Abstract
Background
Data regarding long-term mortality and factors influencing appropriate therapies in Japanese patients with implantable cardioverter defibrillators (ICD), who satisfy the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) criteria for primary prevention, remain scarce.
Methods
A total of 118 consecutive patients who underwent ICD implantation without any prior ventricular arrhythmic event, from January 2000 to December 2012, were enrolled based on the MADIT II criteria: left ventricular ejection fraction (LVEF) of ≤30% with ischemic heart disease and at least 4 weeks after a myocardial infarction. We investigated the mortality and factors influencing appropriate ICD therapies in this population.
Results
The mean age was 69±10 years, and the mean LVEF was 25.1±4.5%. During the median follow up of 1406 days, the mortality rate was 20%, and the incidence of appropriate ICD therapy was 37% at 3 years. Multivariate analysis by using Cox regression model showed that left ventricular diastolic diameter ≥60 mm (hazard ratio [HR], 2.31; 95% confidence interval [CI], 1.07–5.38; P=0.033) and the presence of non-sustained ventricular tachycardia (NSVT) before implantation (HR, 2.26; 95% CI, 1.17-4.39; P=0.015) were independent predictors of appropriate ICD therapy.
Conclusions
The mortality and incidence of appropriate ICD therapy were 20% and 37%, respectively, at 3 years in Japanese patients who met the MADIT II criteria during ICD implantation for primary prevention of sudden cardiac death. The presence of NSVT and dilated left ventricle independently predicted the incidence of appropriate ICD therapy after implantation.
Keywords: Implantable cardioverter defibrillator, Primary prevention, Ventricular tachyarrhythmia
1. Introduction
The second Multicenter Automatic Defibrillator Implantation Trial (MADIT II) demonstrated in a recent report that an implantable cardioverter defibrillator (ICD) for primary prevention of sudden cardiac death (SCD) reduces mortality in patients with a history of myocardial infarction (MI) and left ventricular ejection fraction (LVEF) of ≤30% [1], [2] during an extended 8-year follow-up period [3]. However, the significant risks and high cost of ICD therapy have led some to question what kind of patients with low LVEF after MI should receive ICD implantation for the primary prevention of SCD without prior ventricular arrhythmic event. Improved risk stratification may identify patients whose ventricular arrhythmic event risk is too low to benefit from ICD implantation. In addition, some reports demonstrated that Asian populations have a lower rate of SCD compared with Caucasians [4]. Therefore, the interest still remains regarding what proportion of Japanese patients with MADIT II-like criteria will experience ventricular arrhythmic events and what clinical factors may predict these events during long-term follow up. The purpose of this study was to investigate mortality, incidence of appropriate ICD therapy administration, and factors influencing ICD therapy in Japanese patients with ICDs for primary prevention who fulfilled the MADIT II criteria.
2. Materials and methods
2.1. Study population
From January 2000 to December 2012, 436 consecutive patients without prior ventricular arrhythmic event underwent ICD implantation for primary prevention of SCD in Kokura Memorial Hospital based on the clinical guidelines [5], [6]. Among these patients, we enrolled 118 patients who satisfied the MADIT II criteria for prophylactic ICD implantation as follows: left ventricular ejection fraction (LVEF) of ≤30% with ischemic heart disease and at least 4 weeks after MI.
Patients were also excluded from enrollment if they belong to New York Heart Association (NYHA) functional class IV, had undergone coronary revascularization within the past 3 months, were less than 21 years old, had advanced cerebrovascular disease, as well as in the original MADIT II. Details of the design, methods, and results of the MADIT II have been reported previously [1]. The present study was performed as a single-center retrospective analysis of a prospectively maintained database. All data were collected to evaluate mortality rate, incidence of the appropriate ICD therapies, and factors influencing baseline clinical characteristics on appropriate ICD therapies in accordance with institutional ethics guidelines. The study was approved by the ethical committee of Kokura Memorial Hospital.
2.2. Definitions
The presence of ischemic heart disease was determined based on MI history perceived from clinical manifestations, electrocardiogram (ECG) findings, and echocardiography and coronary angiography results. Non-sustained ventricular tachycardia (NSVT) was defined as the observation of at least three ventricular premature beats but spontaneously terminated within 30 s in Holter monitoring, 12-lead ECG, implantable loop recorders, or the recording of pacemaker. All patients who were referred to our center underwent at least one 24-h Holter-monitoring session before being assessed for ICD implantation.
Data on deaths within the follow-up period were retrieved from the medical records and discharge summaries from our hospital and other institutions, and these were classified based on the modified Hinkle–Thaler scheme used in the MADIT II [2]. The modified Hinkle–Thaler death categories included sudden cardiac, non-sudden cardiac, unclassified cardiac, non-cardiac, and unknown/unclassified causes of death when insufficient information was available to make a reasonable decision as to the cause of death. If patients were unable to visit our hospital because of the long distance, medical interviews were conducted by calling or sending mail to the patients or doctors in the local hospital who are responsible for them to obtain clinical information regarding mortality and incidence of appropriate ICD therapies.
2.3. ICD therapy analysis
Patients in our study population underwent an ICD interview every 3 to 6 months as well as interim visits as their symptoms presented. We classified ICD therapy occurring for VT or VF as appropriate. Appropriateness of therapy was determined by independent review of device-stored electrograms (EGMs) by two independent expert reviewers. VT was differentiated from supraventricular tachycardia by using standard criteria, including a change in EGM morphology, sudden onset, and the atrioventricular relationship, when atrial EGMs were available.
The VF zone was typically set to >200 bpm, with at least one tracing of anti-tachycardia pacing (ATP) before shock, whereas the VT zone was typically set to >150 bpm, with at least three tracings of ATP before shock. Programming was changed for patients who underwent therapy and customized to their specific circumstances at the discretion of the each physician. Devices were not programmed based on the cycle length or presence of NSVT before the implantation.
2.4. Statistical analysis
Continuous variables were presented as mean±SD, and categorical data were summarized as frequencies and percentages. Differences in baseline characteristics between the patients with and without appropriate ICD therapy were analyzed using the independent sample t- or Fisher׳s exact test. Several baseline characteristics were divided into two groups by using clinically useful cutoff points, such as LVEF ≤25% and left ventricular diastolic diameter (LVDd)≥60 mm, which corresponded to the approximate median value.
Univariate and multivariate analyses using Cox proportional hazards model were performed to assess the relationship between baseline characteristics and incidence of appropriate ICD therapy administration. Multivariate analyses were performed by using all variables with P≤0.10 (based on the univariate analysis). Calculations were performed by using JMP 12.0.1 software (SAS Institute Inc., Cary, NC). A P-value of <0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Baseline clinical characteristics are summarized in Table 1. The mean age was 69±10 years, and 84% were male. All patients underwent coronary angiography during their therapeutic course. A total of 114 patients (97%) had a medical history of undergoing coronary revascularization therapies: percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery. The study population was divided into two groups based on the occurrence of appropriate ICD therapy administration: those who received appropriate ICD therapy (40 patients) and those who did not (78 patients). Clinical characteristics were compared between the two groups. Implantation of biventricular pacing was less frequent in patients who received appropriate ICD therapy than in those who did not (38% vs. 60%, P=0.02). The presence of NSVT (55% vs. 33%, P=0.02) and the prevalence of dilated left ventricle (LVDd≥60 mm) (73% vs. 54%, P=0.04) before implantation were more frequent in patients who received appropriate ICD therapy than in those who did not. The prevalence of severely impaired left ventricular systolic function (LVEF≤25%) (57% vs 41%, P=0.09) tended to be more frequent in patients who received appropriate ICD therapy. The proportion of patients with diabetes tended to be lower among patients who received appropriate ICD therapy (32% vs. 49%, P=0.09). The QRS width before ICD implantation, including biventricular pacing was comparable between the two groups (148±36 ms vs. 143±31 ms). Distribution of other clinical characteristics was similar between the two groups.
Table 1.
Comparison of clinical characteristics between patients with appropriate ICD therapy and those without.
| Overall population (n=118) | With appropriate ICD therapy (n=40) | Without appropriate ICD therapy (n=78) | P-value | |
|---|---|---|---|---|
| Age, year | 69±10 | 68±11 | 70±10 | 0.33 |
| Male, (%) | 99 (84) | 36 (90) | 67 (82) | 0.20 |
| QRS width, ms | 145±33 | 148±36 | 144±32 | 0.58 |
| LVEF, % | 25.1±4.5 | 24.8±4.5 | 25.3±4.5 | 0.57 |
| LVEF≤25%, (%) | 55 (47) | 23 (57) | 32 (41) | 0.09 |
| LVDd, mm | 61.4±5.7 | 62.2±5.4 | 60.9±5.8 | 0.22 |
| LVDd≥60 mm, (%) | 71 (60) | 29 (73) | 42 (54) | 0.04 |
| NYHA class, (%) | 0.54 | |||
| I | 31 (26) | 13 (33) | 18 (23) | |
| II | 46 (39) | 14 (35) | 32 (41) | |
| III | 41 (35) | 13 (33) | 28 (36) | |
| Hypertension, (%) | 60 (51) | 19 (48) | 41 (53) | 0.60 |
| Diabetes, (%) | 51 (43) | 13 (32) | 38 (49) | 0.09 |
| Atrial fibrillation, (%) | 8 (7) | 1 (3) | 7 (9) | 0.18 |
| Medication, (%) | ||||
| Amiodarone | 19 (16) | 6 (15) | 13 (17) | 0.81 |
| ACE inhibitor/ARB | 86 (73) | 31 (78) | 55 (71) | 0.41 |
| Beta blocker | 88 (75) | 31 (78) | 57 (73) | 0.60 |
| Statin | 72 (61) | 23 (58) | 49 (63) | 0.57 |
| Digitalis | 51 (43) | 20 (50) | 31 (39) | 0.29 |
| Diuretics | 79 (67) | 30 (75) | 49 (63) | 0.18 |
| Prior CABG, (%) | 46 (39) | 18 (45) | 28 (36) | 0.34 |
| Prior PCI, (%) | 97 (82) | 31 (78) | 66 (85) | 0.34 |
| Biventricular Pacing, (%) | 62 (52) | 15 (38) | 47 (60) | 0.02 |
| Non-sustained VT, (%) | 48 (41) | 22 (55) | 26 (33) | 0.02 |
Data are presented as the mean±standard deviation or n (%).
ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; ICD, implantable cardioverter defibrillator; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; VT, ventricular tachycardia.
3.2. Mortality and adverse events
During the median follow-up period of 1409 days, 50 patients (44%) died, wherein 33 (28%) died because of cardiovascular causes, including 7 (6%) due to sudden cardiac death (Table 2). The mortality of the present study population was 5%, 15%, and 20% at 1, 2, and 3 years, respectively, as shown by the Kaplan–Meier curve (Fig. 1). Major adverse events of ICD implantation occurred in at least one administration of inappropriate ICD, ATP, and shock therapy in 35 (30%), 28 (24%) and 21 patients (18%), respectively. Due to cardiac device infection, lead extraction was performed in 4 patients (3%) during the follow-up period.
Table 2.
Follow-up data.
| Present study (n=118) | |
|---|---|
| Median follow-up, days | 1409 |
| Appropriate ICD therapy (ATP or shock), (%) | 40 (34) |
| ATP, (%) | 33 (28) |
| ATP only, (%) | 22 (19) |
| Shock, (%) | 18 (15) |
| Shock only, (%) | 7 (6) |
| Total cardiac death, (%) | 33 (28) |
| Sudden cardiac death, (%) | 7 (6) |
| Non-sudden cardiac death, (%) | 21 (18) |
| Unclassified cardiac death, (%) | 5 (4) |
| Non-cardiac death, (%) | 15 (13) |
| Unknown cause of death, (%) | 2 (2) |
| All-cause mortality, (%) | 50 (42) |
Data are presented as the mean±standard deviation or n (%).
ATP, anti-tachycardia pacing; ICD, implantable cardioverter defibrillator.
Fig. 1.
The Kaplan–Meier curve of mortality rate of patients in the present study. The number of patients at risk at each time point is indicated below the graph.
3.3. Appropriate ICD therapy
At least one appropriate ICD, ATP, and shock therapy was administered in 40 (34%), 33 (28%), and 18 patients (15%), respectively.
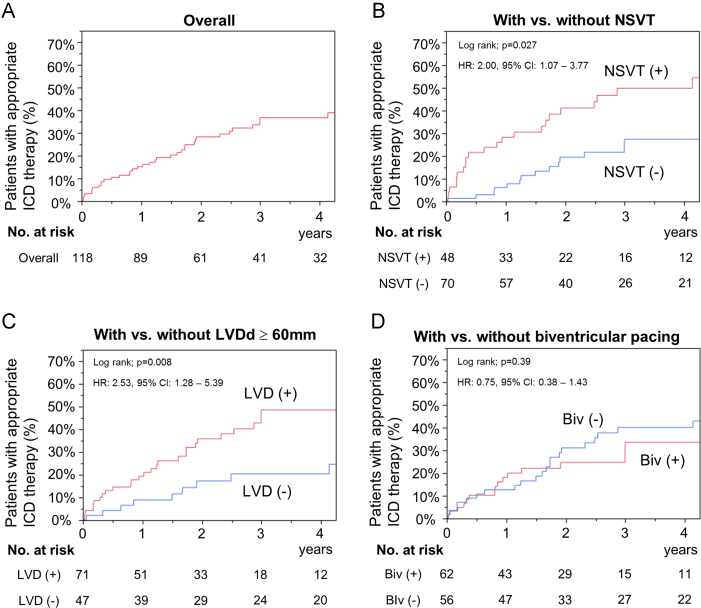
The Kaplan–Meier curve for the incidence of appropriate ICD therapy (ATP and/or shock) administration showed 15%, 28%, and 37% at 1, 2, and 3 years (Fig. 2A), respectively, and that of appropriate ICD shock therapy showed 7%, 9%, and 12% at 1, 2, and 3 years, respectively (Table 3).
Fig. 2.
The Kaplan–Meier curves of probability of appropriate ICD therapy in the present study. The number of patients at risk at each time point is indicated below the graph. (A) Overall population. (B–D) Each subgroup analysis based on the presence of non-sustained ventricular tachycardia (NSVT), the prevalence of left ventricular dilatation (LVD) defined as left ventricular diastolic dimension (LVDd)≥60 mm, and biventricular pacing (Biv).
Table 3.
Incidence of appropriate ICD therapy: shock and anti-tachycardia pacing.
| At implant | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|
| Appropriate therapy | ||||||
| ATP and/or shock | 15% | 28% | 37% | 37% | 41% | |
| (Patients at risk) | (118) | (89) | (61) | (41) | (32) | (24) |
| Shock | 7% | 9% | 12% | 16% | 18% | |
| (Patients at risk) | (118) | (97) | (78) | (62) | (48) | (37) |
The multivariate analyses using Cox proportional hazards model (Table 4) showed that the presence of NSVT (hazard ratio [HR], 2.26; 95% CI, 1.17–4.39; P=0.015) (Fig. 2B) and the prevalence of dilated left ventricle (LVDd≥60 mm) (HR, 2.31; 95% confidence interval [CI], 1.07–5.38; P=0.033) (Fig. 2C) before implantation could independently predict further requirement of appropriate ICD therapy, whereas biventricular pacing was not independently associated with the incidence of appropriate ICD therapy (Fig. 2D).
Table 4.
Univariate and multivariate analyses for incidence of appropriate ICD therapy administration.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Non-sustained VT | 2.00 (1.07–3.77) | 0.030 | 2.26 (1.17–4.39) | 0.015 |
| LVDd≥60 mm | 2.53 (1.28–5.39) | 0.007 | 2.31 (1.07–5.38) | 0.033 |
| LVEF≤25% | 2.63 (1.38–5.12) | 0.003 | 1.84 (0.94–3.73) | 0.077 |
| Diabetes | 0.54 (0.27–1.04) | 0.072 | 0.73 (0.36–1.43) | 0.37 |
| Biventricular pacing | 0.75 (0.38–1.43) | 0.39 | ||
CI, confidence interval; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia.
4. Discussion
4.1. Main findings
In the present study, we investigated long-term mortality and predictors of appropriate ICD therapy administration in Japanese patients with ischemic heart disease (IHD), who fulfilled the MADIT II criteria for ICD implantation. The mortality rate and incidence of appropriate ICD therapy administration at 3 years after implantation were identified as 20% and 37%, respectively. In addition, left ventricular diastolic diameter (LVDd)≥60 mm and the presence of NSVT before implantation were independent predictors of appropriate ICD therapy in patients with reduced left ventricular (LV) systolic function due to IHD.
4.2. SCD rate due to IHD in Japan compared with Western countries
A previous study has reported that the Asian population has a lower SCD rate compared with Caucasians [4]. Since the 1.5-fold increase in mortality rate in patients with ischemic cardiomyopathy (ICM) compared with that in those without ICM in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) [7] has been reported, the differences in the prevalence of ICM may have some effects on the different SCD rates. Indeed, some clinical trials conducted on the Western population reported that 60–75% of heart failures were caused by IHD [8], [9], [10], whereas IHD prevalence in Japanese patients was only 30–34% in a nationwide observational cohort survey [11], [12]. In addition, based on previous reports, some differences in IHD severity may be found, particularly between Western and Japanese populations. The prevalence of severely impaired LV systolic function (LVEF≤30%) after MI in Japanese patients was reported in 199 (4.8%) of 4122 consecutive patients in an observational cohort of 18 medical centers [13], whereas the prevalence was reported to be more frequent in Western countries: 342 (13.4%) of 2544 patients and 79 (15%) of 513 patients in the Netherlands and United Kingdom, respectively [14]. Furthermore, based on the previous report [15], the survival rate of Japanese patients with severely impaired LV systolic function (LVEF≤30%) was superior to that of Western populations. However, data are still limited regarding the long-term mortality and incidence of appropriate therapy administration in Japanese patients with IHD after ICD implantation for primary prevention of SCD.
4.3. ICD implantation for primary prevention of SCD in Japan
The 11th world survey of cardiac pacing and ICD in 2009 [16] revealed that the number of new ICD implantations per million people in Japan was approximately one fifth and one-tenth of that in developed European countries and the United States, respectively, although ICD implantations increase yearly. The Japan Cardiac Device Therapy Registry database [17], which was established by a nationwide survey by the Japanese Heart Rhythm Society, has recently reported that the prevalence of ischemic heart disease was 36% in 7016 patients treated with ICD: 28% in 1801 patients and 39% in 5215 patients underwent primary and secondary prevention of SCD, respectively [18]. These results demonstrate that the proportion of patients with IHD who underwent ICD implantation is lower in Japan compared with approximately 80% in the United States.
In the present study, we retrospectively investigated 118 consecutive patients, who satisfied the MADIT II criteria, who underwent prophylactic ICD implantation for primary prevention of SCD in our hospital for more than 13 years. The Kaplan–Meier estimates of mortality rate showed similar curves between the present study population and the original MADIT II ICD group [3]. This result differed from the improved mortality rate observed in MADIT II-eligible Japanese patients reported in a previous study [15]; this difference may be due to a number of reasons. The population of the previous study consisted of 79% and 18% of patients without any symptom of heart failure (NYHA I) and with mild symptoms of heart failure (NYHA II), respectively. On the other hand, comparison of the present study with the original MADIT II ICD group showed a similar distribution of symptomatic heart failure (NYHA II, 39%; and NYHA III, 35%). In the previous study, 90 consecutive MADIT II-eligible patients were investigated retrospectively among 3258 patients who underwent cardiac catheterization at a university hospital, where patients underwent well-specialized therapies. This method of patient enrollment may have led to a lower proportion of patients with severe heart failure. In addition, the mean age of the patients in the previous study was lower than that in the present study (64±10 years vs 69±10 years). These differences may have played a part in mortality rate discrepancy.
4.4. Predictors of ICD appropriate therapy
Incidence of appropriate ICD therapy administration in patients with ICD for primary prevention was also similar in the present study (37%) compared with the original MADIT II ICD group (34%) [19], 3 years after implantation. Furthermore, the present study demonstrated that the presence of NSVT and dilated left ventricle (LVDd≥60 mm) before implantation could independently predict future appropriate ICD therapy administration.
Implantation of ICD is performed often in developed Western countries for primary prevention of SCD according to current guidelines established based on previously reported criteria, LVEF≤30% or LVEF≤35%, which have been demonstrated to be the gold standard of risk stratification in randomized control trials, such as the MADIT II [1] and SCD-HeFT [7]. However, the number of new ICD implantations per million people was lower in Japan than that in developed Western countries [16], and other Asian countries were even lesser. This may be due to differences in clinical background, frequency, and severity of ischemic heart disease, economic conditions of health care, and different beliefs regarding life and death. With an aging society, ICD therapy for primary prevention of SCD may be more frequently considered in clinical settings, particularly in heavily populated countries such as China and India. Given the increasing medical expenses, accurate risk stratification will be more important in addition to the criteria of the current guidelines. Data on predictors of appropriate ICD therapy are still limited, especially in Asian countries, although sub-analysis in some randomized control trials have been reported [20], [21].
In the JCS guidelines, patients with both NSVT and LVEF≤35% and those with LVEF≤35% alone are classified as I and IIa indications, respectively [22], whereas the presence of NSVT is not mandatory for class I indication in the ACC/AHA and ESC guidelines [5], [6]. The results of this study indicated that the presence of prior NSVT could be helpful for further stratification of the incidence of ventricular arrhythmic (VA) events in patients with reduced LV systolic function. In addition, the Chronic Heart Failure Analysis and Registry in the Tohoku District-2 (CHART-2) study, which was conducted in Japan as a multicenter, prospective observational cohort study, recently reported that chronic atrial fibrillation and LVDd≥65 mm were identified as independent predictors of VA events [23]. In contrast, the presence of prior NSVT was not independently associated with VA events in the study. The differences of the results between the present study and the CHART-2 study may be due to the differences in the baseline characteristics of the patients and the methods used. Indeed, 24-h Holter monitoring at enrollment was performed in only 60% of the patients in the CHART-2 study, whereas at least one 24-h Holter monitoring was always done before ICD implantation in the present study. Because only 2% of the entire population in the CHART-2 study underwent ICD implantation before and after enrollment, the incidence of VA events after enrollment also might be evaluated in a different manner.
Amiodarone did not influence the incidence of appropriate ICD therapy in the present study, whereas the Optimal Pharmacological Therapy in Cardioverter Defibrillator Patients (OPTIC) study [24], which was conducted as a multicenter randomized trial, showed the effectiveness of amiodarone on shock reduction as appropriate therapy in patients with poor LV function after ICD implantation.
This may be partly because of the low proportion of amiodarone use, which was only 16% in the present study. In addition, the differences in baseline characteristics may be associated with the different results. The patients of the present study consisted those who received ICD implantation for primary prevention of SCD without any prior VA event. In contrast, almost 70% of patients in the OPTIC study had a past VA history.
To the best of our knowledge, the present study is the first report on the mortality rate and predictors of appropriate ICD therapy, which was investigated in patients who received ICD implantation for primary prevention of SCD in a clinical setting of an Asian country. The present results may provide important complementary information that may contribute to proper risk stratification, and help clinicians in understanding the benefits and limitations associated with ICD therapy for primary prevention.
4.5. Study limitation
The present study has several limitations. First, the present study was a single-center cohort study with a limited number of patients. As for this study population, Kokura Memorial Hospital, which is located in the center of Kitakyushu City, with a total population of about one million, is one of the representative referral cardiac centers in Japan, where more than 2000 cases of PCI per year have been performed in the past 20 years. Thus, the present cohort represents a series of patients with severely impaired LV function in a typical urban community in Japan because most of the patients with IHD in this district are referred to our hospital. In the near future, further investigation, such as Japan Implantable Devices in Coronary Artery Disease study [25], which was already launched as a multicenter, nationwide observational study, will provide helpful and detailed information on the prevalence and risk factors of VA events in Japanese patients with reduced LV systolic function due to IHD after ICD implantation. Second, the present study also included patients treated with biventricular pacing, unlike the original MADIT II. Generally, approximately 70% of patients treated with biventricular pacing respond to the device with improved echocardiographic LV function. Based on the recent report from MADIT-CRT, biventricular pacing reduced the risk of further VA events, and the device particularly improved the response of LV function [26]. On the other hand, some potential mechanisms for the proarrhythmic role of biventricular pacing might account for the reversal of LV activation and increased transmural dispersion of repolarization with epicardial pacing, resulting in the development of re-entrant circuits [27]. In the present study, biventricular pacing was not independently associated with the incidence of appropriate ICD therapy. Finally, the age of our study population was older than that of the original MADIT II ICD group (mean age: 69±10 vs. 64±10 years). Based on the reports of the World Health Organization, the Japanese had an average life expectancy of 82 and 83 years in 2006 and 2012, respectively, whereas Americans had a life expectancy of 78 years and 79 years in 2006 and 2012, respectively. Comparison with the original MADIT II ICD group shows that different life expectancies and periods, wherein patients underwent ICD implantation, between the two groups should be taken into consideration.
5. Conclusions
Our study demonstrated that mortality rate and incidence of appropriate ICD therapy in Japanese patients who fulfilled the MADIT II criteria were identified, and the presence of dilated left ventricle (LVDd≥60 mm) and NSVT before ICD implantation could be helpful for further risk stratification of the incidence of VA events.
Source of funding
None.
Conflict of interest
All authors declare no conflict of interest related to this study.
References
- 1.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg H., Case R.B., Moss A.J. Analysis of mortality events in the multicenter automatic defibrillator implantation trial (MADIT II) J Am Coll Cardiol. 2004;43:1459–1465. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg I., Gillespie J., Moss A.J. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the multicenter automatic defibrillator implantation trial II. Circulation. 2010;122:1265–1271. doi: 10.1161/CIRCULATIONAHA.110.940148. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z.J., Croft J.B., Giles W.H. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 5.Zipes D.P., Camm A.J., Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace: Eur Pacing, Arrhythm Cardiac Electrophysiol: J Work Groups Card Pacing, Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 6.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 7.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.Pulignano G., Del Sindaco D., Tavazzi L. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry) Am Heart J. 2002;143:45–55. doi: 10.1067/mhj.2002.119608. [DOI] [PubMed] [Google Scholar]
- 9.Mosterd A., Cost B., Hoes A.W. The prognosis of heart failure in the general population: the Rotterdam study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 10.Senni M., Tribouilloy C.M., Rodeheffer R.J. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 11.Kawashiro N., Kasanuki H., Ogawa H. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circ J. 2008;72:2015–2020. doi: 10.1253/circj.cj-08-0323. [DOI] [PubMed] [Google Scholar]
- 12.Tsutsui H., Tsuchihashi-Makaya M., Kinugawa S. Characteristics and outcomes of patients with heart failure in general practices and hospitals. Circ J. 2007;71:449–454. doi: 10.1253/circj.71.449. [DOI] [PubMed] [Google Scholar]
- 13.Shiga T., Hagiwara N., Ogawa H. Sudden cardiac death and left ventricular ejection fraction during long-term follow-up after acute myocardial infarction in the primary percutaneous coronary intervention era: results from the HIJAMI-II registry. Heart. 2009;95:216–220. doi: 10.1136/hrt.2008.145243. [DOI] [PubMed] [Google Scholar]
- 14.Foley P.W., Addison C.E., Whinney S.B. Implantable cardioverter defibrillator therapy for primary prevention of sudden cardiac death after myocardial infarction: implications of international guidelines. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S131–S134. doi: 10.1111/j.1540-8159.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanno K., Miyoshi F., Watanabe N. Are the MADIT II criteria for ICD implantation appropriate for Japanese patients? Circ J. 2005;69:19–22. doi: 10.1253/circj.69.19. [DOI] [PubMed] [Google Scholar]
- 16.Mond H.G., Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009—a World Society of Arrhythmia׳s project. Pacing Clin Electrophysiol. 2011;34:1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu A., Nitta T., Kurita T. Actual conditions of implantable defibrillation therapy over 5 years in Japan. J Arrhyth. 2012;28:263–272. [Google Scholar]
- 18.Greenberg S.M., Epstein A.E., Deering T. A comparison of ICD implantations in the United States versus Italy. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S143–S146. doi: 10.1111/j.1540-8159.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 19.Brodine W.N., Tung R.T., Lee J.K. Effects of beta-blockers on implantable cardioverter defibrillator therapy and survival in the patients with ischemic cardiomyopathy (from the Multicenter Automatic Defibrillator Implantation Trial-II) Am J Cardiol. 2005;96:691–695. doi: 10.1016/j.amjcard.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 20.Singh J.P., Hall W.J., McNitt S. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the multicenter automatic defibrillator implantation trial II (MADIT-II) J Am Coll Cardiol. 2005;46:1712–1720. doi: 10.1016/j.jacc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 21.Chen J., Johnson G., Hellkamp A.S. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2013;61:2161–2168. doi: 10.1016/j.jacc.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group JCSJW Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2011) Circ J. 2013;77:249–274. doi: 10.1253/circj.cj-66-0054. [DOI] [PubMed] [Google Scholar]
- 23.Satake H., Fukuda K., Sakata Y. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure—a report from the CHART-2 study. Circ J. 2015;79:381–390. doi: 10.1253/circj.CJ-14-0925. [DOI] [PubMed] [Google Scholar]
- 24.Connolly S.J., Dorian P., Roberts R.S. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC study: a randomized trial. JAMA. 2006;295:165–171. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu A., Mitsuhashi T., Nitta T. Japan implantable devices in coronary artery disease (JID-CAD) study design. J Arrhyth. 2015;31:83–87. doi: 10.1016/j.joa.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barsheshet A., Wang P.J., Moss A.J. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (multicenter automatic defibrillator implantation trial—cardiac resynchronization therapy) J Am Coll Cardiol. 2011;57:2416–2423. doi: 10.1016/j.jacc.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 27.Medina-Ravell V.A., Lankipalli R.S., Yan G.X. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740–746. doi: 10.1161/01.cir.0000048126.07819.37. [DOI] [PubMed] [Google Scholar]