Abstract
The entirely subcutaneous implantable cardioverter-defibrillator (ICD) system was developed to provide a life-saving defibrillation therapy that does not affect the heart and vasculature. The subcutaneous ICD is preferred over the transvenous ICD for patients with a history of recurrent infection presenting major life-threatening rhythms. In this case report, we describe the first successful intermuscular implantation of a completely subcutaneous ICD in a Japanese patient with pectus excavatum. There were no associated complications with the device implantation or lead positioning. Further, the defibrillation threshold testing did not pose any problem with the abnormal anatomy of the patient.
Keywords: Subcutaneous implantable cardioverter defibrillator, Infection, Intermascular, Pectus excavatum
1. Introduction
The entirely subcutaneous implantable cardioverter-defibrillator (ICD) system (S-ICD™, Boston Scientific Corp., Marlborough, MA, USA), was developed to provide life-saving defibrillation therapy that does not affect the heart and vasculature, and thus these can overcome transvenous lead-associated complications [1]. The subcutaneous ICD system is preferred over the transvenous ICD for active patients, or for those having a history of recurrent transvenous lead positioning, those without or limited vascular access, or those with congenital heart disease, ion channelopathies or primary electrical disease with ventricular fibrillation presenting with major life-threatening rhythms. This system was approved for use in Japan in February 2016.
2. Case report
A 38-year-old patient with pectus excavatum received a dual chamber ICD after resuscitated cardiac arrest due to ventricular fibrillation during myocardial infarction following vasculitis (Fig. 1, Panel A). He developed fever and chill after the ICD implantation, consistent with the growth of methicillin-sensitive Staphylococcus aureus in blood cultures. This suggests that the patient had occult bacterial infection with septic shock. Sixteen days after the primary ICD implantation, the device and the lead were explanted under local anesthesia. As there were no signs of infection in the subcutaneous ICD pocket, we decided to implant the ICD subcutaneously. Prior to implantation, the patient passed a pre-implant screening with two vectors.
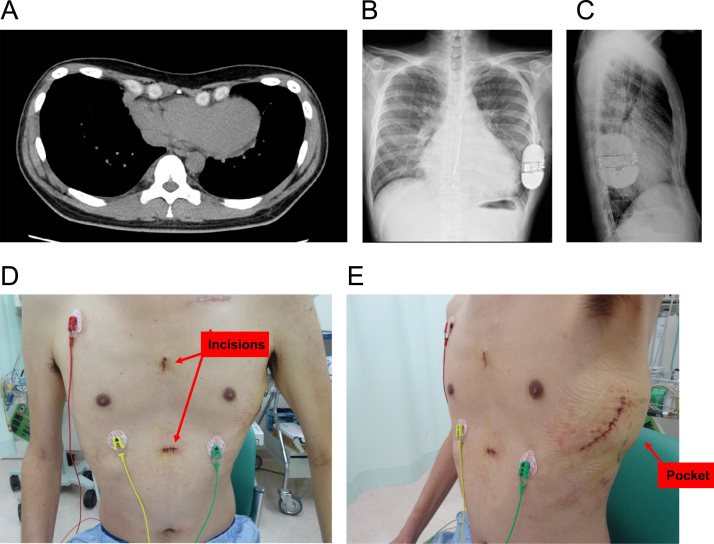
Fig. 1.
(A) Computed tomography demonstrating typical sunken appearance of the chest with pectus excavatum. (B) and (C) Radiography of anteroposterior view (B) and lateral view (C) confirmed lead and device final position. (D) and (E) Complete healing of the wound of 2 parasternal incisions (D) and the generator pocket (E) at follow-up.
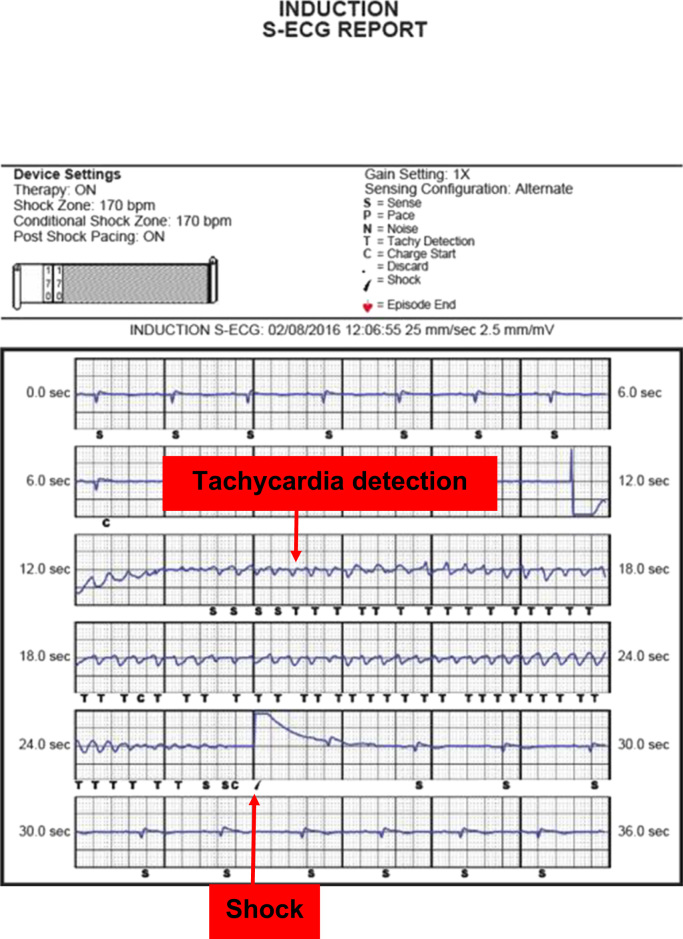
Thirty-four days following the ICD device removal, he received a second-generation subcutaneous ICD. The pulse generator was placed dorsally between the anterior surface of the serratus and the posterior surface of the latissimus dorsi over the left sixth rib and below the mid-axillary line. Via two parasternal incisions, a 3-mm tripolar parasternal electrode was positioned parallel to and 1 cm to the left of the sternal midline with the distal sensing electrode localized adjacent to the manubriosternal junction and the proximal sensing electrode positioned 2 cm above the xiphoid process. At the end of the procedure, ventricular fibrillation (50 Hz stimulation between shock coil and generator) was induced and successfully terminated by a 65 J shock (15 J safety margin). The generator automatically selected the optimal sensing and gain selection configuration. The time from initial detection to the shock delivery was about 11.5 seconds (Fig. 2). Post-operative chest radiography confirmed the lead position (Fig. 1, Panels B and C), as evident from an uneventful post-operative recovery and complete healing of wounds (Fig. 1, Panels D and E).
Fig. 2.
At the end of the procedure, ventricular fibrillation (50 Hz stimulation between shock coil and generator) was induced and successfully terminated by a 65 J shock (15 J safety margin). The time from initial detection to the shock delivery was about 11.5 s.
3. Discussion
The cardiac implantable electronic device infection rate in Japan was similar to that previously reported in the USA and Europe [2]. Complete subcutaneous ICD was developed to provide an alternative to the transvenous ICD system, as it is implanted without any transvenous or epicardial leads. Infection rates involving this new device resulting in explant or revision were not lower than those previously reported in the ICD registries. However, it should be emphasized that no systemic infections were recorded with the use of any of the documented devices [3]. In recent studies, the simplicity of implantation that avoided vascular access was reflected in the very low rates (2%) of major and acute complications, such as the device system infection [4].
The first pilot phase of human studies of the subcutaneous ICD commenced in 2008, followed by subsequent regulatory and post-market studies. Approved by the FDA in September 2012 to provide defibrillation therapy for the treatment of ventricular tachyarrhythmias, the Subcutaneous ICD system was developed over 10 years of defibrillation and sensing research, acute human feasible studies, and chronic clinical studies [1], [4], [5], [6]. This system demonstrated a very high shock efficacy for spontaneous ventricular arrhythmias and a decreasing incidence of inappropriate shocks [4]. The second-generation subcutaneous ICD System (EMBLEM™, Boston Scientific Corp., Marlborough, MA, USA), which is 20% thinner and is projected to last 40% longer than previously described subcutaneous ICD System, is available in Japan from February 2016.
The limitations of the current subcutaneous ICD include its inability to provide antitachycardia pacing (ATP) for ventricular tachycardia, limited bradycardia pacing support (only 30 s post-shock pacing) and the absence of endovascular monitoring ability for collateral data gathering, such as the impedance monitoring for chronic heart failure. Magnetic resonance imaging (MRI) scans in patients with the current system are still an off-label procedure. One estimate of potential candidates for the subcutaneous ICD might include every patient, especially the younger patients indicated for the primary and secondary sudden cardiac death prevention without a pacing indication.
Additionally, the selection for subcutaneous ICD implant is based on pre-implant electrographic body surface mapping. The sensing algorithm for this system depends on the surface elecrtrocardiogram (ECG) morphology, specifically the R-and T-wave amplitudes, R/T and R/P ratios, QRS duration and QT interval. A pre-implant screening tool developed by the manufacturer (Boston Scientific Corp., Marlborough, MA, USA) is used in all patients under consideration for this device to select individuals with ECG morphology that offers appropriate signal configuration to satisfy the requirements of the subcutaneous ICD sensing algorithm, which are critical for an appropriate and effective delivery of the ICD implantation therapy [7].
In this article, we report the intramuscular implantation of a subcutaneous ICD device using a left dorsal configuration with an 8-cm-single coil electrode in a patient with pectus excavatum. There were no associated complications with the device implantation or lead positioning, and also the defibrillation threshold testing did not indicate any problem resulting from the abnormal anatomy of the study subject. There is a relatively high rate of wound complications in the Asian population [8]. Therefore, the intramuscular approach for the subcutaneous ICD appears feasible and a safe alternative to the standard subcutaneous placements in thin and active patients. Also, the more dorsal placement of the pulse generator may provide an improved vector toward the shocking coil capturing of the large part of the left ventricle in comparison to the conventional subcutaneous approach.
4. Conclusions
In this study we achieved the first successful intermuscular implantation of a completely subcutaneous ICD in a Japanese patient with pectus excavatum. There were no associated clinical complications with the device implantation.
Conflict of interest
Dr. Kondo has received a research grant from the St. Jude Medical. Dr. Winter is a consultant and is on the advisory board for Boston Scientific and Cameron Health, and also a part of the speakers bureau of Boston Scientific and Medtronic. Dr. Kobayashi received research grants from the Medtronic, St. Jude Medical, Boston Scientific, and Biotronik.
References
- 1.Bardy G.H., Smith W.M., Hood M.A. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima H., Taki M. Incidence of cardiac implantable electronic device infections and migrations in Japan: results from a 129 institute survey. J Arrhythm. 2015 doi: 10.1016/j.joa.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boersma L., Burke M.C., Neuzil P. Infection and mortality after implantation of the subcutaneous ICD following transvenous ICD extraction. Heart Rhythm. 2016;13:157–164. doi: 10.1016/j.hrthm.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Burke M.C., Gold M.R., Knight B.P. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and effortless registry. J Am Coll Cardiol. 2015;65:1605–1615. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Lambiase P.D., Barr C., Theuns D.A. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the effortless S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo Y., Ueda M., Kobayashi Y. New horizon for infection prevention technology and implantable device. J Arrhythm. 2016 doi: 10.1016/j.joa.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeb M., Curzen N., Allavatam V. Sensitivity and specificity of the subcutaneous implantable cardioverter defibrillator pre-implant screening tool. Int J Cardiol. 2015;195:205–209. doi: 10.1016/j.ijcard.2015.05.082. [DOI] [PubMed] [Google Scholar]
- 8.Hai J.J., Lim E.T., Chan C.P. First clinical experience of the safety and feasibility of total subcutaneous implantable defibrillator in an Asian population. Europace. 2015;17(Suppl. 2):ii63–ii68. doi: 10.1093/europace/euv144. [DOI] [PubMed] [Google Scholar]