Abstract
Puerarin was a major isoflavonoid derived from the Chinese medical herb radix puerariae (Gegen). In present study effect of puerarin on cisplatin nephrotoxicity was evaluated. Rat model of nephrotoxicity was established by a single intraperitoneal injection of cisplatin (7mg/kg). Puerarin was administrated through caudal vein injection once per day at the dose of 10mg/kg, 30mg/kg and 50mg/kg. Biochemical assays showed that after cisplatin treatment the serum urea and creatinine increased significantly compared with control (P<0.05). Cisplatin treatment significantly increased xanthine oxidase (XO) activity and malondialdehyde (MDA) formation, and significantly decreased the levels and /or activities of enzymatic and non-enzymatic antioxidants (GSH, GPx, GST, GR, SOD, CAT), in the kidney tissues. Renal levels of TNF-α and IL-6, two important inflammatory cytokines, were also upregulated by cisplatin. Histopathological examination indicated that cisplatin treatment resulted in severe necrosis and degeneration, hyaline casts in the tubules, intertubular hemorrhage, congestion and swelling in glomerulus and leukocytes infiltration in the kidney tissues. Western blot results demonstrated that cisplatin increased TLR4 and NF-κB protein expression in the kidney tissues. However, all these changes induced by cisplatin were significantly attenuated by puerarin treatment in dose-dependent manner, which indicated the renal protective effect of puerarin. Cell culture experiments illustrated that puerarin alone treatment concentration-dependently inhibited COLO205 and HeLa tumor cell growth and dose-dependently promoted the antitumor activity of cisplatin in COLO205 and HeLa tumor cells. The promotion effects might be attributed to suppression of cisplatin-increased NF-κB p65 expression by puerarin. Taken together, findings in this study suggested that puerarin exhibited renal protection against cisplatin nephrotoxicity via inhibiting TLR4/NF-κB signaling, with no inhibition but promotion effect on the antitumor activity of cisplatin. Puerarin might be a promising adjuvant agent for cisplatin chemotherapy.
Introduction
As one of most potent anti-tumor drugs, cisplatin was used for treatment of a wide variety of solid tumors including testicular, ovarian, bladder, endometrial, cervical, and lung cancers [1–5]. However, due to the severity of side toxicities including nephrotoxicity, clinical use of cisplatin was constrained in 25%–35% of the hospitalized patients undergoing chemotherapy [6]. Owing to the fact that cisplatin exerted potent antitumor activity and couldn’t be abandoned at present in clinic, an urgent demand existed for cancer researchers to develop new adjuvant therapy to ameliorate toxicities of cisplatin without inhibitory effects on the antitumor activity of cisplatin. Recently, phytochemicals attracted more and more eyeballs of cancer scientists [7, 8] and some herbal compounds have been studied to attenuate cisplatin nephrotoxicity [6, 9–12], which might potentially help to expand the clinical application of cisplatin.
Puerarin, a major isoflavonoid derived from the Chinese medical herb radix puerariae (Gegen), has been reported to harbor a wide spectrum of pharmacological properties including antioxidant and anti-inflammatory, present many beneficial effects on various medicinal purposes including treating neuronal disease, cardiovascular and cerebrovascular disease [13, 14]. Recent investigations showed that puerarin had in vitro and in vivo antitumor activities through induction of mitochondria-mediated apoptosis pathway [13, 15–17], which demonstrated that puerarin might be a potential antitumor candidate chemical and was worthy of more investigations. What’s more, Wang L. et al reported that puerarin provided renal protection against lead-induced nephrotoxicity in rat through regulating apoptosis and autophagy in proximal tubular cells [18–21]. Nevertheless, so far, no reports existed about the pharmacological effect of puerarin on cisplatin nephrotoxicity. The present study was aimed to answer this question, which would help to justify the future clinical application of puerarin in cancer treatment in combination with cisplatin as adjuvant therapy to reduce toxicities. Furthermore, the molecular mechanisms of puerarin action were also investigated in this study.
Materials and methods
Animals
Male Sprague-Dawley rats weighing about 200 grams were purchased from Vital-River (Beijing, China). Animals were maintained at a constant temperature (22 ± 2℃), humidity (55%), and light-dark conditions (12/12 h light/dark cycle). Animals were acclimatized from three days before experiments and were fed with food and tap water ad libitum. Animals were cared according to the Guide for the Care and Use of Laboratory Animals published by the National Research Council of the National Academies. The protocol of present study was checked and approved by the animal ethics committee at the People's Hospital of Zhengzhou University.
Experimental design
Twenty-eight rats were used in this study (n = 7/group). According to previous report [22], cisplatin-induced nephrotoxicity was performed by a single intraperitoneal (i.p.) cisplatin injection (7mg/kg in 0.9% saline). Puerarin was purchased from Sigma-Aldrich (St Louis, MO, USA), dissolved in 0.9% saline and administrated through caudal vein injection once per day. The dose of puerarin used in this study was chosen based on previous report [14].The rats were grouped as follows:
Control group: Rats were intraperitoneally injected with 0.9% saline without cisplatin (once per day).
Cisplatin group: Cisplatin (Sigma-Aldrich Co, St Louis, MO, USA) in 0.9% saline was injected on the third day of study in a 7mg/kg dose, intraperitoneally.
Cisplatin+puerarin group: Three days before cisplatin treatment 10mg/kg, 30mg/kg or 50mg/kg puerarin in 0.9% saline was administrated through the caudal vein. And cisplatin was administrated as in the cisplatin group. Thereafter, puerarin administration was continued for another five days.
At day 5 after cisplatin administration the rats were decapitated under anesthesia. Kidneys were removed and stored at -80℃ for further analysis. Blood samples were collected for serum urea and creatinine measurements.
Biochemical assays of oxidative stress
Malondialdehyde (MDA) levels in the kidney tissues were determined using the method reported by Utley et al., in 1967 [23]. Reduced glutathione (GSH) content, glutathione reductase (GR), glutathione peroxidase (GPx), glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) and xanthine oxidase (XO) activities in the kidney tissues were measured according to the methods as previously reported [1, 24, 25]. Serum creatinine and urea levels were determined using commercially available kits (Nanjing Jiancheng, Nanjing, China).
Histopathology of kidney tissues
Kidney tissues were fixed in 10% formalin solution and embedded in paraffin. 4 um thick sections were cut and stained with hematoxylin-eosin (H.E) according to standard method. The sections were examined under light microscope. The necrosis and degeneration were determined in 10 randomly selected areas under 20× magnification and graded as follows according to the percentage of necrosis and degeneration: none (score: 0) = normal, mild (score: 1) = <10%, moderate (score: 2) = 10% ~ 25%, severe (score: 3) = 26% ~ 75% and extremely severe (score: 4) = >75%, as previously reported [26].
Determination of TNF-α and IL-6 levels in kidney tissues
Kidney tissue homogenate of 100 mg protein per milliliter was prepared with phosphate buffer saline (50 mM, pH 7.4) containing 1% protease inhibitor cocktail (Sigma-Aldrich Co, St Louis, MO, USA). After centrifugation at 4000 ×g for 20 min at 4℃, the supernatant was collected and used for measurement of TNF-α and IL-6 with commercially available kits from R&D Systems, USA. The principle of the assays was sandwich ELISA. Absorbance was taken at 450 nm. TNF-α and IL-6 levels in the supernatant were expressed as pg / mg protein.
Western blot analysis
Protein was extracted from rat kidney tissues and collected cultured cells using Radio Immunoprecipitation Assay (RIPA) Lysis Buffer (Beyotime Institute of Biotechnology, Nanjing, China). Total protein concentration was measured by BCA procedure (Cat: 23225, Pierce, Rockford, IL, USA). About 100ug protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a 0.4 μm- polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). Then the membranes were blocked in 5% defatted milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 hour at room temperature, washed twice with TBST and incubated with mouse anti-TLR4 monoclonal antibody (1: 500 dilution, Cat: ab89455, Abcam, Shanghai, China), rabbit anti-NF-κB p65 monoclonal antibody (1:1000 dilution, Cat: 8242, Cell Signaling Technology, Denver, Colorado, USA) and mouse anti-β-actin monoclonal antibody (1:1000 dilution, Cat: MAB1501, Millipore, Bedford, MA, USA) at 4°C overnight. After washing with TBST, membranes were incubated with goat anti-rabbit or goat anti-mouse secondary antibody (1:20000 dilution, Jackson ImmunoResearch, Baltimore, Maryland, USA) at room temperature for 1 hours. Membranes were developed with SuperSignal Western reagent (Cat: 34080, Pierce, Pierce, Rockford, IL, USA). Protein bands were imaged using a GelDoc XR System (Bio-Rad, Shanghai, China). The density of protein bands was quantified and analyzed using the free software of Image J (http://rsb.info.nih.gov/ij/).
Evaluation of tumor cell proliferation
In order to determine whether puerarin interfere in the antitumor activity of cisplatin, in vitro human colon and cervical tumor cell experiments were performed. The dose of puerarin (1–5μg/ml) was chosen based on previously published methods [15]. Human colon cancer cell line COLO205 and cervical cancer cell line HeLa were bought from American Type Culture Collection (ATCC, Manassas, VA, USA). All the cells were cultured in DMEM (Gibco-BRL, Gasthersburg, MD, USA) supplemented with 10% fetal bovine serum (Gibco-BRL, Gasthersburg, MD, USA), 2% penicillin/streptomycin (10,000 U/ml penicillin, 10 mg/ml streptomycin) and grew under the humidified condition of 37°C, 5% CO2 and 95% air atmosphere. When evaluating tumor cell proliferation, 2×104 cells in 200 μl medium per well were seeded in a 96-well plate. Next day, cells were treated by cisplatin in the presence or absence of puerarin for another 24 hours. Then, 20 μl CCK-8 reagent (Dojindo, Kumamoto, Japan) were added to each well and the plate was incubated at 37℃ for additional 2 hours. At last the optical density at 450nm (OD450nm) was determined. The cell viability was expressed at percent of the control group.
Statistical analysis
The statistical analysis was done by using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (one-way ANOVA) followed by post hoc Tukey’s test was performed to detect the difference among different groups. P<0.05 was considered as statistically significant.
Results
Effect of puerarin on cisplatin-induced renal injury
Table 1 showed that, compared with control cisplatin treatment induced significant increases in serum urea (from 6.81±0.73 mmol/L to 59.24±16.98 mmol/L, P<0.05) and creatinine (from 34.18±6.83 μmol/L to 412.52±128.97 μmol/L, P<0.05). All these changes induced by cisplatin were inhibited by puerarin in a dose-dependent manner. At the dose of 50mg/kg puerarin nearly reversed serum urea and creatinine to normal levels. Compared with control puerarin alone treatment showed no substantial influence on serum biomarkers of renal function.
Table 1. Effects of puerarin on serum urea and creatinine levels in rats with cisplatin-induced nephrotoxicity.
| Parameters\Groups | Con. | Puer. (50mg/kg) | Cispt | Cispt+10mg/kg Puer. | Cispt+30mg/kg Puer. | Cispt+50mg/kg Puer. |
|---|---|---|---|---|---|---|
| Urea(mmol/L) | 6.81±0.73 | 7.12±0.41a | 59.24±16.98b | 52.49±10.61c | 38.36±9.12d | 15.43±11.37d |
| Creatinine(umol/L) | 34.18±6.83 | 38.81±5.79a | 412.52±128.97b | 397.48±81.69c | 261.19±79.33d | 81.89±58.91d |
a P>0.05 vs. Control group
b P<0.05 vs. Control group
c P>0.05 vs. Cisplatin group
d P<0.05 vs. Cisplatin group.
Note: Con.: control; Cispt: cisplatin; Puer.: Puerarin.
Effect of puerarin on cisplatin-induced oxidative stress in kidney tissues
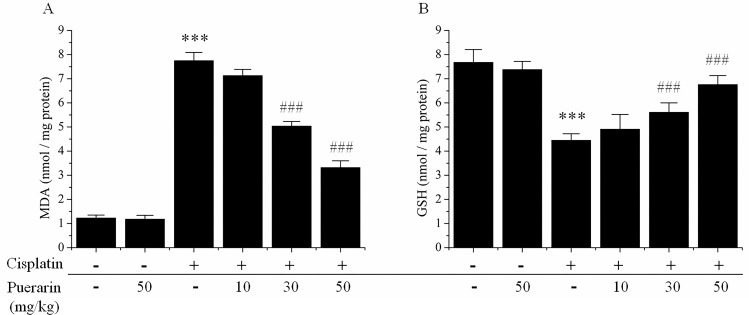
As indicated in Fig 1A, in contrast to control cisplatin administration induced a statistically significant increase (P<0.05) in malondialdehyde (MDA) formation, the biomarker of lipid peroxidation. Compared with control cisplatin treatment rendered a statistically significant decrease (P<0.05) in non-enzymatic antioxidant, reduced glutathione (GSH) content in kidney tissues (Fig 1B). All these changes in kidney tissues induce by cisplatin were dose-dependently reversed by puerarin treatment (P<0.05) (Fig 1). Puerarin alone treatment presented no substantial influences on MDA formation and GSH content in kidney tissues (Fig 1).
Fig 1.
Effects of puerarin on changes of (A) malondialdehyde (MDA) levels and (B) glutathione (GSH) content in the kidney tissues of cisplatin-treated rats. ***P<0.05 versus control group; ###P<0.05 versus cisplatin alone treated group.
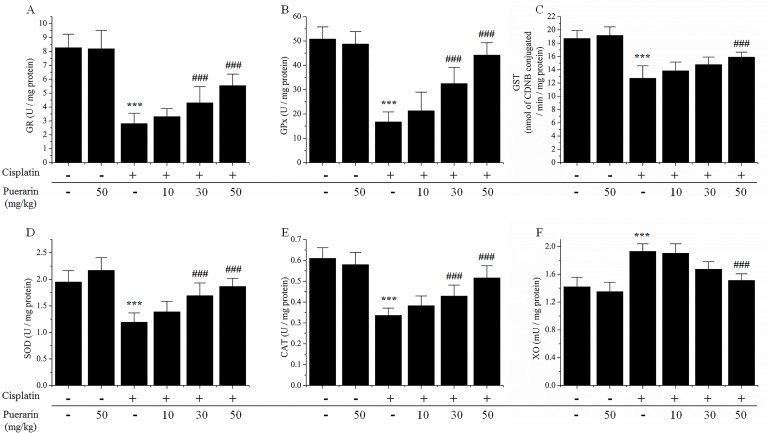
Fig 2 showed that the enzymatic anti-oxidants (GR, GPx, GST, SOD, CAT) activities in kidney tissues were significantly suppressed by cisplatin treatment in contrast to control (P<0.05). While the enzymatic oxidant activity, including xanthine oxidase (XO) in kidney tissues was significantly enhanced by cisplatin administration. However, all these changes induced by cisplatin were significantly inhibited by puerarin treatment in a dose-dependent manner (P<0.05), and for some parameters, the changes induced by cisplatin were almost completely reversed by 50 mg/kg puerarin treatment.
Fig 2. Effects of puerarin on changes of oxidative biomarkers in kidney tissues of cisplatin-treated rats.
(A) glutathione reductase (GR) activities; (B) glutathione peroxidase (GPx) activities; (C) glutathione-S-transferase (GST) activities; (D) superoxide dismutase (SOD)activities; (E) catalase (CAT) activities; (F) xanthine oxidase (XO) activities. ***P<0.05 versus control group; ###P<0.05 versus cisplatin alone treated group.
Effect of puerarin on cisplatin-induced renal histopathological alternations
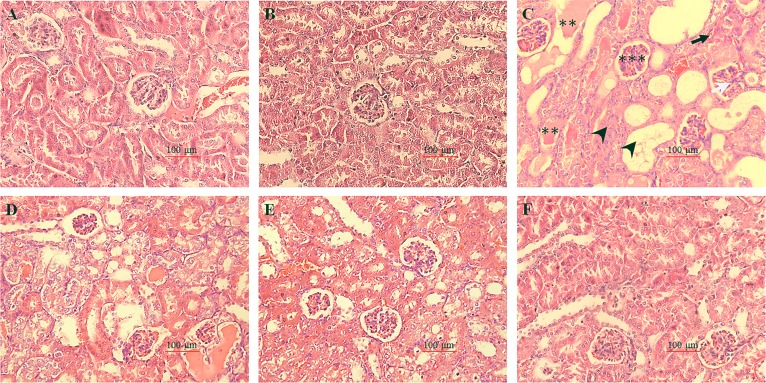
Histopathological alternations were illustrated in Fig 3. Kidney sections in control group and puerarin alone treatment group showed normal morphology (Fig 3A and 3B). While in cisplatin group severe necrosis and degeneration, hyaline casts in the tubules, intertubular hemorrhage, congestion and swelling in glomerulus and infiltration of leukocytes were observed (Fig 3B). However, all these phenomenons caused by cisplatin treatment were dose-dependently suppressed by puerarin treatment in cisplatin + puerarin group (Fig 3 and Table 2). What’s more, when the dose of puerarin was 50mg/kg the histological alternations of kidney tissues resulted from cisplatin treatment were almost completely ameliorated (Fig 3F).
Fig 3. Photomicrographs of rat kidney sections stained with hematoxylin and eosin (H.E) (200×).
(A) Kidney sections from control group showed normal morphological view; (B) Kidney tissues section from puerarin alone treated rats (50mg/kg) showed normal morphological view. (C) Kidney tissue section from cisplatin alone treated rats (7 mg/kg) showed severe tubular degeneration and necrosis, hyaline casts in the tubules, intertubular hemorrhage, congestion and swelling in glomerulus; (D-F) Sections from puerarin plus puerarin treated rats. The respective dose of puerarin was (D) 10mg/kg, (E) 30mg/kg and (F) 50 mg/kg. Kidney sections (F) from cisplatin plus 50mg/kg puerarin treated rats showed predominantly normal renal histology with occasional degenerative changes when compared with cisplatin alone treated rats. Black arrow head indicates necrosis and degeneration; Black arrow indicates intertubular hemorrhage; White arrow indicates leukocytes infiltration; Two asterisks indicate hyaline casts in the tubules. Three asterisks indicate congestion and swelling in glomerulus.
Table 2. Assessment of the necrosis and degeneration.
| Alternations/Groups | Con. | Puer. (50mg/kg) | Cispt | Cispt+10mg/kg Puer | Cispt+30mg/kg Puer | Cispt+50mg/kg Puer. |
|---|---|---|---|---|---|---|
| Necrosis and degeneration | 0.00±0.00 | 0.00±0.00 | 3.48±0.26a | 3.15±0.18b | 2.31±0.15c | 1.22±0.19c |
a P<0.05 vs. Control group
b P>0.05 vs. Cisplatin group
c P<0.05 vs. Cisplatin group. Values are presented as the mean ± SD.
Note: Con.: control; Cispt: cisplatin; Puer.: Puerarin.
Effect of puerarin on cisplatin-induced changes of inflammation mediators in the kidney tissues
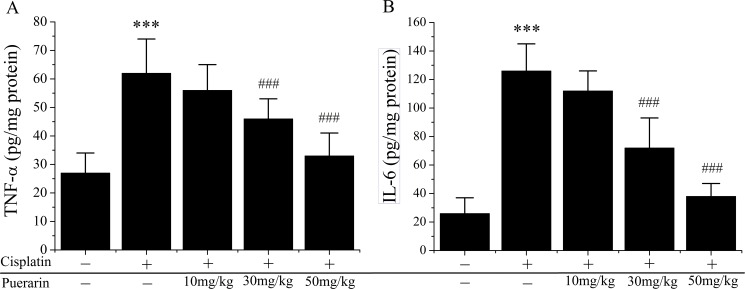
Fig 4 showed the changes of TNF-α (Fig 4A) and IL-6 (Fig 4B), two pro-inflammatory cytokines in kidney tissues. Compared with control, cisplatin treatment evoked significant increases in TNF-α (P<0.05) and IL-6 (P<0.05) production, which was reduced by puerarin treatment in a dose-dependent manner (P<0.05). Furthermore, puerarin administration at 50mg/kg reversed cisplatin-upregulated TNF-α and IL-6 levels to normal.
Fig 4. Effects of puerarin on inflammatory cytokines production in kidney tissues of cisplatin treated rats.
(A) Tumor necrosis factor-α (TNF-α); (B) Interleukin-6 (IL-6). ***P<0.05 versus control group; ###P<0.05 versus cisplatin alone treated group.
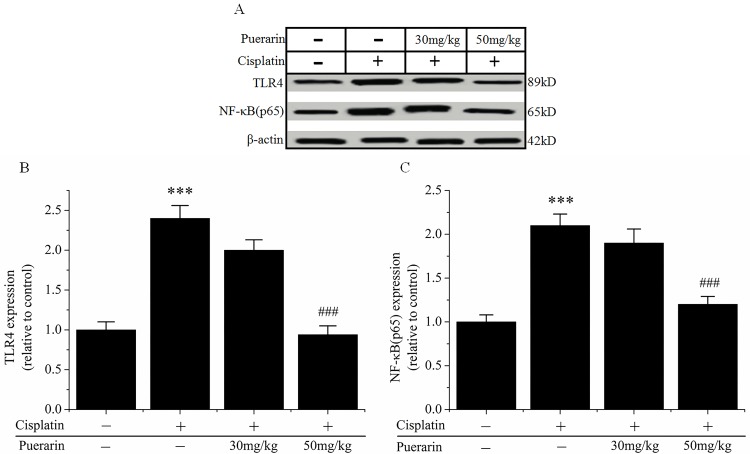
Effect of puerarin on proteins expression of TLR4 and NF-κB p65 in kidney tissues
Western blot analysis showed that cisplatin administration significantly increased the expression of TLR4 and NF-κB p65 proteins in kidney tissues in cisplatin group. However, in cisplatin + puerarin group cisplatin-induced increases in TLR4 and NF-κB p65 proteins expression were significantly reduced by puerarin treatment (30mg/kg, 50mg/kg) (P<0.05) (Fig 5).
Fig 5. Expression of TLR4 and NF-κB p65 proteins in kidney tissues in each groups.
(A) Representative Western blot picture, showing the expression levels of TLR4 and NF-κB p65 proteins. β-actin was used as an internal control. (B) Changes in the expression level of TLR4 protein. (C) Changes in the expression level of NF-κB p65 protein. Data are presented as mean ± SD (n = 4). ***P<0.05 versus control group; ###P<0.05 versus cisplatin alone treated group.
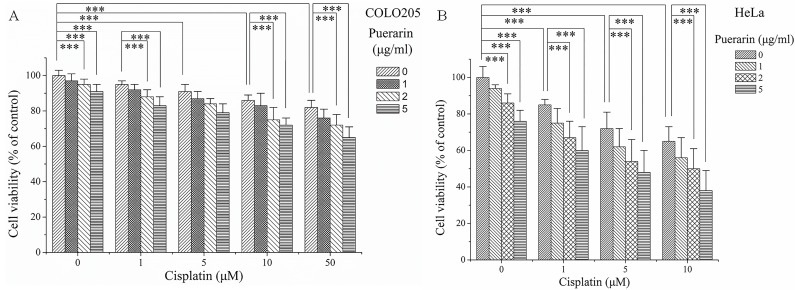
Effect of puerarin on the action of cisplatin
Fig 6 demonstrated how puerarin and cisplatin influence tumor cell proliferation. Puerarin alone treatment dose-dependently inhibited the growth of the colon cancer COLO205 cells and cervical cancer HeLa cells (P<0.05). Cisplatin alone treatment also dose-dependently inhibited the growth of the colon cancer COLO205 cells and cervical cancer HeLa cells (P<0.05). When combined with puerarin, the inhibitory effects of cisplatin on tumor cell proliferation were not negatively influenced by puerarin; however, some additive effects might be seen between cisplatin and puerarin.
Fig 6. Influence of puerarin on the inhibitory activity of cisplatin in human COLO205 and HeLa cancer cells.
***P<0.05.
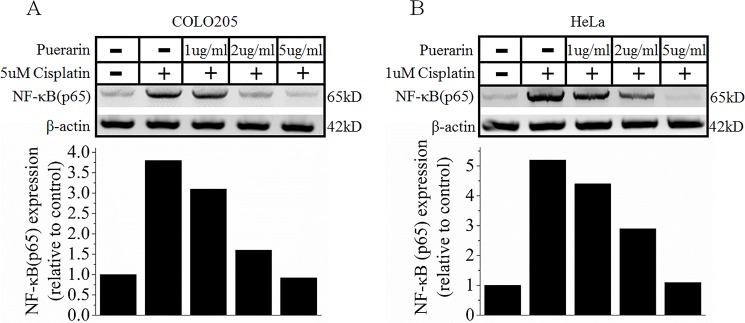
Effect of puerarin on NF-κB p65 expression in COLO205 and HeLa cancer cells
Western blot analysis showed that compared with control cisplatin treatment induced a significant increase of NF-κB p65 expression in COLO205 and HeLa cells. On the contrary, when puerarin treatment was concomitant with cisplatin treatment, NF-κB p65 expression in COLO205 and HeLa cells presented a significant reduction compared with cisplatin alone treatment (Fig 7A and 7B).
Fig 7.
Western blot analysis about the effects of puerarin on NF-κB signaling pathway in COLO205 colon cancer cells (A) and HeLa cervical cancer cells (B).
Discussion
In this study, puerarin was investigated in cisplatin-induced rat nephrotoxic model for the first time. The results showed that cisplatin-induced significant increases in serum urea and creatinine (biomarkers of renal function), lipid peroxidation (MDA level), oxidant (XO), inflammation mediators (TNF-α, IL-6) and significant decreases in some enzymatic and non-enzymatic antioxidants (GSH, GPx, GST, GR, SOD, CAT) in kidney tissues were dose-dependently inhibited by puerarin treatment, which indicated the renal protective effects of puerarin. Findings from histopathological examinations further supported the conclusions from biochemical assays. Cisplatin-induced morphological changes in kidney tissues were ameliorated by puerarin treatment in concentration-dependent manner. At high dose (50mg/kg) puerarin almost completely suppressed cisplatin-induced morphological changes in kidney tissues. Western blot analyses demonstrated that cisplatin-increased TLR4 and NF-κB p65 proteins expression in kidney tissues were reduced by puerarin in concentration-dependent manner, which indicated that it was via inhibiting TLR4/NF-κB pathway puerarin exerted renal protection.
Previous reports investigated the role of puerarin in lead-induced chronic nephrotoxicity [27, 28]. Their results demonstrated that puerarin could attenuate lead-caused renal cells apoptosis and morphologic damages via modulating PI3K/Akt/eNOS pathway [27, 28]. In present study puerarin was studied in cisplatin nephrotoxicity, a frequent devastating side effect of chemotherapy. Puerarin treatment dose-dependently reduced serum urea and creatinine levels increased by cisplatin administration and provided renal protection. The mechanisms of this renal protection were further investigated. The etiology of cisplatin-induced nephrotoxicity was complicated, uncertain and involved multiple factors and numerous signaling pathways. However, the role of oxidative stress, particularly production of reactive oxygen species (ROS) and antioxidant system dysfunction, was well established [29–31]. Cisplatin produced ROS mainly through targeting two subcellular organelles, microsomes [32] and mitochondria [33]. And then, ROS in turn damaged the mitochondria leading to cell death via apoptosis and necrosis in cisplatin-induced nephrotoxicity [30, 34]. ROS might also attack multiple target molecules including lipids, proteins, leading to increased malondialdehyde (MDA) formation and oxidants (XO), decreased.enzymatic and non-enzymatic antioxidants levels or activities (GSH, GPx, GST, GR, SOD, CAT) [10, 35]. In present study, cisplatin-induced increases in formation of MDA and XO oxidase activity in kidney tissues were attenuated by puerarin treatment in concentration-dependent manner (Fig 1). Puerarin treatment also dose-dependently restored renal antioxidants including the levels and /or activities of GSH, GPx, GST, GR, SOD and CAT (Fig 2). These findings strengthened the hypothesis that renal protective effect of puerarin could be attributed to its free radicals scavenging and strong antioxidant properties. However, as puerarin could scavenge ROS and ROS played an important role in the antitumor activity of cisplatin [36], there would be a question that whether puerarin compromise the antitumor activity of cisplatin? Maybe the answer was no. The reasons were as followings. Puerarin could exerted antitumor effect on gastric carcinoma in vivo synergistically with 5-fluorouracil, although no combination treatment with cisplatin was in vivo investigated at present. ROS did not always play positive role in antitumor action of cisplatin, elevated ROS might also cause cisplatin-resistance and antioxidants might also sensitize tumor cell to cisplatin [37]. For some herbal drugs similar to puerarin harboring renal protective activity against cisplatin nephrotoxicity through antioxidant activity might also increase ROS production in cancer cells to potentiate the antitumor activity of cisplatin, such as curcumin [22, 38–40]. What’s more, results from the tumor cell experiments in present study indicated that puerarin did not compromise the inhibitory effect of cisplatin on tumor cell proliferation in vitro. Nevertheless, verification of this postulation and unveiling the real answer warranted further investigations.
A growing body of evidences suggested that inflammation was closely associated with the pathogenesis of cisplatin nephrotoxicity [41]. Over the past decade a plenty of inflammatory mediators, including TNF-α, IL-6, IL-1β, TGF-0058, MIP2, MCP1, were identified to be upregulated in cisplatin-induced nephrotoxicity [41, 42]. However, only TNF-α was verified to play a functional role in cisplatin nephrotoxicity [43]. Ramesh reported that TNF-α was induced by ROS generated by cisplatin and was also an inducer of ROS; Treatment with TNF-α inhibitor alleviated cisplatin-induced renal injury; TNF-α-deficient mice were unsusceptible to cisplatin-induced nephrotoxicity [43]. In present study cisplatin administration induced significant increase of TNF-α and IL-6 levels in kidney tissues, which was greatly decreased by puerarin treatment in dose-dependent manner (Fig 4). These findings indicated that puerarin exerted renal protection through inhibiting inflammation reactions.
Toll-like receptor 4 (TLR4) was a pattern recognition receptor belonging to the TLR family. It recognized the damage-associated molecular pattern molecules (DAMPs) released by damaged tissues to “alert” the immune system to tissue injury [44]. TLR4 expression in murine peritoneal macrophage was increased by cisplatin treatment in vitro [45]. TLR4 was essential to the initiation of intrarenal inflammatory mediators production in cisplatin-induced nephrotoxicity [42]. Remesh also demonstrated that cisplatin synergistically acted with TLR4-specific ligand, lipopolysaccharides (LPS), to produce inflammatory cytokines such as TNF-α and IL-6, thereby leading to nephrotoxicity [46]. In TLR4-deleted mice, cisplatin-induced inflammation and renal injury were significantly reduced compared with wild-type mice [42]. Downstream TLR4 signaling was NF-κB, an critical bridge to inflammatory mediators production [41]. TNF-α production was highly dependent on ROS and NF-κB activation [43]. ROS generated by cisplatin activated the transcript factor NF-κB, which in turn induced the production of proinflammatory cytokines such as TNF-α and IL-6 [47]. Inhibition of NF-κB activation reduced cisplatin nephrotoxicity without affecting its oncolytic action [48, 49], which might be explained by the observation that cisplatin nephrotoxicity was mediated via TNFR2, whereas the anti-tumor effect of TNF-α was mediated by TNFR1. In present study, Western blot results showed that cisplatin treatment increased TLR4 and NF-κB proteins expression in kidney tissues. However, puerarin treatment significantly reduced cisplatin-increased TLR4 and NF-κB proteins expression (Fig 5A–5C); cisplatin-increased TNF-α production in the kidney tissues was also reduced by puerarin treatment. These findings suggested suppression of cisplatin-induced inflammation by puerarin could be partially attributed to its inhibitory effect on TLR4/NF-κB signaling pathway, although other pathways involved in renal inflammation reaction or injury such as MAPKs p38, ERK1/2, and JNK could also be activated by TLR4 and their role in puerarin action couldn’t excluded by presents results [50, 51].
A variety of cells including circulating and resident immune cells and renal parenchymal cells expressed TLR4. Activation of TLR4 in any cells could lead to inflammation reaction and subsequent renal injury [52]. As mentioned above, puerarin treatment could attenuate inflammation through decreasing TLR4 and NF-κB expression, leukocyte infiltration during cisplatin nephrotoxicity. Then, what was the exact site of puerarin action? Maybe the immune cells and parenchymal cells were all targets of puerarin action because previous reports have showed that under other conditions puerarin could inhibit inflammation in immune cells and parenchymal cells in some other organs [53, 54]. However, in order to clearly elucidate the targets and mechanisms of puerarin action future further investigations were warranted.
According to the results mentioned above, puerarin might be a potential adjuvant agent for inhibiting cisplatin nephrotoxicity. However, as treating cancer was the main purpose of cisplatin, it would be unpractical for future puerarin application in cancer patients if puerarin interfere in the action of cisplatin. So, in order to find out whether puerarin interfered the action of cisplatin, human colon cancer COLO205 cells and cervical cancer HeLa cells were employed and in vitro cell culture experiments were performed. Results indicated that puerarin alone treatment exerted inhibitory effects on tumor cell proliferation in vitro, consistent with previous reports [13, 15–17]. What’ more, when combined with cisplatin, the in vitro inhibitory effects of cisplatin on tumor cell proliferation were not negatively influenced by puerarin. These findings suggested that puerarin might harbor no interference in cisplatin action in vitro. Protein analyses by Western blot also provided further supportive evidence for this conclusion (Fig 7). NF-κB, a ubiquitous transcription factor, was constitutively activated in many human cancers [55, 56]. NF-κB activation was associated with tumor promotion, progression and chemoradiotherapy resistance, and so it was proposed as a target for cancer therapy [57]. Targeting NF-κB activation was a strategy to overcome resistance to chemotherapy [58]. In present study, cisplatin treatment increased NF-κB p65 protein expression in both COLO205 and HeLa cells (Fig 7), which might resulted in potential cisplatin resistance [58, 59]. However, puerarin treatment greatly reduced cisplatin- increased NF-κB p65 protein expression in COLO205 and HeLa cells (Fig 7), which might exert inhibitory effects on NF-κB signaling pathway and facilitate cisplatin treating cancer more efficiently according to current point of view [58].
Conclusion
In summary, findings in this investigation suggested that puerarin harbored renal protection by inhibiting oxidative stress and inflammation in cisplatin-induced nephrotoxicity. Inhibition of TLR4/NF-κB signaling pathway was involved in the renal protective activity of puerarin. Puerarin showed no inhibitory influence on the antitumor action of cisplatin while protecting kidney from cisplatin toxicity. On the contrary, puerarin further promoted the antitumor action of cisplatin via inhibiting cisplatin-induced NF-κB activation.
Supporting information
There were seven figures in the “data set.rar” file. Caption for each figure was presented below the paragraph in which the figure was cited.
(RAR)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sultana S, Verma K, Khan R. (2012) Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J Pharm Pharmacol. 64: 872–881. 10.1111/j.2042-7158.2012.01470.x [DOI] [PubMed] [Google Scholar]
- 2.Sanatani MS, Lazo-Langner A, Al-Rasheedy IM. (2013) Cisplatin and short-term 5-Fluorouracil infusion for paraneoplastic microangiopathic hemolytic anemia in gastric cancer: a case report and review of the literature. Case Rep Oncol Med. 2013: 594787 10.1155/2013/594787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang G, Fu C, Zhang Y, Wang J, Qiao N, Yang Q, et al. (2012) Extended-field intensity-modulated radiotherapy and concurrent cisplatin-based chemotherapy for postoperative cervical cancer with common iliac or para-aortic lymph node metastases: a retrospective review in a single institution. Int J Gynecol Cancer. 22: 1220–1225. 10.1097/IGC.0b013e3182643b7c [DOI] [PubMed] [Google Scholar]
- 4.Petrelli F, Zaniboni A, Coinu A, Cabiddu M, Ghilardi M, Sgroi G, et al. (2013) Cisplatin or not in advanced gastric cancer: a systematic review and meta-analysis. PLoS One. 8: e83022 10.1371/journal.pone.0083022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang L, Yang KH, Guan QL, Mi DH, Wang J. (2012) Cisplatin plus etoposide versus other platin-based regimens for patients with extensive small-cell lung cancer: a systematic review and meta-analysis of randomised, controlled trials. Intern Med J. 42: 1297–1309. 10.1111/j.1445-5994.2012.02821.x [DOI] [PubMed] [Google Scholar]
- 6.Sahu BD, Kuncha M, Sindhura GJ, Sistla R. (2013) Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine. 20: 453–460. 10.1016/j.phymed.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Zhu J, Zhang W. (2014) Antitumor effect of traditional Chinese herbal medicines against lung cancer. Anticancer Drugs. 25: 983–991. 10.1097/CAD.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 8.Wang C-Y, Bai X-Y, Wang C-H. (2014) Traditional Chinese Medicine: A Treasured Natural Resource of Anticancer Drug Research and Development. The American Journal of Chinese Medicine. 42: 543–559. 10.1142/S0192415X14500359 [DOI] [PubMed] [Google Scholar]
- 9.Sahu BD, Tatireddy S, Koneru M, Borkar RM, Kumar JM, Kuncha M, et al. (2014) Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: Possible mechanism of nephroprotection. Toxicology and Applied Pharmacology. 277: 8–20. 10.1016/j.taap.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, et al. (2014) Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS One. 9: e105070 10.1371/journal.pone.0105070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahu BD, Rentam KK, Putcha UK, Kuncha M, Vegi GM, Sistla R. (2011) Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem Toxicol. 49: 3090–3097. 10.1016/j.fct.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 12.Hagar H, Medany AE, Salam R, Medany GE, Nayal OA. (2015) Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Experimental and Toxicologic Pathology. 67: 133–141. 10.1016/j.etp.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Zhang H, Peng C. (2014) Puerarin: A Review of Pharmacological Effects. Phytotherapy Research. 28: 961–975. 10.1002/ptr.5083 [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Mei Z, Qian J, Zeng Y, Wang M. (2013) Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen Res. 8: 3203–3215. 10.3969/j.issn.1673-5374.2013.34.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Ma Y, Zheng Y, Song J, Yang X, Bi C, et al. (2013) In vitro and in vivo anticancer activity of a novel puerarin nanosuspension against colon cancer, with high efficacy and low toxicity. Int J Pharm. 441: 728–735. 10.1016/j.ijpharm.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Chen H, Wang Y, Zhang J. (2016) In vitro and in vivo antitumour activities of puerarin 6″-O-xyloside on human lung carcinoma A549 cell line via the induction of the mitochondria-mediated apoptosis pathway. Pharmaceutical Biology. 54: 1793–1799. 10.3109/13880209.2015.1127980 [DOI] [PubMed] [Google Scholar]
- 17.Zhang WG, Liu XF, Meng KW, Hu SY. (2014) Puerarin inhibits growth and induces apoptosis in SMMC-7721 hepatocellular carcinoma cells. Mol Med Rep. 10: 2752–2758. 10.3892/mmr.2014.2512 [DOI] [PubMed] [Google Scholar]
- 18.Liu C-M, Ma J-Q, Sun Y-Z. (2012) Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicology and Applied Pharmacology. 258: 330–342. 10.1016/j.taap.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Lin S, Li Z, Yang D, Wang Z. (2013) Protective effects of puerarin on experimental chronic lead nephrotoxicity in immature female rats. Human & Experimental Toxicology. 32: 172–185. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Li Z, Wang J, Wang H, Wang Z, Wang L. (2014) Puerarin protects against lead-induced cytotoxicity in cultured primary rat proximal tubular cells. Human & Experimental Toxicology. 33: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 21.Song X, Li Z, Liu F, Wang Z, Wang L. (2016) Restoration of autophagy by puerarin in lead-exposed primary rat proximal tubular cells via regulating AMPK–mTOR signaling. Journal of Biochemical and Molecular Toxicology. 0: 1–6 [DOI] [PubMed] [Google Scholar]
- 22.Ugur S, Ulu R, Dogukan A, Gurel A, Yigit IP, Gozel N, et al. (2015) The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren Fail. 37: 332–336. [DOI] [PubMed] [Google Scholar]
- 23.Utley HG, Bernheim F, Hochstein P. (1967) Effect of sulfhydryl reagents on peroxidation in microsomes. Archives of Biochemistry and Biophysics. 118: 29–32. [Google Scholar]
- 24.Hermenean A, Ardelean A, Stan M, Herman H, Mihali CV, Costache M, et al. (2013) Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem Biol Interact. 205: 138–147. 10.1016/j.cbi.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Zheng J, Zhu W, Jia N. (2015) Nephroprotective Effect of Gelsemine Against Cisplatin-Induced Toxicity is Mediated Via Attenuation of Oxidative Stress. Cell Biochemistry and Biophysics. 71: 535–541. 10.1007/s12013-014-0231-y [DOI] [PubMed] [Google Scholar]
- 26.Kandemir FM, Ozkaraca M, Yildirim BA, Hanedan B, Kirbas A, Kilic K, et al. (2015) Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Ren Fail. 37: 518–525. 10.3109/0886022X.2015.1006100 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Lin S, Li Z, Yang D, Wang Z. (2013) Protective effects of puerarin on experimental chronic lead nephrotoxicity in immature female rats. Hum Exp Toxicol. 32: 172–185. 10.1177/0960327112462729 [DOI] [PubMed] [Google Scholar]
- 28.Liu CM, Ma JQ, Sun YZ. (2012) Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 258: 330–342. 10.1016/j.taap.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Santos NA, Catao CS, Martins NM, Curti C, Bianchi ML, Santos AC. (2007) Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 81: 495–504. 10.1007/s00204-006-0173-2 [DOI] [PubMed] [Google Scholar]
- 30.Cetin R, Devrim E, Kilicoglu B, Avci A, Candir O, Durak I. (2006) Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: possible protective roles of natural antioxidant foods. J Appl Toxicol. 26: 42–46. 10.1002/jat.1103 [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Horvath B, Zsengeller Z, Zielonka J, Tanchian G, Holovac E, et al. (2012) Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 52: 497–506. 10.1016/j.freeradbiomed.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Baliga R. (2003) Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 63: 1687–1696. 10.1046/j.1523-1755.2003.00908.x [DOI] [PubMed] [Google Scholar]
- 33.Pabla N, Dong Z. (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73: 994–1007. 10.1038/sj.ki.5002786 [DOI] [PubMed] [Google Scholar]
- 34.Bai J, Cederbaum AI. (2001) Mitochondrial catalase and oxidative injury. Biol Signals Recept. 10: 189–199. [DOI] [PubMed] [Google Scholar]
- 35.Sahu BD, Kuncha M, Sindhura GJ, Sistla R. (2013) Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine. 20: 453–460. 10.1016/j.phymed.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 36.Brozovic A, Ambriović-Ristov A, Osmak M. (2010) The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Critical Reviews in Toxicology. 40: 347–359. 10.3109/10408441003601836 [DOI] [PubMed] [Google Scholar]
- 37.Wangpaichitr M, Sullivan EJ, Theodoropoulos G, Wu C, You M, Feun LG, et al. (2012) The Relationship of Thioredoxin-1 and Cisplatin Resistance: Its Impact on ROS and Oxidative Metabolism in Lung Cancer Cells. Molecular Cancer Therapeutics. 11: 604–615. 10.1158/1535-7163.MCT-11-0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topcu-Tarladacalisir Y, Sapmaz-Metin M, Karaca T. (2016) Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren Fail. 1–8. [DOI] [PubMed] [Google Scholar]
- 39.Jariyawat S, Kigpituck P, Suksen K, Chuncharunee A, Chaovanalikit A, Piyachaturawat P. (2009) Protection against cisplatin-induced nephrotoxicity in mice by Curcuma comosa Roxb. ethanol extract. J Nat Med. 63: 430–436. 10.1007/s11418-009-0345-5 [DOI] [PubMed] [Google Scholar]
- 40.Park BH, Lim JE, Jeon HG, Seo SI, Lee HM, Choi HY, et al. (2016) Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget. 7: 63870–63886. 10.18632/oncotarget.11563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh GS, Kim HJ, Shen A, Lee SB, Yang SH, Shim H, et al. (2016) New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. Biomed Res Int. 2016: 4048390 10.1155/2016/4048390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. (2008) TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 19: 923–932. 10.1681/ASN.2007090982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramesh G, Reeves WB. (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 110: 835–842. 10.1172/JCI15606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kono H, Rock KL. (2008) How dying cells alert the immune system to danger. Nat Rev Immunol. 8: 279–289. 10.1038/nri2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarang S, Sodhi A, Chauhan P. (2007) Differential expression of Toll-like receptors in murine peritoneal macrophages in vitro on treatment with cisplatin. Int Immunol. 19: 635–643. 10.1093/intimm/dxm029 [DOI] [PubMed] [Google Scholar]
- 46.Ramesh G, Kimball SR, Jefferson LS, Reeves WB. (2007) Endotoxin and cisplatin synergistically stimulate TNF-alpha production by renal epithelial cells. Am J Physiol Renal Physiol. 292: F812–819. 10.1152/ajprenal.00277.2006 [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI. (2011) An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol. 41: 803–821. 10.3109/10408444.2011.602662 [DOI] [PubMed] [Google Scholar]
- 48.Li G, Sha SH, Zotova E, Arezzo J, Van de Water T, Schacht J. (2002) Salicylate protects hearing and kidney function from cisplatin toxicity without compromising its oncolytic action. Lab Invest. 82: 585–596. [DOI] [PubMed] [Google Scholar]
- 49.Ramesh G, Reeves WB. (2004) Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 65: 490–499. 10.1111/j.1523-1755.2004.00413.x [DOI] [PubMed] [Google Scholar]
- 50.Kuper C, Beck FX, Neuhofer W. (2012) Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol. 302: F38–46. 10.1152/ajprenal.00590.2010 [DOI] [PubMed] [Google Scholar]
- 51.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. (2005) MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 67: 458–466. 10.1111/j.1523-1755.2005.67102.x [DOI] [PubMed] [Google Scholar]
- 52.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. (2008) TLR4 Signaling Mediates Inflammation and Tissue Injury in Nephrotoxicity. Journal of the American Society of Nephrology. 19: 923–932. 10.1681/ASN.2007090982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Y, Zhou H, Wu QQ, Li FF, Bian ZY, Deng W, et al. (2016) Puerarin attenuates the inflammatory response and apoptosis in LPS-stimulated cardiomyocytes. Exp Ther Med. 11: 415–420. 10.3892/etm.2015.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu W, Yang X, Zhe C, Zhang Q, Sun L, Cao K. (2011) Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-kappaB activation in LPS-induced RAW264.7 macrophage cells. Pharmacol Rep. 63: 781–789. [DOI] [PubMed] [Google Scholar]
- 55.Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. (2003) NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 22: 50–58. 10.1038/sj.onc.1206043 [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Liu Z, Wang L, Zhang X. (2009) NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 6: 327–334. 10.1038/cmi.2009.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erstad DJ, Cusack JC Jr. (2013) Targeting the NF-κB Pathway in Cancer Therapy. Surgical Oncology Clinics of North America. 22: 705–746. 10.1016/j.soc.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 58.Godwin P, Baird AM, Heavey S, Barr MP, O'Byrne KJ, Gately K. (2013) Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front Oncol. 3: 120 10.3389/fonc.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eichholtz-Wirth H, Sagan D. (2000) IkappaB/NF-kappaB mediated cisplatin resistance in HeLa cells after low-dose gamma-irradiation is associated with altered SODD expression. Apoptosis. 5: 255–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There were seven figures in the “data set.rar” file. Caption for each figure was presented below the paragraph in which the figure was cited.
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.