Abstract
Objectives
Genetic factors contribute substantially to variability in warfarin dose requirements and are important in the dose-titration phase; their effects on the stability of anticoagulation later in therapy are not known.
Methods
Using de-identified electronic medical records linked to a DNA-biobank, we studied 140 African-Americans and 943 European-Americans after the warfarin dose-titration phase. We genotyped 12 SNPs in genes (CYP2C9, VKORC1, CYP4F2, GGCX, EPHX1, CALU) associated with altered warfarin dose-requirements and tested their associations with INR variability (INRVAR) and percent time in therapeutic range (TTR) in European-Americans and African-Americans.
Results
One allele copy of rs2108622 in CYP4F2 was associated with a 15 percent (95% CI, 1, 26, p = 0.03) decrease in median INRVAR in European-Americans. In African-Americans, GGCX variants rs11676382 and rs699664 were associated with 4.16 (95% CI, 1.45, 11.97, p = 0.009) and 1.50 (95% CI, 1.07, 2.08, p = 0.02) fold changes in median INRVAR per variant allele copy respectively; rs11676382 was also significantly associated with a 23.19 (95%CI, 5.89, 40.48, p = 0.01) percent decrease in TTR. The total variation in INRVAR explained by both clinical factors and rs2108622 was 5.2% for European-Americans. In African-Americans, the inclusion of GGCX variants rs11676382 and rs699664, and CYP2C9*8 variant rs7900194 explained approximately 29% of the variation in INRVAR.
Conclusions
The stability of anticoagulation after the warfarin dose-titration phase is differentially affected by variants in CYP4F2 in European-Americans and GGCX loci in African-Americans.
Introduction
Warfarin, a vitamin K antagonist, is a cornerstone of oral anticoagulation therapy; however, it has a narrow therapeutic index and patients on warfarin who are under- or over-anticoagulated have increased risk of thrombosis and bleeding, respectively. Consequently, anticoagulation is monitored by measurement of the international normalized ratio (INR), and warfarin dose is adjusted to maintain the INR within a target range, between 2 and 3 for most patients.
There is, however, substantial interindividual variability in the INR response to the same dose of warfarin, and several clinical and genetic factors affect dose requirements [1–4]. Moreover, once the dose of warfarin that an individual patient requires to attain the target INR has been established (the dose-titration phase), the INR can fluctuate despite an unchanged warfarin dose. Thus, the INR is monitored throughout therapy and dosing is adjusted to maintain the INR within the target range.
Approximately 50% of the variability in warfarin dose requirement among individuals is attributable to genetic variations notably those that occur in CYP2C9 that metabolizes S-warfarin, and in VKORC1, the target of warfarin [5]. Consequently, dosing algorithms that incorporate a patient’s genotype have evolved [6], although the added contribution of genotype to improvement in quality of anticoagulation is controversial [7, 8]. We and others have shown that the contribution of genotype to predicting an individual’s stable warfarin dose is lost within 2 weeks because the stable dose is identified rapidly by empiric titration of dose against the INR [9–11]. However, several lines of evidence [12–15] suggest that the effects of genotype on clinical outcomes persist after an individual’s warfarin dose requirement has been determined.
For example, the risk of bleeding or thrombosis was increased long after the initial 2 week dose-titration phase in patients with genotypes associated with altered warfarin sensitivity [12, 13]. The mechanisms whereby genotype might affect complications of warfarin therapy beyond the dose-titration phase are not known. We hypothesized that long-term increased risk associated with genotype is due to greater instability of warfarin response (and thus of INR) in patients with variant genotypes, throughout therapy.
The lack of stability of the INR response despite a stable dose of warfarin in individual patients is a major clinical problem. As a consequence, patients require regular INR monitoring and time above or below the therapeutic range increases the risk of bleeding and thrombosis, respectively. Stability of the INR response during warfarin therapy is most often assessed using two measurements: percent of time spent in therapeutic INR range (TTR), [16] and INR variability [17, 18]. TTR is a standard measure of the quality of anticoagulation; lower values are associated with increased risk of adverse outcomes in patients on warfarin [19]. However, because TTR does not capture the full amplitude of INR fluctuations within and outside the target INR range, INR variability is also measured. As is the case with lower TTR, higher INR variability is also associated with adverse outcomes during warfarin therapy [18, 20].
Little is known about the effect of genetic variants that alter warfarin dose-requirements on the stability of the INR response after the dose-titration phase. Therefore, we tested the hypotheses that variants in genes (CYP2C9, VKORC1, CYP4F2, GGCX, EPHX1, CALU) [4, 21–25] associated with altered warfarin dose are associated with INR variability and TTR after the warfarin dose-titration phase.
Methods
Study Population
The study was performed using BioVU, the clinical practice-based biobank at Vanderbilt University Medical Center (VUMC) that stores DNA samples linked to a de-identified version of the electronic medical record (EMR). The enrollment and biobanking approaches have been described [26, 27]. Notably, since January 2015, accrual into BioVU has been switched from an opt-out to an opt-in approach, to comply with evolving federal regulations on use of DNA samples in biomedical research. The Institutional Review Board of VUMC approved this study.
We utilized a cohort of 1170 patients who received warfarin for at least three months, achieved a stable dose of warfarin during therapy and who had been successfully genotyped for the variants of interest [22]. Patients were required to have at least 6 INR values after the study start date. Of the 1170 potential subjects, 1083 (140 African-Americans and 943 European-Americans) qualified.
Phenotyping
For each patient in the cohort we identified a study start date. We designated the study start date as occurring 30 days after the first INR ≥ 1.8 (in order to exclude the warfarin dose-titration phase). Subsequently, INRs for the next 360 days were included in the analysis unless the patient discontinued warfarin earlier. Temporary warfarin discontinuation of up to two weeks was allowed, for example, as occurs before surgery. We identified periods of temporary warfarin discontinuation by examining all INR values for each patient during the 360 day study period, identifying values ≤ 1.2, and reviewing the EMR to ascertain the cause for the low INR. If review of the EMR determined that the low INR was due to temporary discontinuation of warfarin, the time between the last INR while receiving warfarin and the study resumption start date was excluded from the study time. The study resumption start date after a temporary discontinuation of warfarin was set to 10 days after the next INR of ≥ 1.8.
The primary outcome was INR variability (INRVAR) calculated for each patient using the variance of the INRs according to the modified Fihn Variance Growth Rate (VGR) method [18]. INRVAR measures the time-weighted variance of the INR and reflects the degree to which each INR value deviates from the previous one, thus reflecting INR variability, irrespective of the intensity of anticoagulation (as measured by TTR) [17, 18].
The secondary outcome was TTR calculated by Rosendaal’s linear interpolation method [16]. TTR reflects the sum of a patient’s time within the therapeutic range divided by the total time of observation and is expressed as percent.
SNP Selection and Genotyping
We studied 12 preselected candidate single nucleotide polymorphisms (SNPs) in six genes CYP2C9, VKORC1, EPHX1, GGCX, CALU, and CYP4F2 known to influence warfarin dose that had been previously genotyped in this cohort. Details of genotyping procedures and quality control measures have been described [22]. Briefly, SNPs were genotyped using either the Sequenom (Sequenom, Inc., CA, USA) or Taqman (Applied Biosystems, Inc., CA, USA) genotyping technologies and subjected to standard genotyping quality control procedures. All SNPs passed tests for Hardy Weinberg Equilibrium at a significance level of p < 0.05.
Statistical Analyses
Patient demographics and baseline clinical characteristics data were summarized with 10th, 50th and 90th percentiles for continuous variables and percentages for categorical variables. To examine the association of SNPs with INRVAR and TTR, unadjusted and adjusted analyses were performed separately in European-Americans and African-Americans due to differences in SNP minor allele frequencies. In unadjusted analyses, simple linear models were constructed with each target SNP in the model without adjusting for clinical covariates. In adjusted analyses, multiple linear regression models were constructed using all 12 prespecified SNPs while adjusting for pre-specified clinical risk factors for warfarin response: age, sex, BMI, smoking, amiodarone use, concurrent use of enzyme inducers and indication for warfarin use including atrial fibrillation, pulmonary embolism or venous thrombosis (PE/DVT), and stroke. We utilized an additive genetic model and, if indicated by the putative modes of inheritance for the alleles of interest, also examined recessive or dominant models. Prior to model fitting, INRVAR was log-transformed due to skewness, and the reported exponentiated SNP associations with INRVAR should be interpreted as the fold-change in the geometric mean or the median INRVAR associated with a one minor allele increase. We also analyzed the relationship between both INRVAR and TTR and categories of warfarin response (normal, sensitive or highly sensitive) using combinations of VKORC1 and CYP2C9 SNPs (and genotypes) to categorize individuals [28].
All analyses were performed using R version 3.1.2; a two-sided significance level of 0.05 was used for statistical inferences.
Results
Study Cohort Characteristics
Baseline cohort characteristics are shown in Table 1. Of the 1083 subjects, 140 (12.9%) were African-American and 483 (44.6%) were female. The median (10th, 90th percentile) age and BMI was 67 (43, 83) years and 28.4 (22.2, 38.3) kg/m2, respectively. The most frequent indication for warfarin therapy in European-Americans was atrial fibrillation (56.5%), followed by pulmonary embolism or deep vein thrombosis (PE/DVT, 22.4%). In African-Americans, the most frequent indication was PE/DVT (49.3%) followed by atrial fibrillation (31.4%).
Table 1.
Cohort Characteristics
| European-Americans (N = 943) |
African-Americans (N = 140) |
Overall (N = 1083) |
|
|---|---|---|---|
| Age (years) | 67.0 (45.0, 83.8) | 60.5 (32.0, 79.0) | 67.0 (43.0, 83.0) |
| Gender (female) | 42.7 | 57.1 | 44.6 |
| BMI (kg/m2) | 28.2 (22.2, 38.0) | 30.3 (22.5, 43.1) | 28.4 (22.2, 38.3) |
| Current smokers | 9.8 | 21.4 | 11.3 |
| Amiodarone use | 8.3 | 8.6 | 8.3 |
| Enzyme inducers | 2.7 | 2.9 | 2.7 |
|
Indication for warfarin |
|||
| Atrial fibrillation | 56.5 | 31.4 | 53.3 |
| Thrombosis | 22.4 | 49.3 | 25.9 |
| Stroke | 5.4 | 5.0 | 5.4 |
| Orthopedic | 0.2 | 0.7 | 0.3 |
| Other | 17.3 | 15.0 | 17.0 |
Continuous variables are shown as 10th, 50th and 90th percentiles. Categorical variables are shown as percentages
Outcomes and Association with Genotype
TTR was statistically significantly higher in European-Americans than in African-Americans 62.9 (29.9, 88.3) vs 56.1 (23.1, 83.0), with p = 0.01 using the Wilcoxon rank sum test, though there was no significant difference in INRVAR between the two ethnicities. (p = 0.12).
In European-Americans subjects, the only variant associated with INRVAR was rs2108622 (V433M) in CYP4F2. Each copy of the minor T allele was associated with a 15 percent decrease (95% CI, 1, 26, p = 0.03) in median INRVAR (Table 2).
Table 2.
Association between variants and INR variability
| Variant (SNP) |
Gene | European Americans | African Americans | ||
|---|---|---|---|---|---|
| Effect (95% CI) | P-value | Effect (95% CI) | P-value | ||
| rs339097 | CALU | - | - | 1.33 (0.76, 2.33) | 0.32 |
| rs1799853 | CYP2C9 (*2) | 0.98 (0.80, 1.20) | 0.82 | 0.76 (0.27, 2.10) | 0.59 |
| rs1057910 | CYP2C9 (*3) | 1.09 (0.82, 1.45) | 0.54 | 2.76 (0.67, 11.30) | 0.16 |
| rs9332131 | CYP2C9 (*6) | - | - | 1.19 (0.33, 4.25) | 0.79 |
| rs7900194 | CYP2C9 (*8) | - | - | 1.59 (0.81, 3.12) | 0.18 |
| rs28371685 | CYP2C9 (*11) | - | - | 0.45 (0.14, 1.43) | 0.18 |
| rs2108622 | CYP4F2 (*3) | 0.85 (0.74, 0.99) | 0.03 | 0.85 (0.49, 1.47) | 0.56 |
| rs2292566 | EPHX1 | 1.03 (0.84, 1.25) | 0.80 | 1.14 (0.71, 1.83) | 0.60 |
| rs11676382 | GGCX | 1.13 (0.90, 1.42) | 0.28 | 4.16 (1.45, 11.97) | 0.009 |
| rs699664 | GGCX | 0.97 (0.85, 1.12) | 0.72 | 1.50 (1.07, 2.08) | 0.02 |
| rs9923231 | VKORC1 | 1.03 (0.89, 1.18) | 0.70 | 0.88 (0.51, 1.55) | 0.67 |
| rs2359612 | VKORC1 | 1.02 (0.89, 1.17) | 0.78 | 1.05 (0.70, 1.57) | 0.81 |
INR variability data were log transformed prior to conducting simple (without clinical data adjustments) linear regression analyses. Effect estimates and confidence intervals were exponentiated and results shown should be interpreted as the ratio of the geometric mean or the median associated with a one minor allele change. Blank entries for variants in European Americans are for SNPs represented only in African Americans.
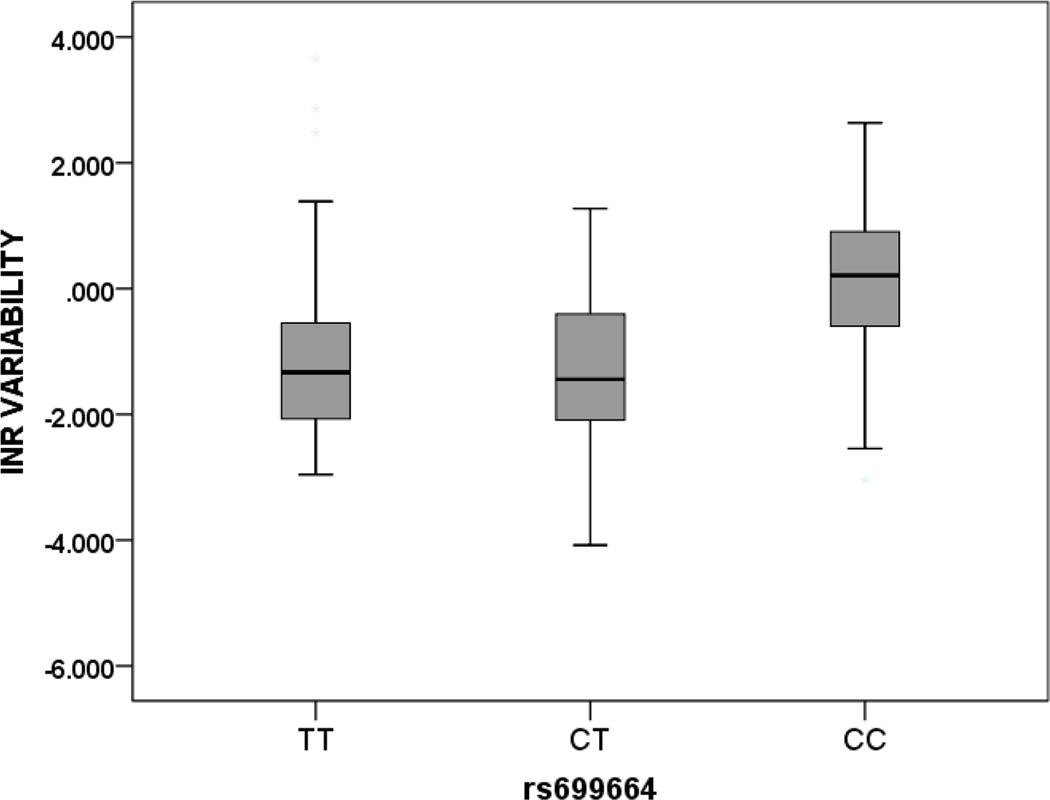
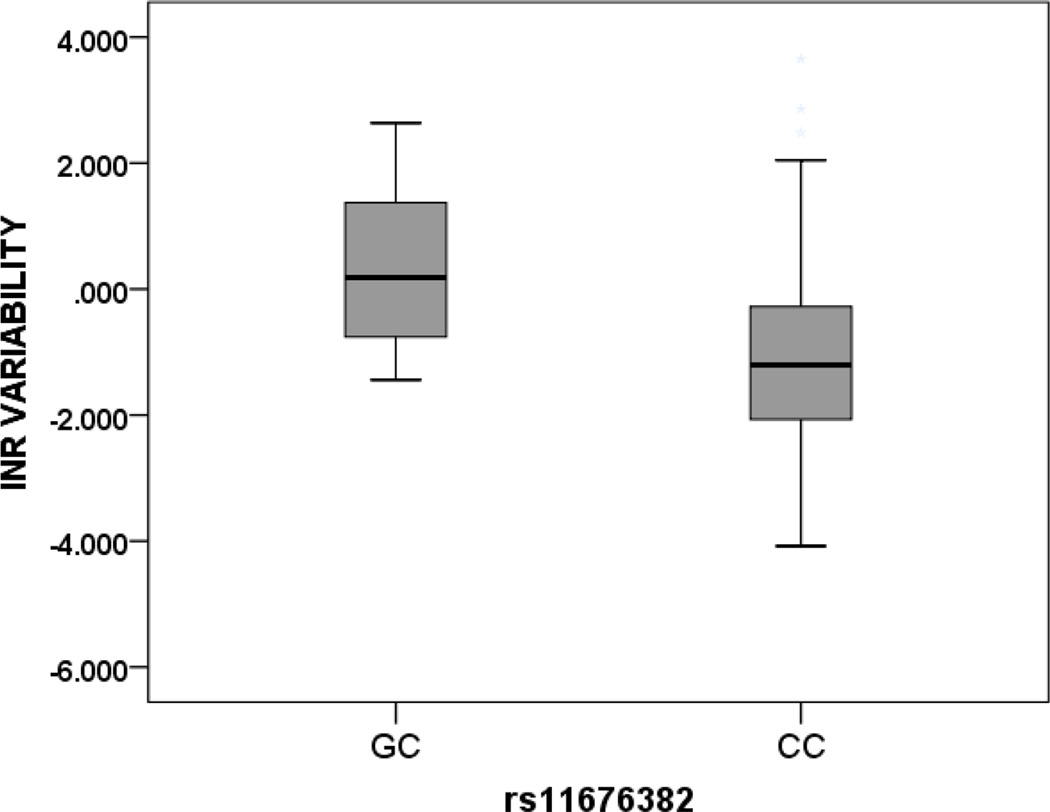
In African-Americans, two SNPs in the GGCX locus were significantly associated with INRVAR, rs11676382 and rs699664 (Figure 1). For rs11676382, there was a 4.16-fold change (95% CI, 1.45, 11.97, p = 0.009) in median INRVAR per copy of the minor G allele. For rs699664, there was a 1.50 fold change (95% CI, 1.07, 2.08, p = 0.02) in median INRVAR per copy of the minor C allele. With this SNP, testing with the recessive model also showed a highly significant association (p < 0.001) in African-Americans.
Figure 1. INR Variability and GGCX Variant Genotypes in African Americans.
Footnote Data shown for GGCX variants rs11676382 and rs699664 and depicted as median (solid dark line) and interquartile range (box) and 1.5 times the interquartile range (whiskers).
Rs11676382; GG = 0, GC = 7, CC = 131 (p = 0.009)
Rs 699664; CC = 21, CT = 63, TT = 56 (p = 0.02)
No SNPs were significantly associated with TTR in European-Americans (Table 3). However, in African-Americans, a GGCX SNP (rs11676382) was significantly associated with TTR. We estimated a 23.19 (95% CI, 5.89, 40.48, p = 0.01) percentage point decrease in mean TTR per copy of the minor G allele.
Table 3.
Association between variants and time in therapeutic range
| Variant (SNP) |
Gene | European Americans | African Americans | ||
|---|---|---|---|---|---|
| Effect (95% CI) | P-value | Effect (95% CI) | P- value |
||
| rs339097 | CALU | - | - | −2.17 (−11.28, 6.94) | 0.64 |
| rs1799853 | CYP2C9 (*2) | −1.79 (−4.83, 1.25) | 0.25 | −11.48 (−28.00, 5.05) | 0.18 |
| rs1057910 | CYP2C9 (*3) | −2.90 (−7.16, 1.35) | 0.18 | −13.62 (−36.57, 9.33) | 0.25 |
| rs9332131 | CYP2C9 (*6) | - | - | 4.53 (−16.19, 25.26) | 0.67 |
| rs7900194 | CYP2C9 (*8) | - | - | −2.90 (−13.85, 8.04) | 0.60 |
| rs28371685 | CYP2C9 (*11) | - | - | −2.50 (−21.50, 16.50) | 0.80 |
| rs2108622 | CYP4F2 (*3) | 0.92 (−1.26, 3.10) | 0.41 | 5.25 (−3.64, 14.13) | 0.25 |
| rs2292566 | EPHX1 | −1.06 (−4.02, 1.9) | 0.48 | −0.07 (−7.82, 7.69) | 0.99 |
| rs11676382 | GGCX | 1.31 (−2.11, 4.73) | 0.45 | −23.19 (−40.48, −5.89) | 0.01 |
| rs699664 | GGCX | −0.38 (−2.49, 1.73) | 0.72 | 0.06 (−5.44, 5.55) | 0.98 |
| rs9923231 | VKORC1 | 1.56 (−0.55, 3.67) | 0.15 | 0.20 (−8.91, 9.32) | 0.97 |
| rs2359612 | VKORC1 | 1.33 (−0.77, 3.43) | 0.22 | −1.42 (−7.94, 5.11) | 0.67 |
P-values for SNP associations with TTR were calculated using simple linear regression (without clinical data adjustments). Effect estimates and confidence intervals capture the additive change in the mean percent TTR associated with a one minor allele change. Blank entries for variants in European Americans are for SNPs represented only in African Americans.
Adjusted Analysis - Full Regression with Genotype and Clinical Factors
In models that included all 12 SNPs and prespecified clinical risk factors, in European-Americans, INRVAR was significantly associated with female gender, concurrent use of amiodarone and enzyme inducers while TTR was associated with older age. In African-Americans, age and BMI were strongly associated with both INRVAR and TTR (Supplementary Table 2). In these models, the effects of the GGCX variants on INRVAR in African-Americans were abrogated but a SNP in the CYP2C9 locus, rs7900194 (CYP2C9*8) became borderline significant (2.09-fold increase in the geometric mean (or median) of INRVAR per copy of the minor allele, p = 0.05). For TTR, the effects of rs11676382 remained significant in African-Americans but the effect of rs2108622 (V433M) in European-Americans did not.
In European Americans, female age, amiodarone use, and use of enzyme inducers explained 4.7% and 2.4% of the variation in INRVAR and TTR, respectively and inclusion of rs2108622 (V433M) into the model only served to explain 5.2% of the total variation in INRVAR.
In African-Americans, age and BMI accounted for 15% of the variation in both INRVAR and TTR. With the inclusion of the significantly associated GGCX variants rs11676382 and rs699664, and the CYP2C9*8 variant rs7900194 (borderline significance in the regression model) the model explained approximately 29% of the variation in INRVAR. For TTR, adding rs11676382 into the model with prespecified clinical factors increased the percent of variation explained from 14.7 % to 19.6%.
Comparison by Warfarin Sensitivity
Subjects were categorized into 3 genotype functional classes (normal, sensitive and highly sensitive) that corresponded to the FDA warfarin response categories based on combinations of VKORC1 and CYP2C9 genotypes. There were 690 normal responders, 280 sensitive and 32 highly sensitive responders. There was no significant differences in either TTR (p = 0.86) or INRVAR (p = 0.93) among the three groups.
Discussion
The major finding is that some genetic variants in the vitamin K recycling pathway contribute to interindividual variations in stability of INR after the dose titration phase of warfarin therapy. Specifically, rs2108622 (V433M) in CYP4F2 contributed to variation in European-Americans, and rs11676382 and rs699664 in GGCX contributed significantly to INR variation in African-Americans.
The CYP4F2 locus encodes the CYP4F2 protein, a key regulator of vitamin K levels through NADPH-dependent oxidation and inactivation that ensures effective negative feedback regulation. The rs2108622 (V433M) variant in this gene, a C>T missense SNP with global MAF 0.24, designated as the *3 allele (CYP allele nomenclature website at http://www.cypalleles.ki.se/) has been associated with warfarin dose. Several studies have reported an association between the T allele and increased warfarin dose requirements [29–32]. A recent meta-analysis estimated that subjects with the rs2108622 T allele required an 8.3% higher warfarin dose than those with the CC genotype [29].
We found an association between rs2108622 (V433M) in CYP4F2 and INRVAR in European-Americans; subjects bearing one or more copies of the T allele had significantly lower INR variability compared with those with the C allele. This association was not significant in African-Americans, likely due to a lack of statistical power (only 1 TT homozygous subject). The finding of decreased INR variability associated with the T allele of this CYP4F2 variant is concordant with findings in recent studies of genetic risk factors for major bleeding in patients receiving warfarin that suggested a lower risk of bleeding in subjects bearing the minor T allele [13, 15].
The GGCX locus encodes gamma-glutamyl carboxylase which catalyzes the gamma-carboxylation of glutamic acid residues on vitamin K-dependent clotting factors. Because of this role in vitamin K recycling, many studies have examined the effects of GGCX variants on warfarin dose. Variants in GGCX have been associated with warfarin dose requirements in cohorts of varying ethnicities [23, 33]. Rs11676382 C>G in GGCX (intronic variant, global MAF = 0.0256) accounted for a 2–6% reduction in warfarin dose per copy of the minor G allele [33]. In the present study, we report an association between increased INR variability and the G allele of rs11676382 in African-Americans. There were no African-American subjects homozygous for the G allele but heterozygotes (n= 7) had a 4 fold change in median INRVAR and almost two fold numerical decrease in TTR than homozygous CC subjects. This difference appears clinically meaningful in terms of risk associated with both decreased intensity and stability of anticoagulation on warfarin [17].
The second GGCX variant, rs699664 C>T (missense SNP, global MAF 0.38), has also been associated with warfarin dosing, largely in Asian populations [34, 35] but not in European-Americans [23]. The differences in outcomes from various studies could be due to the marked differences in minor allele frequency among various ethnicities. The minor (C) allele in African-Americans (MAF 0.39) is the major allele in populations of European ancestry (MAF 0.67). We found significant differences in INRVAR in African-American subjects bearing two copies of the minor allele compared with those bearing one and zero copies. Subjects with the CC genotypes had significantly greater INR variability but did not have a lower TTR compared with CT and TT genotypes. Of interest, the GCCX variants associated with INRVAR or TTR in African-Americans were not associated with either outcome in European-Americans.
It is interesting that the significant genetic associations with stability of anticoagulation were with variants involved in vitamin K recycling rather than warfarin metabolism, suggesting that variants related to vitamin K pathways are more likely to contribute to variability in anticoagulation, perhaps because of variability in dietary vitamin K intake. Associations between VKORC1 and CYP2C9 variants and measurements of warfarin anticoagulation have typically been found to be most relevant early in therapy, particularly in the dose-titration phase. A recent study reported significant differences in TTR and bleeding during warfarin therapy among subjects categorized as normal, sensitive or highly sensitive according to genotype, but differences did not persist beyond 90 days [28]. We found no associations between the same warfarin sensitivity categories and INRVAR or TTR, perhaps because many patients were exposed to warfarin for longer than 90 days. We did however find a borderline significant association between a CYP2C9*8 variant (rs7900194) and INRVAR. The minor A allele has been associated with decreased warfarin clearance and therefore higher dose requirements in African-Americans [36, 37]
Clinical factors also affected the stability of anticoagulation. Concurrent use of amiodarone and enzyme inducing drugs, and female gender were associated with greater INRVAR (in European-Americans) while age and BMI were linked to lower INRVAR (in African-Americans). Use of amiodarone, a potent CYP2C9 inhibitor is known to be associated with lower warfarin dose requirements, while concurrent use of enzyme inducers is associated with higher dose requirements. Older age has been associated with lower warfarin dose requirements and was also associated with INRVAR in our cohort, an association that remained significant after adjustment for other variables. Higher BMI was associated with lower INRVAR in African-Americans. Vitamin K is a fat soluble vitamin and if the dietary intake of vitamin K was lower or more variable in African-Americans it is possible that vitamin K in fat could protect against INR fluctuation under those circumstances. While there were no significant differences in INRVAR, TTR tended to be higher in European-Americans compared to African-Americans. In addition to genetic factors, these variables would be affected by diet, adherence to medication and other factors.
Overall, this study sought to explain the variance observed in stability of the INR during long-term warfarin treatment using genetic and clinical factors. The total amount of variation explained by all factors was approximately 30% in African-Americans, and 5% in European-Americans. One reason for the difference may be that we did not study factors that contribute substantially to variability in European-Americans.
Our study had several limitations; we utilized a retrospective case-only design and thus were not able to prospectively study variables of interest such as diet or other clinical and environmental factors. African-American subjects represented a relatively small cohort and thus we had limited statistical power to detect small effects. Also, we could not measure adherence to warfarin but this should not be differentially affected by genotype.
In conclusion, we report for the first time an association between genetic variants in the vitamin K pathway other than VKORC1 and CYP2C9 on anticoagulation stability (INRVAR) and intensity (TTR) in patients on long-term warfarin. The effects were modest and much of the variability in the stability of anticoagulation in patients receiving chronic warfarin remains unexplained.
Supplementary Material
Acknowledgments
Source of Funding:
This work was supported in part by funding by the NIH through grants GM109145, U19HL069757, and T32 GM007569 and Vanderbilt University Medical Center’s BioVU which is supported by institutional funding and by the Vanderbilt CTSA grant ULTR000445 from NCATS/NIH
Key Words and Abbreviations
- INR
International Normalized Ratio
- TTR
Time in therapeutic range
- EMR
Electronic Medical Records
- INRVAR
INR Variability
- MAF
Minor Allele Frequency
- SNP
Single Nucleotide Polymorphisms
- VUMC
Vanderbilt University Medical Center
Footnotes
Conflicts of Interest:
None declared for all authors.
References
- 1.D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 2.Spreafico M, Lodigiani C, van Leeuwen Y, Pizzotti D, Rota LL, Rosendaal F, et al. Effects of CYP2C9 and VKORC1 on INR variations and dose requirements during initial phase of anticoagulant therapy. Pharmacogenomics. 2008;9:1237–1250. doi: 10.2217/14622416.9.9.1237. [DOI] [PubMed] [Google Scholar]
- 3.Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H, et al. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–211. doi: 10.1160/TH05-06-0446. [DOI] [PubMed] [Google Scholar]
- 4.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–270. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 5.McClain MR, Palomaki GE, Piper M, Haddow JE. A rapid-ACCE review of CYP2C9 and VKORC1 alleles testing to inform warfarin dosing in adults at elevated risk for thrombotic events to avoid serious bleeding. Genet Med. 2008;10:89–98. doi: 10.1097/GIM.0b013e31815bf924. [DOI] [PubMed] [Google Scholar]
- 6.International Warfarin Pharmacogenetics C. Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A Randomized Trial of Genotype-Guided Dosing of Warfarin. New England Journal of Medicine. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 9.Lenzini P, Wadelius M, Kimmel S, Anderson JL, Jorgensen AL, Pirmohamed M, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther. 2010;87:572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Schwarz UI, Ritchie MD, Roden DM, Stein CM, Kurnik D. Relative contribution of CYP2C9 and VKORC1 genotypes and early INR response to the prediction of warfarin sensitivity during initiation of therapy. Blood. 2009;113:3925–3930. doi: 10.1182/blood-2008-09-176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein RS, Moyer TP, Aubert RE, DJ OK, Xia F, Verbrugge RR, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study) J Am Coll Cardiol. 2010;55:2804–2812. doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Kawai VK, Cunningham A, Vear SI, Van Driest SL, Oginni A, Xu H, et al. Genotype and risk of major bleeding during warfarin treatment. Pharmacogenomics. 2014;15:1973–1983. doi: 10.2217/pgs.14.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mega JL, Giugliano RP. Genotype-guided dosing of warfarin. Clin Chem. 2014;60:920–922. doi: 10.1373/clinchem.2013.220004. [DOI] [PubMed] [Google Scholar]
- 15.Roth JA, Boudreau D, Fujii MM, Farin FM, Rettie AE, Thummel KE, et al. Genetic risk factors for major bleeding in patients treated with warfarin in a community setting. Clin Pharmacol Ther. 2014;95:636–643. doi: 10.1038/clpt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 17.Razouki Z, Ozonoff A, Zhao S, Jasuja GK, Rose AJ. Improving quality measurement for anticoagulation: adding international normalized ratio variability to percent time in therapeutic range. Circ Cardiovasc Qual Outcomes. 2014;7:664–669. doi: 10.1161/CIRCOUTCOMES.114.000804. [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen Y, Rosendaal FR, Cannegieter SC. Prediction of hemorrhagic and thrombotic events in patients with mechanical heart valve prostheses treated with oral anticoagulants. J Thromb Haemost. 2008;6:451–456. doi: 10.1111/j.1538-7836.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 19.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk-adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA) Circ Cardiovasc Qual Outcomes. 2011;4:22–29. doi: 10.1161/CIRCOUTCOMES.110.957738. [DOI] [PubMed] [Google Scholar]
- 20.Lind M, Fahlen M, Kosiborod M, Eliasson B, Oden A. Variability of INR and its relationship with mortality, stroke, bleeding and hospitalisations in patients with atrial fibrillation. Thromb Res. 2012;129:32–35. doi: 10.1016/j.thromres.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Pautas E, Moreau C, Gouin-Thibault I, Golmard JL, Mahe I, Legendre C, et al. Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin Pharmacol Ther. 2010;87:57–64. doi: 10.1038/clpt.2009.178. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez AH, Shi Y, Schildcrout JS, Delaney JT, Xu H, Oetjens MT, et al. Predicting warfarin dosage in European-Americans and African-Americans using DNA samples linked to an electronic health record. Pharmacogenomics. 2012;13:407–418. doi: 10.2217/pgs.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieder MJ, Reiner AP, Rettie AE. Gamma-glutamyl carboxylase (GGCX) tagSNPs have limited utility for predicting warfarin maintenance dose. J Thromb Haemost. 2007;5:2227–2234. doi: 10.1111/j.1538-7836.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voora D, Koboldt DC, King CR, Lenzini PA, Eby CS, Porche-Sorbet R, et al. A polymorphism in the VKORC1 regulator calumenin predicts higher warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:445–451. doi: 10.1038/clpt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–48. doi: 10.1111/j.1752-8062.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mega JL, Walker JR, Ruff CT, Vandell AG, Nordio F, Deenadayalu N, et al. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015 doi: 10.1016/S0140-6736(14)61994-2. [DOI] [PubMed] [Google Scholar]
- 29.Danese E, Montagnana M, Johnson JA, Rettie AE, Zambon CF, Lubitz SA, et al. Impact of the CYP4F2 p.V433M polymorphism on coumarin dose requirement: systematic review and meta-analysis. Clin Pharmacol Ther. 2012;92:746–756. doi: 10.1038/clpt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limdi NA, Brown TM, Yan Q, Thigpen JL, Shendre A, Liu N, et al. Race influences warfarin dose changes associated with genetic factors. Blood. 2015;126:539–545. doi: 10.1182/blood-2015-02-627042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75:1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bress A, Patel SR, Perera MA, Campbell RT, Kittles RA, Cavallari LH. Effect of NQO1 and CYP4F2 genotypes on warfarin dose requirements in Hispanic-Americans and African-Americans. Pharmacogenomics. 2012;13:1925–1935. doi: 10.2217/pgs.12.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G, et al. Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb Haemost. 2010;104:750–754. doi: 10.1160/TH09-11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura R, Miyashita K, Kokubo Y, Akaiwa Y, Otsubo R, Nagatsuka K, et al. Genotypes of vitamin K epoxide reductase, gamma-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb Res. 2007;120:181–186. doi: 10.1016/j.thromres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Liang Y, Chen Z, Guo G, Dong X, Wu C, Li H, et al. Association of genetic polymorphisms with warfarin dose requirements in Chinese patients. Genet Test Mol Biomarkers. 2013;17:932–936. doi: 10.1089/gtmb.2013.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavallari LH, Langaee TY, Momary KM, Shapiro NL, Nutescu EA, Coty WA, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:459–464. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Jeong H, Takahashi H, Drozda K, Patel SR, Shapiro NL, et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther. 2012;91:660–665. doi: 10.1038/clpt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.