Abstract
Background
Elevated hepatic oxidative stress and lipid peroxidation levels caused by increased hepatic Cytochrome P-450 2E1 (CYP2E1) enzyme activity has been speculated to play a role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). But studies of lipid peroxidation and CYP2E1 in children are lacking.
Aim
To compare hepatic lipid peroxidation and hepatic CYP2E1 protein content in liver biopsies from children with NAFLD and two control groups.
Study
Liver biopsies from 59 children with NAFLD (49 with NASH), 10 children with normal liver histology, and 9 children with chronic hepatitis C infection (HCV) were examined. Hepatic malondialdehyde (MDA, a measure of lipid peroxidation) levels and CYP2E1 protein content were quantitated, as a percent of total area, by immunohistochemical staining of liver biopsy material followed by digital image quantitation.
Results
Lipid peroxidation was significantly greater in NAFLD liver biopsies (46.7 ± 20.8%) compared to liver biopsies from children with normal liver histology (7.6 ± 9.4%; p<0.001) or HCV (7.7 ± 7.6%; p<0.001). However, hepatic CYP2E1 was not higher in NAFLD biopsies compared to other groups (p=). Among children with NAFLD, lipid peroxidation and CYP2E1 protein content were not different between biopsies with and without NASH. The BMI was independently associated with hepatic lipid peroxidation (r=0.549; p<0.001).
Conclusions
Hepatic lipid peroxidation is increased in children with NAFLD but this is not related to hepatic CYP2E1. Lack of difference in lipid peroxidation among different NAFLD subgroups argues against its role in the disease progression.
Keywords: Nonalcoholic steatohepatitis, Steatosis, Fibrosis, Oxidative stress, Children
Introduction
The epidemic incidence of overweight and obesity in the pediatric population has likely contributed to the rise of nonalcoholic fatty liver disease (NAFLD) as the most prevalent chronic liver condition in children today1–2. In fact, estimates for the current prevalence of NAFLD in children and adolescents range from 2.6% to 9.6%, depending on the characteristics of the patient population and the diagnostic tests used3. NAFLD, including both nonalcoholic steatohepatitis (NASH) and simple steatosis (non-NASH NAFLD), are of great importance in the pediatric population due to the potential for progression to cirrhosis in young and middle-aged adults4–5.
NAFLD represents an entire spectrum of histological features upon liver biopsy, ranging from simple fatty liver to serious pathological findings including advanced fibrosis and cirrhosis6. The histological findings in adult NAFLD have been well characterized, but diagnostic criteria in children are less clear. Schwimmer et al. first proposed two different types of nonalcoholic steatohepatitis in children: type 1 (“adult”) NASH associated with steatosis, ballooning degeneration, and perisinusoidal fibrosis in the absence of portal features, and type 2 (“pediatric”) NASH characterized by steatosis, portal inflammation, and portal fibrosis in the absence of ballooning degeneration and perisinusoidal fibrosis7. Type 1 NASH was found to be more common in older children, girls, and Caucasians, while type 2 NASH was observed more often in younger and heavier children, boys, and non-Whites7. Findings from subsequent studies have confirmed this unique portal-based pattern of disease in children and suggested that the presence of overlapping features of both type 1 and type 2 NASH may be more commonly observed than one type alone8–12.
Regardless of disease type, understanding of the pathogenesis, progression, and response to treatment of pediatric NAFLD remains a significant unmet medical need. One pathway commonly implicated in the development of adult NAFLD is oxidative stress and inflammation, leading to increased lipid peroxidation. Indeed, both hepatic and systemic lipid peroxidation levels are elevated in adults with non-NASH NAFLD and NASH13–16. A key hepatic enzyme capable of producing oxidative stress and lipid peroxidation is cytochrome P-450 2E1 (CYP2E1), a microsomal enzyme that functions in fatty acid hydroxylation and drug metabolism. Both expression and activity of CYP2E1 is increased in animal models of NASH17–18 and in adults with non-NASH NAFLD and NASH19–22. While the precise contribution(s) of increased oxidative stress and/or elevated CYP2E1 activity to the development of NAFLD in adults is unknown, even less is known about these factors in the pathogenesis of NAFLD in children. Furthermore, differences in histopathology observed between pediatric and adult NAFLD raise the possibility that different mechanisms may be responsible for development of non-NASH NAFLD and NASH in children.
In the current study, our objective was to investigate a potential role for elevated lipid peroxidation and/or increased CYP2E1 protein expression in the pathogenesis of pediatric NAFLD. We measured levels of hepatic lipid peroxidation and CYP2E1 protein expression in our large cohort of children with NAFLD and in children with normal liver biopsies or chronic hepatitis C infection (HCV) for comparison purposes. We also considered patient clinical characteristics, histological findings, and serum parameters for correlative studies.
Materials and Methods
Subjects
Subjects seen at the Pediatric Liver Clinic at Riley Hospital for Children (Indianapolis, IN, USA) who had undergone a liver biopsy for clinical reasons were identified retrospectively from clinical databases. The Institutional Review Board of the Indiana University School of Medicine reviewed the protocol and approved the study. All together, 59 children (age ≤19 years) with biopsy-proven NAFLD (49 with NASH), 10 children (age ≤19 years) with normal liver biopsies (control group), and 9 children (age ≤19 years) with chronic hepatitis C infection with minimal activity (HCV group) were included in this study. Children comprising the control group did have other medical conditions, and indications for these children to have undergone a liver biopsy procedure are shown in Table 1. Exclusion criteria for all groups included alcohol intake and diagnosis of other liver diseases [including viral hepatitis (with the exception of HCV in the HCV group), α1-anti-trypsin disease, autoimmune hepatitis, and Wilson disease].
Table 1.
Indications for liver biopsies of children comprising the control group
| Patient Number | Indication for Liver Biopsy |
|---|---|
|
| |
| 1 | portal hypertension |
| 2 | urea cycle defect |
| 3 | follow-up after acute hepatitis |
| 4 | urea cycle defect |
| 5 | portal hypertension |
| 6 | urea cycle defect |
| 7 | urea cycle defect |
| 8 | portal hypertension |
| 9 | urea cycle defect |
| 10 | portal hypertension |
Clinical and Laboratory Measures
Patient demographic data was obtained and body mass index (BMI) was calculated for each child. For the laboratory evaluations, all assays were performed at the local Clarian Pathology clinical laboratory (Indianapolis, IN, USA). Measures included: serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum albumin, blood glucose and insulin, glycosylated hemoglobin (HbA1c), and plasma lipid panel (total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting blood glucose and insulin levels. Medication histories were examined and no control subjects or NAFLD/HCV patients were taking compounds known to be inducers or inhibitors of CYP2E1.
Liver histology and immunohistochemistry
Percutaneous needle liver biopsies were routinely processed in the Clarian Pathology clinical histology laboratory (Indianapolis, IN, USA). Formalin-fixed, paraffin-embedded histological sections were stained with hematoxylin and eosin (H&E) and Masson Trichrome stains for microscopic evaluation. Biopsies were evaluated by two pathologists blinded to patient characteristics. Five features of NAFLD were graded histologically based on the NASH CRN histological scoring system published by Kleiner et al.8: steatosis (amount), inflammation (portal and lobular), hepatocyte ballooning (grade) and fibrosis (stage). Steatosis was graded on a scale from 0 to 3 according to the amount of fat that was present: grade 0 (<5% macrovesicular fat); grade 1 (15–33%); grade 2 (34–66%); and grade 3 (>66%). Portal inflammation was graded on a scale from 0 to 2: grade 0 (none); grade 1 (minimal inflammatory infiltrate); and grade 2 (greater than minimal inflammatory infiltrate) and lobular inflammation was graded on a scale from 0 to 3: grade 0 (no foci); grade 1 (<2 foci/200× magnification); grade 2 (2–4 foci/200× magnification); and grade 3 (>4 foci/200× magnification). Hepatocyte ballooning was graded on a scale from 0 to 2: grade 0 (none); grade 1 (few ballooning cells); and grade 2 (many ballooning cells). Fibrosis was staged on a scale from 0 to 5: stage 0 (none); stage 1 (perisinusoidal with Trichrome stain required); stage 2 (perisinusoidal easily visible on H&E stain); stage 3 (portal/periportal only); stage 4 (combination of periportal and zone 3); and stage 5 (bridging).
In addition, patients were assigned a diagnosis of non-NASH NAFLD, type 1 NASH, type 2 NASH, or mixed (type 1 and type 2) NASH based on histological evaluations and the classification system put forth by Schwimmer et al.7. Non-NASH NAFLD was defined by steatosis in the absence of both fibrosis and hepatocyte ballooning. Type 1 (or “adult”) NASH was defined by a pattern similar to adult NASH, with steatosis, hepatocyte ballooning, and perisinusoidal fibrosis with zone 3 predominance. Type 2 (or “pediatric”) NASH was defined by steatosis and predominance of portal injury (including inflammation and/or fibrosis). Mixed (type 1 and type 2) NASH was defined by an overlap of features from both type 1 and type 2 NASH (often, this was type 2 NASH with hepatocyte ballooning and/or perisinusoidal fibrosis).
Immunohistochemistry was performed on 4 micron thick sections cut from formalin-fixed paraffin-embedded tissue. Briefly, the sections were deparaffinized and heat-induced antigen retrieval was carried out with EDTA in a pressure cooker. Endogenous peroxidase was quenched by incubating with hydrogen peroxidase. Sections were incubated with anti-MDA antibody (Abcam, Inc., Cambridge, MA, USA) or anti-CYP2E1 antibody (LifeSpan Biosciences, Seattle, WA, USA) and subsequently with a secondary antibody (EnVision+ from DAKO, Carpintenia, CA, USA). The reaction was developed using strepavidin labeled with horse radish peroxidase and 3,3′ diaminobenzidine (DAB) as the chromogen. Appropriate positive and negative control staining was performed. Immunohistochemical staining and steatosis was digitally quantitated and expressed as a percent of total liver biopsy area using SPSS Sigma Scan Pro 5.0 software (SPSS Inc., Chicago, IL, USA).
Statistical analysis
Basic descriptive statistics, including means, standard deviations (SD), and percentages were used to characterize the study patients. Because the data lacked a normal distribution, the Kruskal-Wallis test was used to make comparisons among patient groups and controls. Spearman rank correlations were used to detect the associations between lipid peroxidation levels and CYP2E1 protein content and patient clinical characteristics, histological findings, and serum parameters. When appropriate, stepwise regression analysis was performed to take into account the linear effect of several independent variables predicting the dependent variables (lipid peroxidation levels and CYP2E1 protein content). Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
Results
Immunohistochemical analyses
Immunohistochemistry and digital image quantification was used to measure hepatic lipid peroxidation and CYP2E1 protein content, and a set of representative images for all patient groups and controls is shown in Figure 1. Lipid peroxidation, as measured by MDA staining, was lowest in normal and chronic HCV liver biopsies (panels A and B), and significantly greater in patients with non-NASH NAFLD (panel C), type 1 NASH (panel D), type 2 NASH (panel E), and mixed NASH (panel F). Hepatic CYP2E1 protein expression was not different between the control, HCV, and all NAFLD patient groups (panels G–L). As described below, there were no significant differences in lipid peroxidation or CYP2E1 protein expression levels when comparisons were made across the NAFLD patient cohort.
Figure 1. Immunohistochemical assessment of liver biopsies.
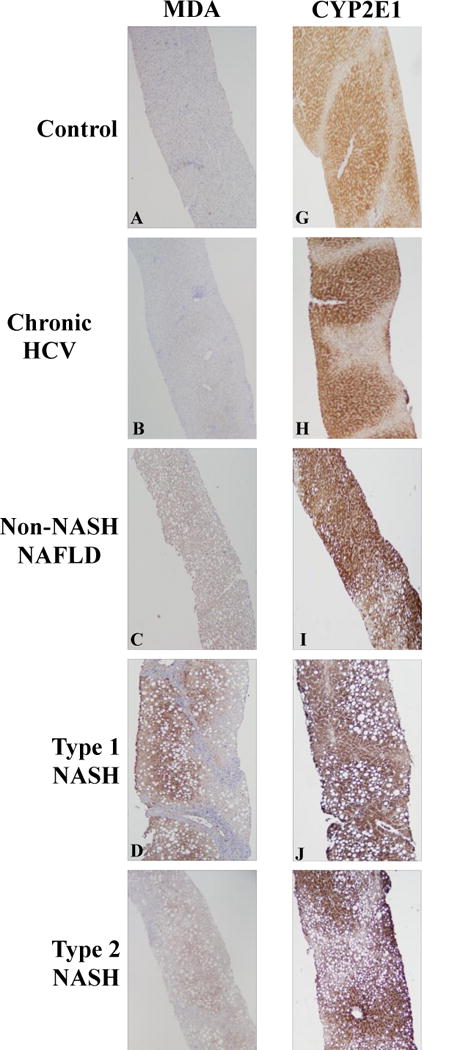
Representative images of lipid peroxidation levels [measured by malondialdehyde (MDA) staining] are shown in the left panels (A–F) and CYP2E1 staining is shown in the right panels (G–L) as follows: (A,G) Control; (B,H) Chronic HCV Infection; (C,I) non-NASH NAFLD; (D,J) Type 1 NASH; (E,K) Type 2 NASH; (F,L) Mixed NASH. Hepatic lipid peroxidation was significantly greater in pediatric NAFLD compared to controls and chronic HCV patients (compare panels A,B with panels C–F). Hepatic CYP2E1 protein content was not different in children with normal biopsies or chronic HCV infection versus those across the spectrum of NAFLD (compare panels G,H with panels I–L).
Comparison of control, HCV, and NAFLD groups
Demographic and histological data from all subjects (control, HCV, and all NAFLD patients) are shown in Table 2. Compared to children with normal liver histology or HCV infection, pediatric NAFLD patients had significantly greater hepatic lipid peroxidation (p<0.001) and steatosis measured by digital quantification (p<0.001). CYP2E1 protein content did not differ among the three groups (p=0.116). It is important to note that children with normal liver biopsies or chronic HCV infection and NAFLD patients did not differ significantly with respect to serum ALT or AST concentrations, and a number of children in the control and HCV groups did display lobular and/or portal inflammation, along with hepatocyte ballooning, upon liver biopsy evaluation.
Table 2.
Comparison of demographic, laboratory, immunohistochemical, and histological data for control subjects and children with chronic HCV or NAFLD
| Control (n=10) | Hepatitis C (n=9) | NAFLD (n=59) | |
|---|---|---|---|
| Male/Female | 5/5 | 4/5 | 46/13 |
| Age (yr) | 6.8 ± 5.5 | 11.6 ± 1.7* | 13.3 ± 3.1*# |
| BMI (kg/m2) | 19.8 ± 5.4 | 19.4 ± 3.7 | 34.9 ± 6.7*# |
| Glycemic Measures | |||
| Glucose (mg/dl) | Not done | 89.7 ± 10.9 | 98.3 ± 21.6 |
| Insulin (mcU/ml) | Not done | Not done | 25.8 ± 16.9 |
| HOMA | Not done | Not done | 6.4 ± 5.0 |
| HbA1c (%) | Not done | Not done | 5.5 ± 1.2 |
| Lipids | |||
| Total Cholesterol (mg/dl) | Not done | Not done | 169.8 ± 34.0 |
| LDL Cholesterol (mg/dl) | Not done | Not done | 69.8 ± 45.3 |
| HDL Cholesterol (mg/dl) | Not done | Not done | 70.2 ± 39.6 |
| Triglycerides (mg/dl) | Not done | Not done | 156.4 ± 99.7 |
| Liver Biochemistries | |||
| ALT (U/L) | 64.8 ± 57.7 | 64.1 ± 24.4 | 112.7 ± 83.4 |
| AST (U/L) | 105.5 ± 105.8 | 49.6 ± 15.6 | 67.1 ± 40.2 |
| Albumin (GM/dl) | 3.5 ± 0.6 | 4.2 ± 0.2* | 4.1 ± 0.4* |
| Immunohistochemistry | |||
| Malondialdehyde (MDA) content (%) | 7.6 ± 9.4 | 7.7 ± 7.6 | 46.7 ± 20.8*# |
| CYP2E1 protein content (%) | 53.5 ± 10.7 | 60.0 ± 11.9 | 60.7 ± 8.7 |
| Steatosis (%) | 0.4 ± 0.5 | 0.6 ± 0.5 | 10.7 ± 6.1*# |
| Histological Scoring | |||
| Steatosis grade | 0 | 0 | 1.8 ± 0.9*# |
| Fibrosis stage | 0 | 0.4 ± 1.1 | 2.4 ± 1.9*# |
| Grade of lobular inflammation | 0.5 ± 1.0 | 1.6 ± 0.7 | 1.1 ± 0.6# |
| Grade of portal inflammation | 0.3 ± 0.5 | 0.9 ± 0.4 | 0.8 ± 0.6 |
| Hepatocyte ballooning | 0.5 ± 1.0 | 0 | 0.8 ± 0.8# |
Values expressed are mean ± SD and immunohistochemical measures are as a percent of total liver biopsy area. Note that diagnoses were based on histological scoring criteria and statistical comparisons between groups should be interpreted accordingly.
Abbreviations: BMI, body mass index; HOMA, Homeostatic Model Assessment Method; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
p<0.05 vs Control;
p<0.05 vs Hepatitis C.
Comparison of non-NASH NAFLD and NASH groups
Demographic and histological data from all NAFLD patients, separated into non-NASH NAFLD and NASH groups, is depicted in Table 3. All liver biochemistries measured (ALT, AST, and albumin) were significantly greater (p<0.05) in the pediatric NASH patients compared to children with non-NASH NAFLD. However, the amount of hepatic lipid peroxidation, CYP2E1 protein content, and steatosis measured by digital quantification did not differ between non-NASH NAFLD and NASH groups.
Table 3.
Comparison of demographic, laboratory, immunohistochemical, and histological data for non-NASH NAFLD patients and children with NASH
| Non-NASH NAFLD (n=10) | NASH (n=49) | p-value | |
|---|---|---|---|
| Male/Female | 7/3 | 39/10 | |
| Age (yr) | 12.7 ± 4.5 | 13.4 ± 2.7 | 0.684 |
| BMI (kg/m2) | 33.6 ± 8.9 | 35.2 ± 6.1 | 0.844 |
| Glycemic Measures | |||
| Glucose (mg/dl) | 105.0 ± 32.6 | 96.8 ± 18.6 | 0.339 |
| Insulin (mcU/ml) | 18.8 ± 9.0 | 27.2 ± 17.8 | 0.719 |
| HOMA | 4.3 ± 2.4 | 6.9 ± 5.3 | 0.582 |
| HbA1c (%) | 6.2 ± 2.2 | 5.3 ± 0.4 | 0.180 |
| Lipids | |||
| Total Cholesterol (mg/dl) | 160.1 ± 19.6 | 171.5 ± 35.8 | 0.321 |
| LDL Cholesterol (mg/dl) | 71.8 ± 34.3 | 69.4 ± 47.7 | 0.618 |
| HDL Cholesterol (mg/dl) | 69.3 ± 38.9 | 70.5 ± 40.3 | 0.896 |
| Triglycerides (mg/dl) | 99.9 ± 21.7 | 167.7 ± 105.4 | 0.053 |
| Liver Biochemistries | |||
| ALT (U/L) | 77.4 ± 41.8 | 119.9 ± 88.1 | 0.045 |
| AST (U/L) | 47.7 ± 28.1 | 71.0 ± 41.3 | 0.035 |
| Albumin (GM/dl) | 3.9 ± 0.2 | 4.1 ± 0.4 | 0.031 |
| Immunohistochemistry | |||
| Malondialdehyde (MDA) content (%) | 38.7 ± 19.7 | 48.3 ± 20.8 | 0.233 |
| CYP2E1 protein content (%) | 58.5 ± 11.4 | 61.1 ± 8.1 | 0.746 |
| Steatosis (%) | 9.5 ± 6.3 | 11.0 ± 6.1 | 0.780 |
| Histological Scoring | |||
| Steatosis grade | 1.6 ± 1.0 | 1.9 ± 0.8 | 0.437 |
| Fibrosis stage | 0 | 2.8 ± 1.8 | <0.001 |
| Grade of lobular inflammation | 0.8 ± 0.6 | 1.1 ± 0.6 | 0.169 |
| Grade of portal inflammation | 0.4 ± 0.5 | 0.8 ± 0.6 | 0.056 |
| Hepatocyte ballooning | 0 | 1.0 ± 0.8 | <0.001 |
Values expressed are mean ± SD and immunohistochemical measures are as a percent of total liver biopsy area. Note that diagnoses were based on histological scoring criteria and statistical comparisons between groups should be interpreted accordingly.
Abbreviations: BMI, body mass index; HOMA, Homeostatic Model Assessment Method; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Comparison within NASH groups
Demographic and histological data from the NASH patient group, divided into subtypes (type 1, type 2, and mixed), is presented in Table 4. Four children were not characterized by NASH subtype due to the small size of their liver biopsy material and therefore were not included in this table. Of the remaining 45 pediatric patients, 21 had type 1 NASH (47%), 19 had type 2 NASH (42%), and 5 had mixed NASH (11%). Children with type 1 NASH were significantly older (p<0.001), but no other demographic or clinical laboratory measures differed significantly among the NASH group subtypes. Although there were no significant differences in CYP2E1 protein content or steatosis, there was a trend (p=0.066) for MDA staining to be greatest in type 1 NASH, lower in type 2 NASH, and lowest in mixed NASH.
Table 4.
Comparison of demographic, laboratory, immunohistochemical, and histological data across NASH subtypes
| Type 1 NASH (n=21) | Type 2 NASH (n=19) | Mixed NASH (n=5) | p-value | |
|---|---|---|---|---|
| Male/Female | 16/5 | 16/3 | 4/1 | |
| Age (yr) | 15.0 ± 1.8* | 11.5 ± 2.7 | 13.8 ± 2.4 | <0.001 |
| BMI (kg/m2) | 35.3 ± 6.1 | 32.7 ± 4.8 | 38.4 ± 4.6 | 0.156 |
| Glycemic Measures | ||||
| Glucose (mg/dl) | 98.5 ± 20.4 | 92.8 ± 17.0 | 104.8 ± 23.6 | 0.442 |
| Insulin (mcU/ml) | 24.7 ± 16.8 | 26.4 ± 17.9 | 41.4 ± 19.0 | 0.204 |
| HOMA | 6.3 ± 5.8 | 6.4 ± 4.3 | 10.8 ± 5.4 | 0.147 |
| HbA1c (%) | 5.5 ± 0.4 | 5.2 ± 0.3 | 5.1 ± 0.3 | 0.126 |
| Lipids | ||||
| Total Cholesterol (mg/dl) | 165.6 ± 37.7 | 178.4 ± 36.5 | 185.4 ± 35.9 | 0.502 |
| LDL Cholesterol (mg/dl) | 65.0 ± 52.0 | 78.9 ± 51.1 | 79.0 ± 35.7 | 0.560 |
| HDL Cholesterol (mg/dl) | 72.6 ± 38.6 | 58.8 ± 39.1 | 76.0 ± 57.9 | 0.587 |
| Triglycerides (mg/dl) | 161.7 ± 88.9 | 207.4 ± 142.4 | 151.6 ± 57.1 | 0.587 |
| Liver Biochemistries | ||||
| ALT (U/L) | 106.4 ± 55.1 | 128.2 ± 118.6 | 182.6 ± 75.6 | 0.109 |
| AST (U/L) | 70.9 ± 36.6 | 70.2 ± 49.7 | 90.4 ± 38.5 | 0.427 |
| Albumin (GM/dl) | 4.1 ± 0.4 | 4.3 ± 0.4 | 4.0 ± 0.2 | 0.286 |
| Immunohistochemistry | ||||
| Malondialdehyde (MDA) content (%) | 53.3 ± 19.5 | 44.3 ± 20.0 | 37.2 ± 8.2 | 0.066 |
| CYP2E1 protein content (%) | 59.4 ± 8.2 | 61.7 ± 8.9 | 60.4 ± 4.8 | 0.764 |
| Steatosis (%) | 10.1 ± 6.1 | 12.7 ± 6.6 | 7.9 ± 5.6 | 0.299 |
| Histological Scoring | ||||
| Steatosis grade | 1.7 ± 0.8 | 2.2 ± 0.9 | 1.4 ± 0.5 | 0.080 |
| Fibrosis stage | 1.9 ± 1.8# | 3.7 ± 0.9 | 2.8 ± 0.8 | 0.001 |
| Grade of lobular inflammation | 1.3 ± 0.6 | 1.0 ± 0.5 | 1.2 ± 0.8 | 0.274 |
| Grade of portal inflammation | 0.5 ± 0.5# | 1.1 ± 0.6 | 1.4 ± 0.5 | 0.004 |
| Hepatocyte ballooning | 1.4 ± 0.7* | 0.5 ± 0.7 | 0.8 ± 0.5 | 0.001 |
Values expressed are mean ± SD and immunohistochemical measures are as a percent of total liver biopsy area. Note that diagnoses were based on histological scoring criteria and statistical comparisons between groups should be interpreted accordingly.
NOTE: 4 NASH patients were not characterized by NASH type and were therefore not included in this analysis.
Abbreviations: BMI, body mass index; HOMA, Homeostatic Model Assessment Method; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
p<0.05 vs type 2 NASH;
p<0.05 vs all other groups.
Variables associated with hepatic MDA content in the entire patient cohort
Univariate analysis revealed a significant correlation between hepatic MDA and age (r=0.433; p<0.001), BMI (r=0.602; p<0.001), and steatosis measured by digital image analysis (r=0.526; p<0.001). Upon stepwise regression, both BMI (r=0.549; p<0.001; Figure 2) and amount of steatosis (r=0.255; p=0.013) were independently associated with hepatic lipid peroxidation levels.
Figure 2. BMI is a significant independent predictor of both hepatic MDA content (r=0.549; p<0.001) and hepatic CYP2E1 protein content (r=0.458; p=0.002).
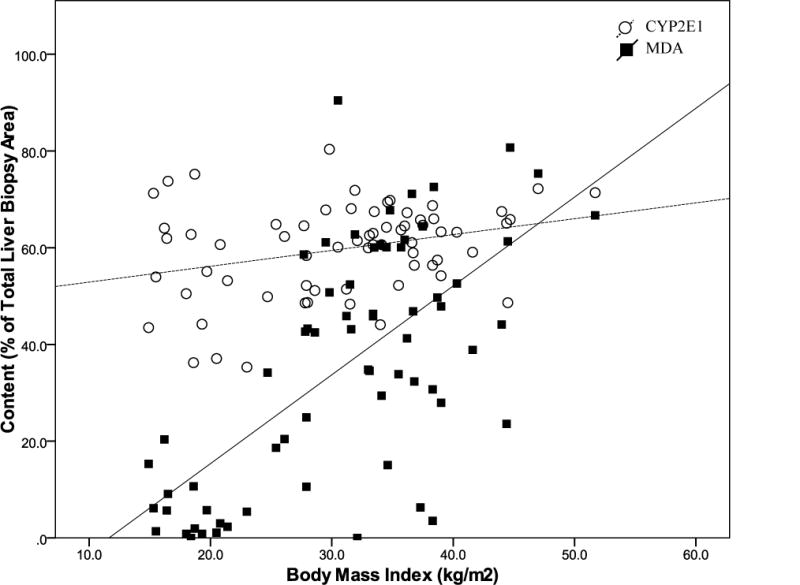
Lipid peroxidation levels [measured by malondialdehyde (MDA) staining] and CYP2E1 protein content was measured in liver biopsies by immunohistochemistry followed by digital image quantification and is represented as the percent (%) of the total liver biopsy area that stained positive for MDA or CYP2E1.
Variables associated with hepatic CYP2E1 protein content in the entire patient cohort
Univariate analysis showed a significant correlation between hepatic CYP2E1 protein expression and BMI (r=0.283; p=0.021) and HDL cholesterol levels (r=−0.309; p=0.041). Upon stepwise regression analysis, only BMI (r=0.458; p=0.002; Figure 2) was identified as a significant independent predictor of hepatic CYP2E1 protein content.
Discussion
As the incidence of NAFLD in both the general and pediatric populations continues to increase, additional studies are clearly needed to define the pathogenesis and progression of these common liver diseases. Importantly, limited evidence suggests that histological lesions and clinical predictors of NASH differ between adults and children. Therefore, factors that play a role in the pathogenesis of adult NAFLD cannot be assumed to be involved in pediatric disease and must be directly examined in this patient subgroup. In the current study, we investigated whether elevated hepatic lipid peroxidation and CYP2E1 protein expression are present in pediatric NAFLD, as is observed in adult disease. We found that, compared to children with normal liver histology or chronic HCV infection, pediatric non-NASH NAFLD and NASH patients had significantly elevated hepatic levels of lipid peroxidation. However, these parameters did not differ across the subtypes of pediatric fatty liver disease (non-NASH NAFLD, type 1 NASH, type 2 NASH, and mixed NASH). We also noted that, similar to adults, there was a significant positive relationship between BMI and hepatic CYP2E1 protein content in our pediatric patient cohort.
Elevations in both hepatic and systemic lipid peroxidation levels in adult NAFLD have been previously reported14–16. In addition, animal models have revealed that CYP2E1 plays a key role in the generation of oxidative stress and lipid peroxidation in the liver18 and several studies have demonstrated that adult NAFLD is associated with increased hepatic CYP2E1 protein expression and activity19–22. In our pediatric cohort, children with NAFLD displayed a significant increase in hepatic MDA content compared to children with normal liver biopsies or chronic HCV infection, but CYP2E1 protein content was not elevated. We also did not observe any significant differences in lipid peroxidation levels or CYP2E1 expression among the different subtypes of pediatric NASH or in children with non-NASH NAFLD. This is consistent with a previous study by Chtioui et al. in which in vivo hepatic CYP2E1 activity (measured by oral chlorzoxazone clearance) and hepatic CYP2E1 protein expression (assessed by immunohistochemical staining) did not differ between 10 adults with non-NASH NAFLD and 30 adults with NASH21. However, within the pediatric NASH subtypes we did detect a non-significant trend (p=0.066) for MDA staining to be greatest in type 1 NASH, lower in type 2 NASH, and lowest in mixed NASH. This is in agreement with the findings of MacDonald et al., in which they reported a predominance of lipid peroxidation (measured by MDA staining) in liver biopsies from adults with varying degrees of steatosis in acinar zone 316, which closely resembles the type 1 NASH pattern of pediatric fatty liver disease. In contrast, Sanyal et al. observed greater 3-nitrotyrosine (a marker similar to MDA) staining in adults with NASH compared to those with simple fatty liver14, and we were not able to recapitulate these findings when comparing pediatric patients with non-NASH NAFLD to those with NASH. Upon multivariate analysis, we found that BMI was independently associated with both hepatic lipid peroxidation levels and hepatic CYP2E1 expression in our pediatric cohort. This is consistent with previous studies demonstrating that obesity, which is often observed in patients with NAFLD, is independently associated with elevations in hepatic CYP2E1 expression and activity22–24. There was also a significant positive relationship between the amount of steatosis and hepatic MDA content. This observation in our pediatric NAFLD cohort is similar to a significant association between steatosis and lipid peroxidation levels previously reported in adults with NAFLD16.
It is important to note that a large difference in lipid peroxidation levels was seen between the control/HCV and NAFLD groups; however, no difference in CYP2E1 protein expression was observed. This indicates that non-CYP-related factors contribute to the elevated oxidative stress that results in increased lipid peroxidation in pediatric NAFLD. One possibility is hepatic steatosis itself, when fatty acids that have accumulated in the liver are oxidized and reactive oxygen species are produced in the process. In addition, iron overload is thought to play an important role in generation of highly reactive hydroxyl radicals from the hydrogen peroxide produced when free fatty acids undergo peroxisomal β-oxidation25. Another possibility is an inadequate defense against oxidative stress via depletion of antioxidant stores26–27. These multiple sources of oxidative stress, all with the potential to induce lipid peroxidation, remain largely uninvestigated in the context of pediatric NAFLD.
We also considered distribution patterns of hepatic MDA and CYP2E1 staining in our pediatric cohort. In adults with NAFLD, a zone 3 predominance for hepatic lipid peroxidation has been reported16, but we did not observe any obvious patterns in MDA distribution across our pediatric NAFLD patient cohort (i.e. confining of MDA staining to a specific acinar zone and/or to lipid droplets). Distribution of CYP2E1 protein expression in our pediatric NAFLD cohort was similar to that observed under conditions of elevated hepatic CYP2E1 content due to induction by alcohol intake, with staining throughout acinar zones 1, 2, and 328–29. Furthermore, hepatic CYP2E1 staining also demonstrated a slight association with lipid droplets in our pediatric patient group, but this relationship did not appear to be as strong as that originally noted by Weltman et al. in adult NASH20 or as that observed in our recent study of CYP2E1 protein expression in a cohort of bariatric patients with NASH30.
There have been several studies aimed at clarifying the histopathology, clinical and laboratory predictors, and progression of pediatric NAFLD7, 9, 12, 31. Although this was not the primary focus of our study, we did compare our patient population with those described in previous reports and found that histopathology features of our pediatric population differed slightly. Specifically, we observed almost equal numbers of children with type 1 and type 2 NASH (n=21 and n=19, respectively), and only 5 children were diagnosed as having a mixed pattern of NASH. In comparison, Schwimmer et al. originally reported a predominance (51%) of type 2 NASH in a group of 100 children across the spectrum of NAFLD7, and Carter-Kent et al. recently found that in a cohort of 108 pediatric patients, 82% displayed overlapping features of both type 1 and type 2 NASH12. However, similar to these other pediatric patient groups, children in our cohort with type 1 NASH were significantly older (p<0.001) than the type 2 NASH or mixed NASH. The differences in histopathology and the subsequent classification observed between our pediatric patient cohort and others reiterates the need for an improved standardized scoring system designed specifically with the patterns of pediatric NASH, including portal inflammation, in mind12.
This study was the first to investigate a role for elevated hepatic lipid peroxidation and/or increased CYP2E1 protein expression in pediatric NAFLD; however, there are several limitations that require mention. First, we included a control group of children with normal liver histology upon biopsy (but with medical conditions listed in Table 1) and children with chronic HCV infection for comparison purposes. Importantly, these children did have elevated transaminase levels and did exhibit inflammation and hepatocyte ballooning upon liver biopsy. In addition, the control and HCV groups were significantly younger than the NAFLD group (p<0.05). It is not likely that this age disparity would affect hepatic CYP2E1 content, as several previous reports have shown no affect of age (≥1 year) on CYP2E1 expression and/or activity32–34. Likewise, although increasing age is likely associated with elevated oxidative stress/lipid peroxidation levels35, the minor age differences between groups does not explain the large increase in MDA content in the NAFLD patient group that was observed. Nonetheless, comparisons with the control and HCV groups should be interpreted with these limitations in mind. Second, a greater number of boys (n=55) were included in our study as compared to girls (n=23), making it difficult to draw conclusions based on gender. Third, due to the retrospective nature of our study, we were unable to obtain enough liver tissue to perform other measures of CYP2E1 content, including mRNA expression, Western blotting for CYP2E1 protein expression, and in vitro CYP2E1 activity assays. Finally, we have tried to prevent any confounding of our study results by standardizing the immunohistochemical staining thresholds when measuring MDA and CYP2E1 staining using our digital quantification technique.
In conclusion, findings from this study demonstrate that hepatic lipid peroxidation, but not CYP2E1 protein content, are increased in children with NAFLD compared to those with normal liver histology or chronic HCV infection. We also identified BMI as a significant independent predictor of both hepatic lipid peroxidation levels and CYP2E1 protein expression. Despite these novel observations, lipid peroxidation levels and CYP2E1 protein content did not differ across the non-NASH NAFLD group and pediatric NASH subtypes; therefore, further study is required to identify additional factors that may play a role in the pathogenesis and progression of these potentially very different patterns of disease.
Acknowledgments
The authors thank Dr. Constance J. Temm and Rashmil Saxena for their assistance with the immunohistochemical techniques.
Financial Disclosures
Supported in part by K24 DK072101 (to NC) and Lauren N Bell is supported by a Clinical Pharmacology Training Grant to Indiana University (T32 GM08425). Dr. Chalasani has financial consulting agreements with several pharmaceutical companies but none pose a potential conflict.
References
- 1.Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD: Summary of a single topic conference. Hepatology. 2006;44:1344–54. doi: 10.1002/hep.21373. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009 doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki D, Hashimoto E, Kaneda K, Tokushige K, Shiratori K. Liver failure caused by non-alcoholic steatohepatitis in an obese young male. J Gastroenterol Hepatol. 2005;20:327–9. doi: 10.1111/j.1440-1746.2005.03724.x. [DOI] [PubMed] [Google Scholar]
- 5.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- 6.Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837–47. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 9.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–71 e2. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226–32. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 11.Nobili V, Marcellini M, Devito R, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 12.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: A multicenter clinicopathological study. Hepatology. 2009 doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–9. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald GA, Bridle KR, Ward PJ, et al. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- 17.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–53. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–75. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–50. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 20.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–33. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 21.Chtioui H, Semela D, Ledermann M, Zimmermann A, Dufour JF. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int. 2007;27:764–71. doi: 10.1111/j.1478-3231.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 22.Emery MG, Fisher JM, Chien JY, et al. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38:428–35. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- 23.Ernstgard L, Warholm M, Johanson G. Robustness of chlorzoxazone as an in vivo measure of cytochrome P450 2E1 activity. Br J Clin Pharmacol. 2004;58:190–200. doi: 10.1111/j.1365-2125.2004.02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raucy JL, Lasker JM, Kraner JC, Salazar DE, Lieber CS, Corcoran GB. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–80. [PubMed] [Google Scholar]
- 25.Machado M, Cortez-Pinto H. Nash, insulin resistance and iron. Liver Int. 2006;26:1159–62. doi: 10.1111/j.1478-3231.2006.01394.x. [DOI] [PubMed] [Google Scholar]
- 26.Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem. 2007;40:776–80. doi: 10.1016/j.clinbiochem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed] [Google Scholar]
- 28.Tsutsumi M, Lasker JM, Shimizu M, Rosman AS, Lieber CS. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10:437–46. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- 29.Buhler R, Lindros KO, von Boguslawsky K, Karkkainen P, Makinen J, Ingelman-Sundberg M. Perivenous expression of ethanol-inducible cytochrome P450 IIE1 in livers from alcoholics and chronically ethanol-fed rats. Alcohol Alcohol Suppl. 1991;1:311–5. [PubMed] [Google Scholar]
- 30.Bell LN, Temm CJ, Saxena R, et al. Bariatric surgery-induced weight loss reduces hepatic lipid peroxidation levels and affects hepatic cytochrome P-450 protein content. Ann Surg. 2010;251:1041–8. doi: 10.1097/SLA.0b013e3181dbb572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period . Eur J Biochem. 1996;238:476–83. doi: 10.1111/j.1432-1033.1996.0476z.x. [DOI] [PubMed] [Google Scholar]
- 33.Blanco JG, Harrison PL, Evans WE, Relling MV. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos. 2000;28:379–82. [PubMed] [Google Scholar]
- 34.Johnsrud EK, Koukouritaki SB, Divakaran K, Brunengraber LL, Hines RN, McCarver DG. Human hepatic CYP2E1 expression during development. J Pharmacol Exp Ther. 2003;307:402–7. doi: 10.1124/jpet.102.053124. [DOI] [PubMed] [Google Scholar]
- 35.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–61. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]


