.
The difference in susceptibility to tolerance between the ventrolateral PAG and DRN provides additional evidence that these are two distinct pain modulatory systems. Antinociception produced by focal electrical stimulation of the DRN (Sanders et al., 1980; Fardin et al., 1984a) or the selective antinociception produced by microinjection of the nicotinic receptor agonist epibatidine into the DRN provides additional evidence that neurons within the DRN produce antinociception independent of the PAG (Cucchiaro et al., 2005). Although the PAG makes numerous connections throughout the brain (Carrive & Morgan, 2012), the antinociceptive effects mediated by the ventrolateral PAG appear to be mediated by a descending projection to the rostral ventromedial medulla (Prieto et al., 1983; Basbaum & Fields, 1984; Sandkuhler & Gebhart, 1984; Tortorici & Morgan, 2002; Morgan et al., 2008). These behavioral data are consistent with anatomical data showing retrograde labeling from the rostral ventromedial medulla to the PAG, but not to the DRN (Kalyuzhny et al., 1996).
The primary output of the DRN is to forebrain structures (Azmitia & Segal, 1978; Hornung, 2003), although projections to the dorsolateral PAG have been demonstrated (Stezhka & Lovick, 1994; Viana et al., 1997) and could contribute to DRN mediated antinociception. Systemic administration of morphine causes serotonin release in a number of forebrain structures—an effect that is blocked by infusion of the opioid receptor antagonist naloxone into the DRN (Tao & Auerbach, 1995; 2002). A rostral pathway for DRN mediated antinociception is consistent with the preservation of stimulation-produced antinociception from neurons in and around the DRN despite transection of descending outputs (Morgan et al., 1989).
In conclusion, the present study provides the most comprehensive comparison of morphine antinociception mediated by the ventrolateral PAG and DRN. Differences in the antinociceptive potency of morphine and the development of morphine tolerance demonstrate that the ventrolateral PAG and DRN are distinct antinociceptive systems. Although the antinociceptive potency of morphine is greater with microinjections into the ventrolateral PAG compared to the DRN, this antinociception is offset by the pronounced tolerance that occurs with repeated morphine injections. Less clear are the specific neurons within each structure that mediate these effects. A wide range of neurons with different inputs and outputs exist within each region (Pollak Dorocic et al., 2014), and molecular and genetic techniques will be needed to provide a more refined understanding of these circuits.
Acknowledgments
Funding provided by NIDA grant DA015498 and by funds provided for medical and biological research by the State of Washington Initiative Measure 171. The technical assistance of Shauna Schoo is greatly appreciated.
Abbreviations
- DRN
dorsal raphe nucleus
- PAG
periaqueductal gray
- D50
dose of half maximal antinociception
Footnotes
Author Contributions
Kyle Campion’s and Kimber Saville’s contributions included experimental design, data collection, and manuscript editing. Michael Morgan’s contributions included experimental design, data analysis, and writing the manuscript.
None of the authors have a conflict of interest with the research presented.
References
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bobeck EN, Chen Q, Morgan MM, Ingram SL. Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology. 2014;39:2142–2152. doi: 10.1038/npp.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Haseman RA, Hong D, Ingram SL, Morgan MM. Differential development of antinociceptive tolerance to morphine and fentanyl is not linked to efficacy in the ventrolateral periaqueductal gray of the rat. J Pain. 2012;13:799–807. doi: 10.1016/j.jpain.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Morgan MM. Periaqueductal gray. In: Paxinos G, Mai JM, editors. The Human Nervous System. Academic Press; Boston: 2012. pp. 368–401. [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33:13978–13988. 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiaro G, Chaijale N, Commons KG. The dorsal raphe nucleus as a site of action of the antinociceptive and behavioral effects of the alpha4 nicotinic receptor agonist epibatidine. J Pharmacol Exp Ther. 2005;313:389–394. doi: 10.1124/jpet.104.079368. [DOI] [PubMed] [Google Scholar]
- Eidson LN, Murphy AZ. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33:15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984a;306:105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984b;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Ferreira MD, Menescal-de-Oliveira L. Nociceptive vocalization response in guinea pigs modulated by opioidergic, GABAergic and serotonergic neurotransmission in the dorsal raphe nucleus. Brain Res Bull. 2014;106:21–29. doi: 10.1016/j.brainresbull.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Ge ZJ, Li C, Zhang LC, Zeng YM, Cao JL, Dai TJ, Wang JK, Cui GX, Tan YF, Zhao YP, Liu GJ. Involvement of local orphanin FQ in the development of analgesic tolerance induced by morphine microinjections into the dorsal raphe nucleus of rats. Neurosci Lett. 2007;413:233–237. doi: 10.1016/j.neulet.2006.11.077. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. CNS GABA neurons express the mu-opioid receptor: immunocytochemical studies. Neuroreport. 1997;8:3367–3372. doi: 10.1097/00001756-199710200-00035. [DOI] [PubMed] [Google Scholar]
- Lane DA, Tortorici V, Morgan MM. Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience. 2004;125:63–69. doi: 10.1016/j.neuroscience.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Mayer DJ. Analgesia produced by electrical stimulation of the brain. Prog Neuro-Psychoph. 1984;8:557–564. doi: 10.1016/0278-5846(84)90015-0. [DOI] [PubMed] [Google Scholar]
- Morgan MM. Differences in antinociception evoked from dorsal and ventral regions of the caudal periaqueductal gray matter. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 139–150. [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Be. 2006;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Liebeskind JC. Site specificity in the development of tolerance to stimulation-produced analgesia from the periaqueductal gray matter of the rat. Brain Res. 1987;425:356–359. doi: 10.1016/0006-8993(87)90519-1. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Reid RA, Saville KA. Functionally selective signaling for morphine and fentanyl antinociception and tolerance mediated by the rat periaqueductal gray. PloS One. 2014;9:e114269. doi: 10.1371/journal.pone.0114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502:61–66. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140:376–386. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. Academic Press; Sydney: 2005. [Google Scholar]
- Pollak Dorocic I, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlen M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Prado WA, Faganello FA. The anterior pretectal nucleus participates as a relay station in the glutamate-, but not morphine-induced antinociception from the dorsal raphe nucleus in rats. Pain. 2000;88:169–176. doi: 10.1016/S0304-3959(00)00326-2. [DOI] [PubMed] [Google Scholar]
- Prieto GJ, Cannon JT, Liebeskind JC. N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res. 1983;261:53–57. doi: 10.1016/0006-8993(83)91282-9. [DOI] [PubMed] [Google Scholar]
- Sanders KH, Klein CE, Mayor TE, Heym C, Handwerker HO. Differential effects of noxious and non-noxious input on neurones according to location in ventral periaqueductal grey or dorsal raphe nucleus. Brain Res. 1980;186:83–97. doi: 10.1016/0006-8993(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Sharpe LG, Garnett JE, Cicero TJ. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behav Biol. 1974;11:303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- Stezhka VV, Lovick TA. Inhibitory and excitatory projections from the dorsal raphe nucleus to neurons in the dorsolateral periaqueductal gray matter in slices of midbrain maintained in vitro. Neuroscience. 1994;62:177–187. doi: 10.1016/0306-4522(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience. 1995;68:553–561. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 2005;1049:70–79. doi: 10.1016/j.brainres.2005.04.076. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM. Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. J Neurophysiol. 2002;88:1707–1715. doi: 10.1152/jn.2002.88.4.1707. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM, Vanegas H. Tolerance to repeated microinjection of morphine into the periaqueductal gray is associated with changes in the behavior of off- and on-cells in the rostral ventromedial medulla of rats. Pain. 2001;89:237–244. doi: 10.1016/s0304-3959(00)00367-5. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Viana MB, Graeff FG, Loschmann PA. Kainate microinjection into the dorsal raphe nucleus induces 5-HT release in the amygdala and periaqueductal gray. Pharmacol Biochem Be. 1997;58:167–172. doi: 10.1016/s0091-3057(96)00451-0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]