Abstract
Importance
Bacteriuria plus pyuria is highly prevalent among older women living in nursing homes. Cranberry capsules are an understudied, non-antimicrobial, prevention strategy used in this population.
Objective
To test the effect of two oral cranberry capsules once per day on presence of bacteriuria plus pyuria among women residing in nursing homes
Design, Setting, and Participants
This study was a double-blind, randomized, placebo-controlled efficacy trial with stratification by nursing home and surveillance of one year. 21 nursing homes with at least 90 beds and within 50 miles of New Haven, CT participated. 185 English-speaking, female, nursing home residents, age 65 or older, with or without bacteriuria and pyuria at baseline, were randomized. The study was conducted from 8/24/12-10/26/15.
Intervention
Two oral cranberry capsules, each capsule containing 36mg of the active ingredient proanthocyanidin (i.e., 72mg total, equivalent to 20 ounces of cranberry juice), versus placebo administered once per day in 92 treatment and 93 control group participants.
Main Outcomes and Measures
The primary outcome was the presence of bacteriuria (i.e., at least 105 cfu/mL of one or two microorganisms on urine culture) plus pyuria (i.e., any number of white blood cells on urinalysis) assessed every two months for a total of six assessments over the one year of surveillance; any positive finding was considered to meet the primary outcome. Secondary outcomes were symptomatic urinary tract infection (UTI), all-cause death, all-cause hospitalization, all multi-drug antibiotic resistant organisms, antibiotics administered for suspected UTI, and total antimicrobial administration.
Results
Among 185 women who were randomized (mean age 86.4 years [± 8.2], 90.3% white, 31.4% with bacteriuria plus pyuria at baseline), 147 completed the study. Overall adherence to capsule administration was 80.1%. Unadjusted results showed the presence of bacteriuria plus pyuria in 25.5% (95% CI 18.6, 33.9) of the treatment group and 29.5% (95% CI 22.2, 37.9) of the control group overall over 1 year. The adjusted GEE model that accounted for missing data and covariates showed no significant difference in the presence of bacteriuria plus pyuria between the treatment and control groups (29.1% vs. 29.0%; OR 1.01, 95% CI 0.61,1.66; p=0.984). There were no significant differences in number of symptomatic UTIs (10 vs. 12 episodes), rates of death (17 vs. 16, 20.4 vs. 19.1 deaths/100 person-years, Rate Ratio [RR] 1.07, 95% CI 0.54, 2.12), hospitalization (33 vs. 50 episodes, 39.7 vs. 59.6 hospitalizations/100 person-years, RR 0.67, 95% CI 0.32, 1.40), bacteriuria associated with multi-drug resistant gram-negative bacilli (9 vs. 24 episodes, 10.8 vs. 28.6 episodes/100 person-years, RR 0.38, 0.10, 1.46), antibiotics administered for suspected UTI (692 vs. 909 antibiotic days, 8.3 vs. 10.8 antibiotic days/person-year, RR 0.77, 95% CI 0.44, 1.33), or total antimicrobial utilization (1415 vs. 1883 antimicrobial days, 17.0 vs. 22.4 antimicrobial days/person-year, RR 0.76, 95% CI 0.46, 1.25).
Conclusions and Relevance
Among older women residing in nursing homes, administration of cranberry capsules, compared with placebo, resulted in no significant difference in presence of bacteriuria plus pyuria over 1 year.
INTRODUCTION
Urinary tract infection (UTI) is the most commonly diagnosed infection among nursing home residents. Distinguishing symptomatic UTI from asymptomatic bacteriuria is problematic among nursing home residents because of challenges with symptom assessment.1 Bacteriuria is prevalent in 25-50% of female nursing home residents and pyuria is present in 90% of those with bacteriuria.2 A randomized trial of antibiotic treatment versus no treatment of bacteriuria in nursing home women showed no decrease in genitourinary morbidity or mortality with treatment.3 This study along with others4 led to the recommendation that bacteriuria should not be treated with antibiotics in older institutionalized adults.3 Bacteriuria plus pyuria is a necessary but not sufficient condition to make the diagnosis of UTI in this population.5,6
Cranberry products represent a potential non-antimicrobial method for UTI prevention. Cranberry proanthocyanidins (PAC) have been shown to inhibit adherence of P-fimbriated Escherichia coli (E.coli) to uroepithelial cells,7 and this effect on virulence is dose dependent.8 Since E.coli represents the majority of urinary isolates among nursing home residents, cranberry products remain an appealing UTI prevention strategy, but evidence is conflicting.9,10 Most published reports have used cranberry juice, and postulated reasons for lack of benefit have included insufficient participant adherence to cranberry juice consumption and insufficient PAC in the tested cranberry product.9 Among older women (ie, mean age 78.5 years), 300 mL (i.e., approximately 10 ounces) of cranberry juice cocktail containing 36mg of PAC reduced bacteriuria plus pyuria over six months.11 However, the acrid flavor of cranberry juice is difficult to tolerate in large volumes,12 especially for nursing home residents because of swallowing disorders, exacerbation of incontinence, and impaired thirst.13
Prior studies showed that cranberry capsule administration and urine collection is feasible, and two cranberry capsules (each containing 36mg PAC [total 72mg PAC], equivalent to 20 ounces of cranberry juice) are an optimal dose to test among women living in nursing homes.14,15 The primary aim of this trial was to test the effect of two oral cranberry capsules once per day, compared to placebo, on the presence of bacteriuria plus pyuria among older women nursing home residents.
METHODS
Study Design and Oversight
This study was a double-blind, randomized, placebo-controlled efficacy trial of two cranberry capsules versus two placebo capsules once per day. The unit of randomization was each participant. Nursing homes targeted for participation had at least 90 beds, within a 50-mile radius of New Haven, CT, and UTI rates and sociodemographic characteristics similar to national averages. The Yale Human Investigation Committee approved the study; all nursing home administrators signed letters of participation and signed consent was obtained from participants or their surrogates.
Participants
Nursing homes were approached sequentially, and all residents in participating facilities were screened. Once enrollment was initiated at one nursing home, screening began at the next home. A HIPAA waiver (http://www.hhs.gov/hipaa/for-professionals/privacy/) was obtained for recruitment purposes. Inclusion criteria were: 1) female; 2) long-term care residents; 3) English speaking; and 4) 65 years or older. Only women were included in this study for the following reasons: 1) a prior study included women only;11 2) women are the majority of nursing home residents; 3) there is no evidence that cranberry products reduce bacteriuria plus pyuria in men; 4) the predominant risk factor for UTI in men is underlying structural or functional abnormalities of the urinary tract; 5) the prevalence of bacteriuria plus pyuria is lower in men. Exclusion criteria were: 1) not expected to be in the nursing home for at least one month (i.e., short term rehabilitation, pending discharge, terminal life expectancy < 1 month); 2) on chronic suppressive antibiotic or anti-infective (i.e., mandelamine) therapy for recurrent UTI; 3) on dialysis for end stage renal disease; 4) unable to produce a baseline clean catch urine specimen; 5) on warfarin therapy, because of a potential interaction with cranberry juice;16 6) history of nephrolithiasis, because cranberry products may increase the risk of nephrolithiasis;17 7) presence of an indwelling bladder catheter; 8) allergy to cranberry products; 9) treatment with cranberry products; 10) nursing home residence for < 4 weeks. Women with and without bacteriuria plus pyuria were eligible for the study. Nursing home staff identified whether a resident was able to provide self-consent or required surrogate consent. Assent was attempted for residents with surrogate consent. After written consent, a baseline urine specimen was obtained to ensure that subsequent clean catch urine collection was possible. Subsequent waves of recruitment occurred in each nursing home every three months.
Randomization and Intervention
Participants were randomly assigned to two cranberry capsules (manufactured by Pharmatoka, each containing 36mg of PAC, confirmed by BL-DMAC)18 or two placebo capsules, once per day, using a permuted block design with a variable block size (randomly set to 4 or 6) and equal allocation. Stratification by nursing home accounted for potentially different standards of care. The trial statistician designed the randomization scheme, the statistical programmer implemented it, and the Investigational Drug Services pharmacist made treatment assignments. Only the statistical programmer and pharmacist had access to the randomization codes during enrollment. Cranberry or placebo capsules were administered for 360 days (i.e., 12 30-day blister packs per participant) and total surveillance of each participant was 365 days.
Data and Outcomes
Research nurses recorded baseline descriptive characteristics from review of the medical chart including age, race, ethnicity, medications, comorbidities, and history of UTI. The primary nurse and/or certified nursing assistant (CNA) was asked questions adapted from the Minimum Data Set (MDS) regarding cognitive status, behavior, activities of daily living, continence, and degree of mobility, similar to previously conducted studies.15,19,20 At study initiation in each nursing home, the Senior Intervention Nurse Educator organized a series of “in-service” training sessions for nurses and CNAs regarding methods of urine specimen collection and capsule administration. Clean catch urine specimens were targeted for collection in containers with Boritex® (boric acid preservative tablet keeping urine stable for up to 96 hours) by nursing home staff between 5-7AM to optimize pharmacokinetic properties of PAC8 and feasibility of collection, then refrigerated once obtained. If the specimen was not collected on the due date, attempts at specimen collection continued for two additional weeks at any time of the day before classifying the specimen as missing. All specimens were transported in a cooler to Yale New Haven Health on the day obtained for processing at a single central laboratory.
Surveillance for the primary outcome (i.e., the presence of bacteriuria plus pyuria) occurred every two months after randomization for a total of six assessments over 12 months. Urine specimens were obtained by clean catch since catheterization of participants requiring surrogate consent was not authorized by the Institutional Review Board (IRB). Presence of bacteriuria was defined as at least 105 cfu/mL of one or two organisms. Absence of bacteriuria was defined as a urine culture with no growth, mixed flora (three or more organisms), or less than the highest quantitation of bacteriuria reported by the laboratory. Pyuria was defined as any number of white blood cells on urinalysis as in a previous study.11 Any positive specimen during the surveillance period was considered a positive primary outcome. Two members of the outcome adjudication committee, blinded to treatment assignment, reviewed the primary outcomes. Urinary tract specific symptoms were assessed at each bimonthly assessment.
Secondary outcomes included symptomatic UTI, all-cause death, all-cause hospitalization, all multi-drug antibiotic resistant organisms, antibiotics for suspected UTI, and total antimicrobial prescriptions. Symptomatic UTI, adapted from National Healthcare Safety Network (NHSN) criteria, was defined as 1) (a) acute dysuria; OR (b) fever or leukocytosis and at least one of the following: acute costovertebral angle pain or tenderness; suprapubic pain; gross hematuria; new or marked increase in incontinence, urgency, or frequency; OR (c) two or more of new or marked increase in: incontinence, urgency, frequency, suprapubic pain, new gross hematuria AND 2) a urine culture with (a) at least 105 cfu/mL of no more than two species of microorganisms in a voided sample OR (b) at least 102 cfu/mL of any number of organisms collected by in-and-out catheter (criteria 1 and 2 must be met).21 All antimicrobials administered during the trial were recorded via chart review. Multi-drug resistant organisms were defined as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), and multi-drug resistant gram negative bacilli (i.e., defined as resistance to at least 3 of the following antibiotics: ampicillin-sulbactam, cefazolin, ceftriaxone, ceftazidime, fluoroquinolones, piperacillin-tazobactam, meropenem, imipenem, and trimethoprim-sulfamethoxazole).22
Adherence was determined by the number of capsules administered to each participant relative to the prescribed number. Thirty-day blister packs were delivered each month; adherence was determined from the number of capsules remaining from the previous 30-day supply. Reasons for lack of adherence were obtained from review of the medication administration record. High adherence was defined as administration of ≥80% of prescribed capsules. All adverse events were recorded based on chart review and discussion with nursing staff on a monthly basis. A safety report was generated every six months and reviewed by an Independent Safety Monitor.
Statistical Analyses
All analyses were performed by intent-to-treat (i.e., participants were analyzed as randomized, regardless of adherence). A p-value of 0.05 (two-sided) was used for the level of statistical significance. SAS® 9.4 statistical software (SAS/STAT Version 14.1) was used for analyses.23 Sample size was determined to detect a difference between the proportion with bacteriuria plus pyuria over time in the treatment versus placebo group using the method of Diggle et al. for repeated binary outcomes.24 The following assumptions were made for this sample size calculation: Type 1 error of 5% (2-sided), 80% power, a serial correlation of 0.35 between six repeated participant outcomes, an overall bacteriuria plus pyuria rate of 0.45 in the control group,15 a 33% lower rate of bacteriuria plus pyuria in the treatment group (i.e., bacteriuria plus pyuria rate of 0.30),11 and 20% inflation for deaths, transfers and missing cultures. Based on these assumptions, the total sample size was 180 participants (90 per group).
The adjusted analysis of the primary outcome was conducted using generalized estimating equations (GEE) with inverse probability weighting (IPW) at the observation level for missing monotone values. The SAS PROC GEE procedure, which permits explicit modeling of the missing data mechanism as the means for determining inverse probability weights, was used instead of the initially planned generalized linear mixed model (GLMM). Missing intermittent values and two-month values for participants with no recorded outcomes were singly imputed using the fully conditional specification (FCR) method, a prerequisite for using the IPW GEE method.25 This regression modeling approach for handling missing values under the assumption of data missing at random (MAR) was chosen because of the unexpected amount and mechanism of the missing data, because it allowed for the explicit modeling of the missing data mechanism without adding covariates to the regression model that were not pre-specified and because it avoided making unreasonable assumptions regarding random effects needed for GLMM with binary outcomes. Pre-specified baseline variables for bacteriuria, incontinence, age at enrollment, and number of comorbidities were included in the model as covariates. A covariate for surveillance time was also added and assessed both for its linear association with the outcome and for its interaction with treatment. An unstructured correlation matrix was used to model the serial correlation of repeated participant outcomes. The adjusted percentage with bacteriuria plus pyuria for each treatment group at each surveillance time point was estimated by transforming the GEE model estimates using an inverse logit link function. The secondary outcomes were analyzed by a generalized linear regression model with a Poisson distribution, a treatment status explanatory variable, a natural logarithm of the time at risk as an offset, and adjustments for overdispersion (see Protocol and Supplemental Methods Appendix for additional details of all Methods). Since the primary proposed mechanism of action of cranberry products is targeted towards E.coli, exploratory analyses examined the percentage of E.coli urinary isolates versus others that met the criteria for bacteriuria in bi-monthly urines. Additionally, exploratory analyses of those participants without bacteriuria plus pyuria at baseline was conducted. No tests of significance were conducted for exploratory analyses.
RESULTS
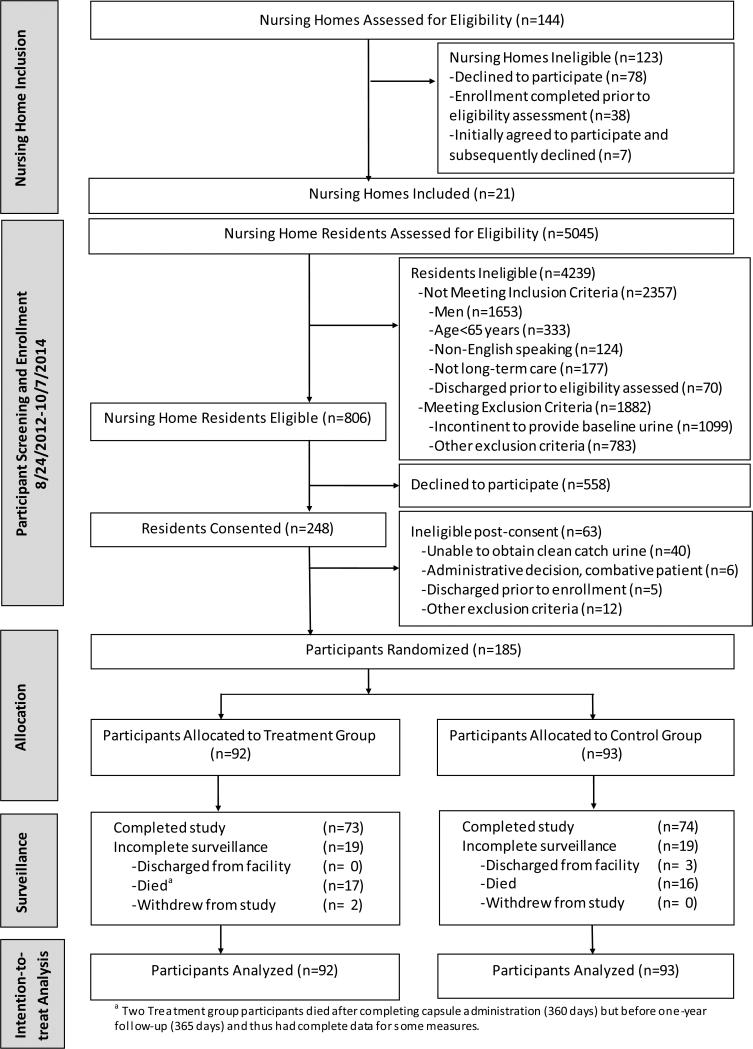
Figure 1 shows the flow of nursing homes and study participants. From August 24, 2012 through October 7, 2014, 5045 nursing home residents in 21 nursing homes were screened for participation, 806 (16.0% of those screened for eligibility) were eligible, 248 (30.8% of eligible) consented, and 185 (74.6% of consented) were able to provide a baseline clean catch urine specimen and be randomized. Surrogate consent had to be obtained in 93.5% of participants. Surveillance for all outcome and safety data ended on October 26, 2015. Table 1 displays the baseline demographic characteristics of participants. The mean age of participants was 86.4 years (± 8.2). The treatment and control groups were generally comparable. Rates of incontinence, bacteriuria, and number of episodes of UTI in the past year were similar; however, the control group had more coexisting conditions, specifically arrhythmia and hemiplegia.
Figure 1.
Flow Diagram of a Randomized Trial Comparing the Effect of Cranberry Capsules vs Placebo on Bacteriuria Plus Pyuria in Women Residents of Nursing Homes.
Table 1.
Baseline Characteristics, Overall and by Treatment Status
| Characteristics | Total (N=185) | Treatment (N=92) | Control (N=93) |
|---|---|---|---|
| Demographics | |||
| Age – yr | 86.4 ±8.2a | 87.1 ±8.4 | 85.6 ±8.0 |
| Hispanic ethnicity – no. (%) | 6 (3.2) | 3 (3.3) | 3 (3.22) |
| White race – no. (%) | 167 (90.3) | 83 (90.2) | 84 (90.3) |
| Coexisting Conditions | |||
| Hypertension – no. (%) | 152 (82.2) | 74 (80.4) | 78 (83.9) |
| Dementia – no. (%) | 145 (78.4) | 70 (76.1) | 75 (80.6) |
| Psychiatric disorder – no. (%) | 125 (67.6) | 57 (62.0) | 68 (73.1) |
| Connective tissue disease – no. (%) | 112 (60.5) | 56 (60.9) | 56 (60.2) |
| Other endocrine disorder – no. (%) | 87 (47.0) | 42 (45.7) | 45 (48.4) |
| Coronary artery disease – no. (%) | 60 (32.4) | 30 (32.6) | 30 (32.3) |
| Congestive heart failure – no. (%) | 57 (30.8) | 30 (32.6) | 27 (29.0) |
| Diabetes – no. (%) | 51 (27.6) | 25 (27.2) | 26 (28.0) |
| COPD – no. (%) | 41 (22.2) | 18 (19.6) | 23 (24.7) |
| Kidney disease – no. (%) | 40 (21.6) | 17 (18.5) | 23 (24.7) |
| Arrhythmia – no. (%) | 38 (20.5) | 12 (13.0) | 26 (28.0) |
| Cancer – no. (%) | 35 (18.9) | 16 (17.4) | 19 (20.4) |
| Peripheral vascular disease – no. (%) | 33 (17.8) | 15 (16.3) | 18 (19.4) |
| Stroke – no. (%) | 26 (14.1) | 14 (15.2) | 12 (12.9) |
| Peptic ulcer disease – no. (%) | 23 (12.4) | 13 (14.1) | 10 (10.8) |
| Hemiplegia – no. (%) | 9 (4.9) | 1 (1.1) | 8 (8.6) |
| Liver disease – no. (%) | 4 (2.2) | 2 (2.2) | 2 (2.2) |
| Number of coexisting conditions – median (IQR)b | 5 (4, 7) | 5 (4, 7) | 6 (5, 7) |
| Incontinence Status | |||
| Bladder incontinence – no. (%) | 126 (68.1) | 57 (62.0) | 69 (74.2) |
| Bowel incontinence – no (%) | 82 (44.3) | 38 (41.3) | 44 (47.3) |
| History of Infection | |||
| Bacteriuria plus pyuria – no (%) | 58 (31.4)c | 26 (28.3)c | 32 (34.4) |
| Number of episodes of UTI in the past year– no. (%) | |||
| 0 | 128 (69.2) | 68 (73.9) | 60 (64.5) |
| 1 | 40 (21.6) | 19 (20.7) | 21 (22.6) |
| 2 | 12 (6.5) | 4 (4.3) | 8 (8.6) |
| 3 or more | 5(2.7) | 1 (1.1) | 4 (4.3) |
| Number of courses of antibiotics in the past year – no. (%) | |||
| 0 | 78 (42.2) | 43 (46.7) | 35 (37.6) |
| 1 | 53 (28.6) | 25 (27.2) | 28 (30.1) |
| 2 | 26 (14.1) | 13 (14.1) | 13 (14.0) |
| 3 or more | 28 (15.1) | 11 (12.0) | 17 (18.3) |
| Functional and Behavioral Status | |||
| Number of ADL disabilities in the past seven days – no. (%)d | |||
| 0 | 126 (68.1) | 65 (70.7) | 61 (65.6) |
| 1 - 4 | 48 (25.9) | 21 (22.8) | 27 (29.0) |
| 5 - 8 | 11 (6.0) | 6 (6.5) | 5 (5.4) |
| Resists care– no. (%) | 16 (8.6) | 9 (9.8) | 7 (7.5) |
| Number of prescribed medications – median (IQR) | 10 (7, 13) | 10 (7, 13) | 10 (8,13) |
| Cognitive and Mental Status | |||
| Non-alert – no. (%) | 7 (3.8) | 4 (4.3) | 3 (3.2) |
| Delirium, Periods of altered perception – no. (%)e | 35 (18.9) | 18 (19.6) | 17 (18.3) |
| Delirium, Periods of lethargy – no. (%)f | 14 (7.6) | 5 (5.4) | 9 (9.7) |
| Delirium, Episodes of disorganized speech – no (%)g | 11 (5.9) | 8 (8.7) | 3 (3.2) |
Abbreviations: COPD Chronic Obstructive Pulmonary Disease; IQR InterQuartile Range; UTI Urinary Tract Infection; ADL Activities of Daily Living;
Plus-minus values are means ± standard deviations.
The coexisting conditions variable was defined as the number of coexisting conditions among the 17 listed in this table.
There was 1 participant with bacteriuria alone at baseline (no pyuria). This one additional participant with bacteriuria was in the treatment group (i.e., 27 participants total).
The ADL disabilities variable was defined as total dependence in the number of 8 ADLs (i.e., bed mobility, transfer, dressing, eating, toilet use, personal hygiene, bathing, and walking in room).
The study participant moves lips or talks to someone not present, believes he/she is somewhere else, or confuses day and night.
The study participant shows sluggishness, staring into space, difficulty to arouse, or little body movement.
The study participant's speech is incoherent, nonsensical, irrelevant, or rambling, or he/she loses a train of thought.
One hundred forty-seven participants completed one year of surveillance, and 33 participants died. Twenty participants, 9 in the treatment group and 11 in the control group, became incontinent prior to the first outcome assessment and were unable to provide any of the scheduled urine specimens. Over the course of the study, 45 participants stopped taking the capsules, 24 in the treatment and 21 in the control groups, for the following reasons: patient refusal (N=21), transitioned to hospice care (N=19), started on warfarin which was an early termination event (N=4), and family refusal (N=1). Overall adherence to capsule administration was 80.1%, 77.5% in the treatment group and 82.6% in the control group. Adherence was 83.7% (N=185) in the first six months and 76.7% (N=168) in the second six months.
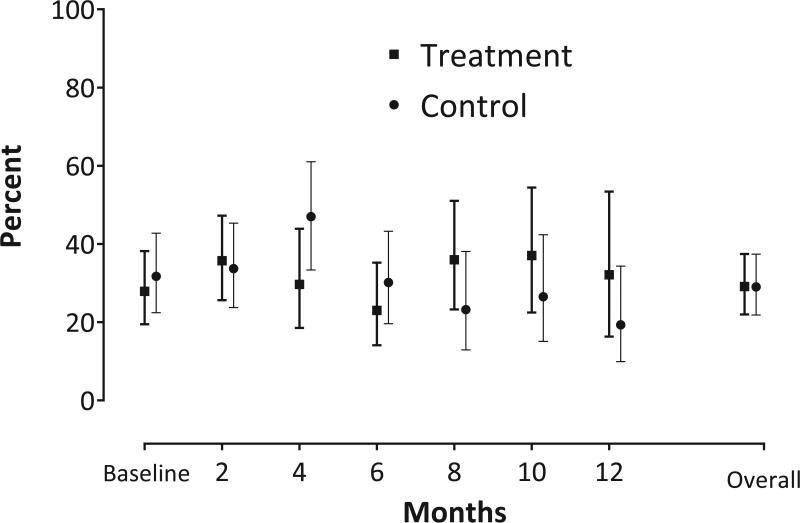
Table 2 depicts the percentage of urine specimens that met the primary outcome of bacteriuria plus pyuria over 12 months of surveillance. The overall unadjusted results showed rates of 25.5% [95% CI 18.6, 33.9] vs 29.5% [95% CI 22.2, 37.9]) in treatment versus control groups, respectively. The adjusted analysis, accounting for missing data and pre-specified covariates, showed no significant difference between the treatment and control groups (29.1% vs. 29.0%; OR 1.01, 95% CI 0.61,1.66; p=0.984, see Figure 2). Of the 723 urinary specimens obtained, 9 participants (7 with one symptom [3 in treatment group, 4 in control group] and 2 with two symptoms [1 in treatment group, 1 in control group]) had urinary tract specific symptoms at a bimonthly assessment.
Table 2.
Bi-Monthly and Overall Observed Counts and Percentages of Bacteriuria Plus Pyuria Specimens by Treatment Status (N=185)
| Treatment | Control | |||||
|---|---|---|---|---|---|---|
| Count/Total | %a | 95% CIc | Count/Total | %a | 95% CIc | |
| Baseline | 26/92 | 28.3 | 20.0, 38.3 | 32/93 | 34.4 | 25.5, 44.6 |
| 2 Months | 21/76 | 27.6 | 18.8, 38.7 | 27/78 | 34.6 | 24.9, 45.8 |
| 4 Months | 15/63 | 23.8 | 14.9, 35.8 | 26/65 | 40.0 | 28.9, 52.3 |
| 6 Months | 13/64 | 20.3 | 12.2, 31.9 | 20/67 | 29.9 | 20.1, 41.8 |
| 8 Months | 14/55 | 25.5 | 15.7, 38.5 | 14/60 | 23.3 | 14.3, 35.6 |
| 10 Months | 15/51 | 29.4 | 18.6, 43.2 | 13/54 | 24.1 | 14.5, 37.2 |
| 12 Months | 12/44 | 27.3 | 16.2, 42.1 | 9/46 | 19.6 | 10.5, 33.5 |
| Ove rail | 90/353b | 25.5b | 18.6, 33.9 | 109/370b | 29.5b | 22.2, 37.9 |
Abbreviations: CI Confidence Interval
These percentages represent the number of study participants at each time point (baseline or follow up time) having a specimen with bacteriuria plus pyuria divided by the number of study participants enrolled and followed at the specified time point multiplied by 100.
This percentage represents the sum of the number of specimens with bacteriuria plus pyuria occurring during the six follow-up periods (months 2-12) divided by the sum of the number of study participant assessments during the six follow-up time points (i.e., the sum of the Total counts) multiplied by 100. 1110 (=185*6) observations were scheduled to be collected across six follow-up time points of which 723 were actually obtained, 353 in the treatment group and 370 in the control group.
Confidence intervals for binomial proportions were estimated using the logit method. For further details, consult the Statistical Methods Appendix.
Figure 2.
Bi-monthly and Overall Adjusted Percentages of Bacteriuria Plus Pyuria Specimens by Treatment Status (N=185)a
a Percentage with bacteriuria plus pyuria and corresponding 95% confidence intervals were adjusted for the following pre-specified baseline variables: bacteriuria, incontinence, age at enrollment, and number of comorbidities (see Supplemental Methods Appendix for additional details). The baseline and bi-monthly data represent study participants with bacteriuria plus pyuria while the overall data represent all specimens of bacteriuria plus pyuria over the six follow-up time points (months 2-12). See Total number of participants contributing data at each time point in Table 2.
Of 350 episodes of clinically suspected UTI in 131 participants, there were 10 symptomatic UTIs in the treatment group (8 participants with 1 episode and 1 participant with 2 episodes) and 12 symptomatic UTIs in the control group (7 participants with 1 episode, 1 participant with 2 episodes, and 1 participant with 3 episodes). Although blood cultures were requested in 35 episodes of clinically suspected UTI, there was only one episode of septicemia, which occurred in the control group. Table 3 shows that there were no significant differences in rates of death, hospitalization, multi-drug resistant gram-negative bacilli bacteriuria (from scheduled and suspected UTI urine cultures), antibiotics administered for suspected UTI, or total antimicrobial utilization. There was one MRSA urinary isolate identified in the treatment group and no VRE urinary isolates identified. There were a total of 3830 adverse events (see eTable 1), 116 serious (83 hospitalizations and 33 deaths, all protocol-unrelated and anticipated) and 3714 non-serious (14 protocol-related and anticipated). The frequency of the 14 protocol-related and anticipated non-serious adverse events (i.e., altered mental status, gastrointestinal disturbance, oral cavity disturbance, skin and soft tissue event, weight loss) was similar in the two treatment groups.
Table 3.
Unadjusted Rates and Rate Ratios for Secondary Outcomesa
| Secondary Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mortalityb | Hospitalizationb | MDR GNB Bacteriuriab,c | Antibiotics for Suspected UTId | Total antimicrobialsd | ||||||
| Treatment Status | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control |
| Count | 17 | 16 | 33 | 50 | 9 | 24 | 692 | 909 | 1415 | 1883 |
| Person years | 83.2 | 84.0 | 83.2 | 84.0 | 83.2 | 84.0 | 83.2 | 84.0 | 83.2 | 84.0 |
| Rate 95% CI |
20.4 12.7, 32.9 |
19.1 11.7, 31.1 |
39.7 22.3, 70.4 |
59.6 37.4,95.0 |
10.8 3.4, 34.2 |
28.6 14.1,57.8 |
8.3 5.5, 12.6 |
10.8 7.6, 15.5 |
17.0 11.7, 24.8 |
22.4 16.2,31.1 |
| Rate Ratio 95% CI |
1.07 0.54, 2.12 |
0.67 0.32, 1.40 |
0.38 0.10, 1.46 |
0.77 0.44, 1.33 |
0.76 0.46, 1.25 |
|||||
| P-value | 0.84 | 0.28 | 0.16 | 0.34 | 0.28 | |||||
Abbreviations: CI Confidence Interval; MDR GNB Multi-Drug Resistant Gram Negative Bacilli; UTI Urinary Tract Infection.
Estimates and confidence intervals are from generalized linear regression models with Poisson distributions, offsets for the natural logarithm for the time at risk, and adjustments for overdispersion. Mortality counts are for individuals; counts for other outcomes are for episodes (i.e., counts of the number of hospitalizations, isolates of MDR GNB bacteriuria, uses of antibiotics for suspected UTI, and uses of total antimicrobials).
Rates and CIs are in counts/100 person-years.
Multi-drug resistant Gram negative bacilli are defined as urinary isolates (E. coli, Klebsiella, Proteus, Providencia, Pseudomonas, Citrobacter, or Enterobacter spp.) having resistance to three or more of the following antibiotics (ampicillin-sulbactam, cefazolin, ceftriaxone, ceftazidime, fluoroquinolones, piperacillin-tazobactam, meropenem, imipenem, or trimethoprim-sulfamethoxazole).
Rates and CIs are in antimicrobial days/person-year. Multiple antimicrobials on a given day are counted as one antimicrobial day.
Antimicrobial/classes include cephalosporins, fluoroquinolones, penicillins, oseltamivir, sulfonamides, macrolides, nitrofurantoin, vancomycin, aminoglycosides, carbapenems, metronidazole, tetracycline, fluconazole, mycostatin, daptomycin, and antivirals: acyclovir/valacyclovir.
Exploratory analyses of the percentage of E.coli isolates showed that in the treatment group, the percentage of E.coli at 0, 2, 4, 6, 8, 10, and 12 months was 19.6%, 25.0%, 20.6%, 15.6%, 20.0%, 17.7%, and 22.7%. In the control group, the percentage of E.coli at 0, 2, 4, 6, 8, 10, and 12 months was 21.5%, 23.1%, 24.6%, 22.4%, 18.3%, 18.5%, and 17.4%. Exploratory analyses in the subset of 127 women (n =66 in the treatment group, n= 61 in the control group) who did not have bacteriuria plus pyuria at baseline showed that the overall rate of bacteriuria plus pyuria over 1 year was 15% (n=39) in the treatment group and 11.4% (n=28) in the control group (see eTable 2).
DISCUSSION
Despite prior studies demonstrating that cranberry juice reduced bacteriuria plus pyuria in older women and that two cranberry capsules with 72mg of PAC (equivalent to 20 ounces of cranberry juice) was an appropriate dose to test,11,15,26 the findings from this trial demonstrated no significant difference in presence of bacteriuria plus pyuria among women who received cranberry capsules vs placebo over 1 year. The lack of statistically significant differences in any of the secondary outcomes is consistent with this finding. Many studies of cranberry products have been conducted over several decades with conflicting evidence of its utility for UTI prevention. The results have led to the recommendation that cranberry products do not prevent UTI overall, but may be effective in older women.9,27 This trial did not show a benefit of cranberry capsules in terms of a lower presence of bacteriuria plus pyuria among older women living in nursing homes.
Some studies evaluated cranberry products solely among older adults. In a recent Cochrane review,9 two reports showed a reduction in bacteriuria plus pyuria,11,26 whereas two others did not show a clinical benefit of cranberry products.14,16 In one study of older women in nursing homes and assisted living facilities, 300mL of cranberry juice cocktail containing 36mg of PAC showed a benefit but the placebo group had a higher rate of prior history of UTI and likely higher risk for bacteriuria plus pyuria.28 A more recent study of one cranberry capsule twice per day (18mg PAC total) in long-term care facility residents with high risk for UTI (i.e., need for long-term catheterization, diabetes mellitus, or at least one UTI in the preceding year) showed that participants receiving cranberry capsules had a lower incidence of clinically defined UTI (i.e., one of the following: specific and nonspecific micturition-related symptoms and signs; a positive nitrite, leukocyte esterase, dipslide, or culture; antibiotic treatment for UTI; or UTI reported in the medical record). The UTI definition used was very broad, and there was no difference between the treatment and control groups using a strict UTI definition, or for either definition in the low risk UTI group. Hence, cranberry capsules have not shown meaningful clinical benefit and have not been cost effective.29,30
There are several potential explanations for why cranberry capsules, compared to placebo, did not result in a difference in the presence of bacteriuria plus pyuria in this trial. First, there appeared to be an initial effect on bacteriuria plus pyuria in the first six months, but these rates returned to baseline in the second six months of study. Slightly lower adherence in the second six months could have contributed to this finding. Additionally, it is possible that because of worsening incontinence and changes to the vaginal microbiome with age, the effects of cranberry capsules were not sustained. Although the exploratory analyses of those without bacteriuria plus pyuria at baseline is limited because the benefit of randomization is lost, it did not support that cranberry capsules prevented bacteriuria plus pyuria over the surveillance period. Second, cranberry capsules do not provide the hydration of cranberry juice. A recent study among women undergoing elective gynecological surgery with urinary catheter removal showed that two cranberry capsules twice per day (equivalent to 16 ounces of cranberry juice) over six weeks were able to reduce the rate of UTI by half.31 However, all participants were instructed to drink 8 ounces of water twice a day with each capsule administration so it is possible that the fluid load was necessary along with cranberry product. Hydration may also be a necessary component to reduce bacteriuria and urinary symptoms in older women.5 Third, although adherence was high as measured by capsules removed from the blister pack, participants might not have actually ingested the capsules.
This trial had several strengths. Nursing home residents exclusively were enrolled, the dose of PAC was standardized, adherence to capsule administration by the planned assessment method was high, and nursing home staff were trained to optimize outcome assessments. However, there were limitations. First, since participants could not be catheterized to obtain bi-monthly urine specimens, only residents capable of providing a clean catch urine specimen were randomized. Exclusion of residents for complete incontinence limited the generalizability of these findings. For randomized participants, 20 became incontinent prior to the first outcome assessment and were unable to provide any urine samples. Others became incontinent or were transitioned to hospice care, so urine samples were not obtained. Nevertheless, 65% of planned urine specimens were collected. Second, 78 nursing homes either did not respond or declined to participate and 7 that agreed to participate but subsequently declined. Third, anti-adhesion of E.coli to uroepithelial cells in the urines of participants in the trial was not tested. Adhesion studies to date have been conducted on patients enrolled for relatively short observation periods.7,8 Since there were multiple assessment time points and it was not possible to ensure adherence to capsule ingestion on the day prior to obtaining a urine specimen for adhesion testing, thus anti-adhesion testing was not feasible. Fourth, the baseline rate of bacteriuria plus pyuria and percentage of E.coli bacteriuria in this trial population was lower than in the pilot dosing study.15 Fifth, this study enrolled women with or without bacteriuria plus pyuria at baseline. Therefore, it was not possible to definitively determine the specific role of cranberry capsules for prevention of new occurrence of bacteriuria plus pyuria among women without bacteriuria plus pyuria at baseline, nor for reduction of bacteriuria plus pyuria among women with prevalent bacteriuria plus pyuria at baseline.
CONCLUSIONS
Among older women residing in nursing homes, administration of cranberry capsules, compared with placebo, resulted in no significant difference in presence of bacteriuria plus pyuria over 1 year.
Supplementary Material
KEY POINTS.
Question: Do cranberry capsules with sufficient proanthocyanadin content affect the presence of bacteriuria (i.e., at least 105 cfu/mL of one or two organisms on urine culture) plus pyuria (i.e., any number of white blood cells on urinalysis) in older women living in nursing homes?
Findings: In this randomized clinical trial of 185 women nursing home residents, after adjusting for missing data and covariates, there was no statistically significant difference in presence of bacteriuria plus pyuria between the treatment (29.1%) and control (29.0%) groups over 1 year.
Meaning: Among older women living in nursing homes, cranberry capsules, compared with placebo, did not have a significant effect on the presence of bacteriuria plus pyuria over 1 year.
ACKNOWLEDGEMENTS
All authors would like to thank Leo M. Cooney, Jr, MD, Humana Foundation Professor of Medicine (Geriatrics), Yale School of Medicine, for serving as the Independent Safety Monitor for this study. Dr. Cooney did not receive compensation for serving in this role.
Funding: This study was supported by the National Institutes of Health, National Institute on Aging, R01 AG041153, as well as K07 AG030093 and the Claude D. Pepper Older Americans Independence Center P30 AG021342. Cranberry and placebo capsules used in this study were manufactured and donated by an independent manufacturer.
Role of Funder: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The cranberry and placebo capsule manufacturer had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author contributions: Dr. Juthani-Mehta and Dr. Van Ness had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Juthani-Mehta, Van Ness, Quagliarello, Peduzzi. Data management: Argraves, Trentalange, Charpentier. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Juthani-Mehta, Van Ness, Quagliarello, Peduzzi. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Trentalange, Van Ness, Peduzzi. Obtaining funding: Juthani-Mehta. Administrative, technical, or material support: Bianco, Acampora. Study supervision: Bianco, Acampora, Juthani-Mehta.
Conflicts of Interest Disclosures: No authors have any potential conflicts of interest.
Trial Registration: ClinicalTrials.gov Identifier NCT01691430
REFERENCES
- 1.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juthani-Mehta M, Datunashvili A, Tinetti M. Tests for urinary tract infection in nursing home residents. JAMA. 2014;312(16):1687–1688. doi: 10.1001/jama.2014.13554. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med. 1987;83(1):27–33. doi: 10.1016/0002-9343(87)90493-1. [DOI] [PubMed] [Google Scholar]
- 4.Abrutyn E, Mossey J, Berlin JA, et al. Does asymptomatic bacteriuria predict mortality and does antimicrobial treatment reduce mortality in elderly ambulatory women? Ann Intern Med. 1994;120(10):827–833. doi: 10.7326/0003-4819-120-10-199405150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mody L, Juthani-Mehta M. Urinary Tract Infections in Older Women: A Clinical Review. JAMA. 2014;311(8):844–854. doi: 10.1001/jama.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 7.Howell AB, Vorsa N, Der Marderosian A, Foo LY. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339(15):1085–1086. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- 8.Lavigne JP, Bourg G, Combescure C, Botto H, Sotto A. In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008;14(4):350–355. doi: 10.1111/j.1469-0691.2007.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988–996. doi: 10.1001/archinternmed.2012.3004. [DOI] [PubMed] [Google Scholar]
- 11.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 12.Wing DA, Rumney PJ, Preslicka CW, Chung JH. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol. 2008;180(4):1367–1372. doi: 10.1016/j.juro.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wotton K, Crannitch K, Munt R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp Nurse. 2008;31(1):44–56. doi: 10.5172/conu.673.31.1.44. [DOI] [PubMed] [Google Scholar]
- 14.Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028–2030. doi: 10.1111/j.1532-5415.2010.03080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180–1181. doi: 10.1111/j.1532-5415.2012.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurdo ME, Bissett LY, Price RJ, Phillips G, Crombie IK. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34(3):256–261. doi: 10.1093/ageing/afi101. [DOI] [PubMed] [Google Scholar]
- 17.Terris MK, Issa MM, Tacker JR. Dietary supplementation with cranberry concentrate tablets may increase the risk of nephrolithiasis. Urology. 2001;57(1):26–29. doi: 10.1016/s0090-4295(00)00884-0. [DOI] [PubMed] [Google Scholar]
- 18.Prior RL, Fan E, Ji H, et al. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90(9):1473–1478. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- 19.Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc. 2009;57(6):963–970. doi: 10.1111/j.1532-5415.2009.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juthani-Mehta M, Van Ness PH, McGloin J, et al. A cluster-randomized controlled trial of a multicomponent intervention protocol for pneumonia prevention among nursing home elders. Clin Infect Dis. 2015;60(6):849–857. doi: 10.1093/cid/ciu935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965–977. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pop-Vicas A, Tacconelli E, Gravenstein S, Lu B, D'Agata EM. Influx of multidrug-resistant, gram-negative bacteria in the hospital setting and the role of elderly patients with bacterial bloodstream infection. Infect Control Hosp Epidemiol. 2009;30(4):325–331. doi: 10.1086/596608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SAS/STAT User's Guide, Version 14.1. SAS Institute Inc.; Cary, NC: 2015. [Google Scholar]
- 24.Diggle P, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; New York: 2002. [Google Scholar]
- 25.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 26.Haverkorn MJ, Mandigers J. Reduction of bacteriuria and pyuria using cranberry juice. JAMA. 1994;272(8):590. doi: 10.1001/jama.272.8.590a. [DOI] [PubMed] [Google Scholar]
- 27.Jepson R, Craig J, Williams G. Cranberry products and prevention of urinary tract infections. JAMA. 2013;310(13):1395–1396. doi: 10.1001/jama.2013.277509. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins WJ, Heisey DM, Jonler M, Uehling DT. Reduction of bacteriuria and pyuria using cranberry juice. JAMA. 1994;272(8):588–589. author reply 589-590. [PubMed] [Google Scholar]
- 29.Caljouw MA, van den Hout WB, Putter H, Achterberg WP, Cools HJ, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons: a double - blind randomized placebo-controlled trial in long-term care facilities. J Am Geriatr Soc. 2014;62(1):103–110. doi: 10.1111/jgs.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hout WB, Caljouw MA, Putter H, Cools HJ, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111–116. doi: 10.1111/jgs.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foxman B, Cronenwett AE, Spino C, Berger MB, Morgan DM. Cranberry juice capsules and urinary tract infection after surgery: results of a randomized trial. Am J Obstet Gynecol. 2015;213(2):194, e191–198. doi: 10.1016/j.ajog.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.