Abstract
Background
Urinary supersaturation is one key determinant of calcium oxalate (CaOx) urinary stone formation, and urinary excretions of oxalate and citrate are two key determinants. Each is influenced by gastrointestinal processes.
Methods
Open label and randomized placebo studies have examined the effect of oral probiotic preparations on urinary supersaturation and oxalate excretion. Cross sectional studies in humans have studied the association of Oxalobacter formigenes colonization status and urinary oxalate excretion and prevalence of urinary stones. The intestinal microbiome of representative animals adapted to a high oxalate diet has been defined.
Results
The fecal content of O. formigenes, the best studied oxalate-degrader, varies depending on stone status. However, trials with probiotics designed to degrade oxalate including those containing O. formigenes, Lactobacillus, and/or Bifidobacterium spp., have been disappointing. Multiple intestinal segments of animals on a high oxalate diet contains diverse communities of microorganisms that can function together to degrade and detoxify a large oxalate load.
Conclusions
Although the intestinal microbiome seems likely to play a role to modify gastrointestinal absorption of lithogenic substances and hence urinary stone risk, whether we can develop tools to manipulate it and decrease this kidney stone risk remains to be determined.
Keywords: Calcium oxalate (CaOx), Lactobacilli, microbiome, nephrolithiasis, Oxalobacter formigenes
Introduction
Biology of calcium oxalate (CaOx) kidney stones
The urine of most humans is supersaturated and favors CaOx crystallization. Thus, perhaps it is not surprising that 70% or more of kidney stones are composed of CaOx (1). Given that the urine of most persons is supersaturated for CaOx, one might indeed wonder why everyone does not form stones. However, although supersaturation is key and requisite for stone formation, other biologic events are also implicated. These include the formation of anchored precursors within the kidney including Randall’s plaque and collecting duct plugs (2-5), macromolecules that control the rates of crystal growth and aggregation (6,7), and crystal internalization and processing by cells (8). These secondary factors are only partially understood, and not subject to therapeutic interventions at the present time.
Fortunately, relatively more is known about the control of the urinary composition of stone forming salts. Key factors that determine urinary supersaturation include the urinary excretion of calcium, oxalate, citrate and water. Of these, evidence is strong that genetics greatly influence urinary calcium excretion (9), although diet is also an important modifier (10). Evidence also suggests that there are heritable components of the amount of urinary oxalate, citrate and even water (the latter likely mediated by thirst) (11). However, most likely environment (diet and fluid intake/losses) are relatively more important for determining the urine composition.
Oxalate biology
Oxalate is a small dicarboxylic acid formed as an end product of metabolism by humans, largely in the liver (12). Oxalate is also found in certain plants, largely as CaOx crystals in the stems and leafs. Whatever oxalate is generated or absorbed from the diet, it must be eliminated in the urine (13). At least three genetic defects are known to cause primary hyperoxaluria (PH), which leads to over-generation of oxalate in the liver (12). Whether or not other genetic variation in these or other genes underlies milder hyperoxaluria in the general population remains unknown.
Because oxalate exists in plants is in the form of CaOx crystals, only a small amount is bioavailable for absorption (typically about 10% or less) (14). The majority of anionic oxalate is thought to be absorbed via a paracellular route (Figure 1) (15). Apical and basolateral transporters have also been demonstrated to have oxalate-transporting activity in vitro. Conversely, the role of transcellular oxalate transport in normal human biology remains unclear. Interestingly, however, knockout of SLC26A6 (the gene that encodes PAT1) results in hyperoxaluria due to decreased secretion of oxalate into the gut lumen in mice (16,17). Thus it has been hypothesized that increased degradation of oxalate by the intestinal microbiome could create a driving force for oxalate section into the gut, and hence reduce urinary excretion. Furthermore, certain bacteria might release soluble factors that increase PAT1 activity (18).
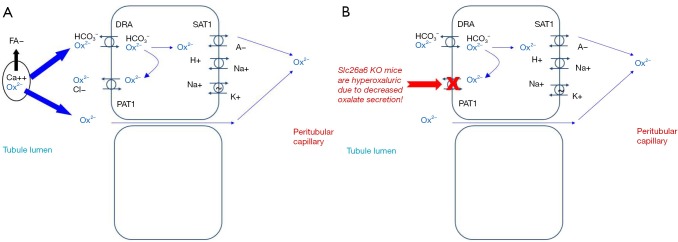
Figure 1.
Oxalate transport in the intestine. (A) It is thought that the majority of oxalate is absorbed paracellularly. In states of fat malabsorption, increased fatty acids can bind calcium resulting in more oxalate ion free for absorption. Paracellular transporters to facilitate transcellular oxalate absorption include DRA (SLC26A3) on the apical surface that can exchange oxalate for bicarbonate, and SAT1 (SLC26A1) on the basolateral surface that can exchange oxalate for sulfate and other anions; (B) PAT1 (SLC26A6) in the apical surface can facilitate oxalate secretion into the lumen in exchange for chloride. SLC26A6 knockout mice are hyperoxaluric, presumably due to loss of this intestinal secretory pathway.
The majority of oxalate is eliminated by the kidney via filtration. A smaller amount can be secreted in the proximal tubule. The amount of oxalate secretion can increase in CKD, perhaps in response to increasing blood concentrations (19). Oxalate is not secreted or reabsorbed past the proximal tubule. Thus the oxalate concentration increases as water is reabsorbed along the nephron, reaching critical thresholds by the collecting duct where it can crystallize with calcium (19). This is undoubtedly a key factor in urinary stone risk, especially in regards to growth upon a preexisting nidus, since a urinary stone cannot develop in the absence of supersaturation.
The gastrointestinal tract is a key player in oxalate biology. In normal individuals, only about 10% of ingested oxalate is absorbed, presumably because it is tightly complexed with calcium within the plant matter ingested (14). Factors that influence oxalate absorption include the amount of calcium and fat in the diet (20). It is thought that fatty acids bind calcium, and thus increase unbounds anionic oxalate that can then be absorbed paracellularly. Free calcium in the gut lumen can in turn bind up this anionic oxalate and prevent its absorption. Patients with any cause of fat malabsorption are thus at risk of enteric hyperoxaluria on this basis.
On average, CaOx stone formers appear to absorb slightly higher percentage of oxalate from their food (14). The reasons are not known. Could this be due genetic alterations in oxalate transport, a tendency towards fat malabsorption, or changes in the intestinal microbiome? To date there are no clear answers. Typical treatments for stone patients with mild hyperoxaluria include a lower oxalate diet with adequate amounts of calcium. Preferably the calcium should be in food sources like dairy products, since calcium supplements might slightly increase stone risk (21). Lower fat intake is also a good idea, although not extensively studied outside of the group with clear enteric hyperoxaluria. It has been hoped that manipulation of the intestinal microbiome might also alter oxalate absorption.
Citrate biology
Citrate is thought to be an important crystallization inhibitor (22). Citrate complexes with filtered calcium and also has independent effects at the crystal surface to inhibit CaOx and brushite crystal growth (22,23). Some filtered citrate is reabsorbed in the proximal tubule, largely regulated by proximal tubule cell pH, with lower intracellular pH increasing citrate reabsorption (24). In the absence of renal tubular acidosis the net absorption of alkali by the gastrointestinal tract is thought to be the most important determinant of net urinary citrate excretion (25). Thus a diet weighted towards protein, chronic malabsorption states, hypokalemia, or distal renal tubular acidosis are the most common causes of hypocitraturia, which is found in 20–60% of calcium stone formers (24). Treatment is of the underlying disorder and/or administration of potassium citrate are the available options (24). Given the key role of gastrointestinal function in citrate homeostasis, is seems likely that the microbiome might influence net alkali absorption, and hence urinary citrate excretion. However, no evidence to this effect has yet been published. Indeed, urinary citrate excretion did not increase in the kidney stone probiotic studies where this value was reported (26-28).
Methods
In this systematic review we present the results of open label and randomized placebo studies that have examined the effect of oral probiotic preparations on urinary CaOx supersaturation and oxalate excretion. We also discuss cross sectional studies in humans that have studied the association of O. formigenes colonization status and urinary oxalate excretion, and prevalence of urinary stones. Finally, we review what is reported regarding potential oxalate-degrading organisms in the intestine of humans and animals.
Results
Trials of Lactobacillus-containing probiotics for stone disease
Investigators have conclusively demonstrated that components of the endogenous digestive microflora can utilize oxalate, potentially limiting its absorption from the intestinal lumen (29). Probiotics containing Lactobacilli spp. have been commonly used to treat gastrointestinal symptoms such as antibiotic-induced diarrhea. Thus one might hope they would have favorable effects on urinary oxalate and/or citrate excretion. Oxadrop® was formulated specifically for potential treatment of hyperoxaluria (28). Each gram of the mix (Oxadrop®) contains 2×1011 bacteria (Lactobacillus acidophilus, L. brevis, Streptococcus thermophilus, and Bifidobacterium infantis). The different strains are mixed in a 1:1:4:4 weight and prepared as a granulate. The organisms were chosen on the basis of their ability to degrade oxalate in vitro.
In an initial pilot study, Oxadrop® reduced urine oxalate excretion by 40% in a group of mildly hyperoxaluric CaOx stone formers (30) (Table 1). The hypo-oxaluric effect even lasted after a 1 month wash out period. In a subsequent study, a group of ten patients with various causes of enteric hyperoxaluria and stones were also treated with Oxadrop® (28). This study was also unblinded and thus lacked a placebo arm. Patients sequentially received 4 g Oxadrop®, 8 g Oxadrop®, and 12 g Oxadrop® for 1 month each. These data suggested a small effect at 4 and 8 g, with a fall in urine oxalate excretion of about 20–25% (Table 1). The third month on 12 g of Oxadrop® the urine oxalate excretion was again close to baseline, after which it fell slightly after another washout month. Thus this study suggested there might be a dose-dependent effect of the preparation, or perhaps that the differences observed in urine oxalate excretion at the lower (and/or higher) doses were nonspecific and not related to the study drug at all.
Table 1. Trials of Lactobacillus-containing probiotics for hyperoxaluria.
| Author | Population | Design | Outcome |
|---|---|---|---|
| Campieri et al. (30) | Mildly hyperoxaluric stone formers (n=6) | Unblinded; 4 g Oxadrop® for 30 days |
Urine oxalate dropped 40% at 30 days and remained down 50% at 60 days |
| Lieske et al. (28) | Patients with enteric hyperoxaluria (n=10) | Unblinded; 4 g Oxadrop® for 30 days; then 4 g for 30 days; then 12 g for 30 days |
Urine oxalate dropped 19% at 30 days; 24% at 60 days; 2% at 30 days; and 20% after a 30-day washout |
| Lieske et al. (26) | Patients with enteric hyperoxaluria (n=40) | Patients on controlled diet randomized to Oxadrop®, AKSB, or placebo for 4 weeks | No change from baseline (controlled diet alone) for any group |
| Goldfarb et al. (31) | Stone patients with mild hyperoxaluria (n=20) | Randomized to placebo vs. Oxadrop® for 56 days | No change in urine oxalate in either group at 28 or 56 days |
| Ferraz et al. (27) | Stone patients (n=14) | Sequentially on high oxalate diet then high oxalate diet plus Lactobacilli and Bifidobacterium preparation | Urine oxalate increased on high oxalate diet with no effect of the probiotic |
AKSB, Agri-King Synbiotic.
Based upon these intriguing data, albeit inconclusive, a more rigorous randomized trial was completed in a population of 40 enteric hyperoxaluria stone formers (26). Patients were randomized to Oxadrop®, placebo, or an alternative probiotic, Agri-King Synbiotic (AKSB) (Agri-King Inc., Fulton, IL, USA). AKSB is a candidate synbiotic preparation extensively studied at Mayo Clinic that was hoped to have beneficial effects on gastrointestinal health, although there was no direct evidence it should influence oxalate metabolism directly (26). Subjects were given two AKSB capsules per day for a total of 1010 organisms containing: (I) fructooligosaccharide (115 mg), manufactured as Ultra-FOS ST by Encore Technologies, Minnetonka, MN, as food-grade quality and is a prebiotic component of AKSB; (II) Enterococcus faecium [(E. faecium) SF68; 4.5 billion] produced by Cerbios-Pharma SA (Barbengo, Switzerland); (III) Saccharomyces cerevisiae subsp. boulardi (300 million), a yeast produced as Levucell SB by Lallemand Biochem International, Ontario, Canada, as ‘food-grade’ quality; and (IV) Saccromyces cerevisiae (200 million), a food-grade yeast produced as active dry yeast by SAF Corporation in Milwaukee, WI, USA. AKSB was developed by Agri-King with the primary aim of improving gut performance in animals so that the routine use of antibiotics in animal feeds could be reduced or eliminated. Studies in farm animals by Agri-King have confirmed that the preparation improves intestinal health and reduces the risk of illness when animals are challenged with food- or water-borne pathogens, and overall growth rates improve.
In this randomized, placebo-controlled trial study patients were placed on a controlled metabolic diet with normal calcium (1,000 mg) and reduced oxalate (80–100 mg), appropriate for their CaOx urinary stone diagnosis. The diet itself was effective, reducing urine oxalate excretion by an average of 36%, with an overall improvement in urinary CaOx supersaturation. However, urine oxalate not fall further from this baseline on controlled diet with either probiotic or placebo. It is possible that the diet was “too effective”, in essence not leaving enough free oxalate within the gut lumen for the probiotics to degrade. Nevertheless, the more rigorous design than the previous studies suggests general use of currently available Lactobacillus-containing preparations may not work as well as initially hoped in patients with enteric hyperoxaluria.
Subsequently, a 56-day randomized, placebo controlled trial of Oxadrop® was completed in 20 mildly hyperoxaluric stone formers, without known enteric hyperoxaluria and on a free choice diet (31). In this study, like the placebo-controlled enteric hyperoxaluria study (26), no effect on urinary oxalate excretion was observed in either arm at 28 or 56 days.
As noted above, it has been hypothesized that oxalate-degrading bacteria may require a certain amount of free oxalate to survive and/or thrive in the intestinal lumen. Thus Ferraz and colleagues studied a population of 14 stone formers (7 men and 7 women) on a low calcium (400 mg) and high oxalate (200 mg) diet (27). Under these dietary conditions urinary oxalate excretion did increase by 30% from the previous baseline on a free choice diet. However, addition of a Lactobacillus/Bifidobacterium preparation had no further effect on urinary oxalate levels.
Thus, on balance one can conclude that evidence from the more rigorous studies does not suggest that currently available Lactobacillus or other probiotic products and/or regimens of their administration can consistently reduce urinary oxalate excretion.
Oxalobacter and oxalate metabolism
O. formigenes is an interesting organism. This obligate anaerobe utilizes oxalate as its sole energy source. The three key genes are the oxalate/formate antiporter (OxlT), a formyl coenzyme A transferase (frc), and oxalyl-coenzyme A decarboxylase (Oxc). Most humans become colonized with O. formigenes during childhood, but colonization can be lost later in adulthood, perhaps in response to antibiotics. The balance of dietary calcium and oxalate (and hence free oxalate in the gut lumen) appears to influence the amount of O. formigenes recovered from the stool (32). Thus, O. formigenes might be an inducible defense against ingestion of a high oxalate diet.
Observational studies support a role for O. formigenes colonization in CaOx urinary stone risk. For example, in a large cross sectional study only 17% of 247 stone patients were colonized with O. formigenes, while 38% of 259 control patients were (33). Previous antibiotic usage was associated with colonization status. However, although the stone patients had higher urine oxalate excretion, colonization status did not correlate with urine oxalate excretion in these patients who were on a self-choice diet.
In a smaller cross sectional study, eleven O. formigenes-colonized stone formers were found to have a lower urine oxalate excretion (0.31±0.10 mM/day) compared to 26 non-colonized stone formers (0.40±0.13 mM/day) (34). Non colonized stone formers were more likely to have a history of multiple stone events. Interestingly, the percent of absorption of an oral radiolabeled oxalate load did not vary between the two groups, despite the fact the plasma oxalate was significantly higher in the O. formigenes colonized group. Together, these observations are consistent with decreased gastrointestinal secretion of oxalate by the patients not colonized with O. formigenes.
Because of evidence that oxalate can be secreted by rodents into their gut lumen (17), there has been great interest to test whether oral administration of O. formigenes might increase oxalate degradation within the gut lumen and possibly promote oxalate secretion amongst hyperoxaluric patients, even those with PH. The hoped-for net effect is to increase gut and decrease urinary oxalate elimination. Studies in a mouse model of type 1 PH support the possible effect of this strategy (35). Indeed, in a small unblinded pilot study of four PH patients, urine oxalate excretion fell up to 50% during 4 weeks on an oral preparation of O. formigenes (36). In this pilot study, three out of five patients with preserved renal function demonstrated a 22–48% reduction of urinary oxalate excretion while taking the first oral formulation of O. formigenes. Two other patients in the study with chronic kidney disease on dialysis experienced a significant reduction in plasma oxalate and amelioration of clinical symptoms. While taking a second O. formigenes formulation, four out of six patients with normal renal function demonstrated a reduction in urinary oxalate ranging from 38.5% to 92%. Although O. formigenes could be detected in the vast majority of subjects on active treatment, fecal recovery dropped at follow up (off therapy), indicating only transient gastrointestinal-tract colonization while subjects were still taking the preparation.
Despite these promising preliminary data, in the decade since no evidence from a follow up controlled trial has been published to further support the use of oral O. formigenes therapy in PH. Potential issues with the therapeutic use of oral O. formigenes include formulation of pharmacologic amounts of this obligate anaerobe, as well as the long term viability of this obligate anaerobe in paste or freeze dried preparations. Furthermore, no studies using oral O. formigenes in groups of patients with enteric or idiopathic hyperoxaluria have yet appeared in the literature. Thus, the role of pharmacologic use of this intriguing bacteria to reduce urinary oxalate excretion, and hence kidney stone risk, remains unclear.
Other oxalate degraders
In vitro studies suggest that O. formigenes is the most efficient oxalate-degrading organism found in the human intestinal tract. For example, under controlled conditions O. formigenes degraded up to 98% of available oxalate (37). On the other hand, in the same studies Lactobacillus and Bifidobacterium spp. also effectively degraded oxalate, albeit somewhat less effectively (11–68%). Furthermore, the key oxalate degrading genes Oxc and frc have been sequenced from Lactobacillus and Bifidobacterium spp. One potentially unique feature of O. formigenes is that this organism can utilize oxalate as a carbon and energy source, and thrives in the presence of the anion (38). Other oxalate-degrading species such as Lactobacilli can detoxify oxalate and survive in its presence, but not necessarily thrive. Thus, the relative importance of various bacteria in oxalate-homeostasis in humans remains ill-defined.
Along these lines, recent studies in the white-throated wood rat Neotoma albigula are of interest (39). This mammal consumes a diet comprised almost entirely of the oxalate-rich Opuntia cactus. These animals have a complicated segmented gut that harbors a diverse microbiome along its length. The foregut microbial community in particular shifts in composition in response to dietary toxins, and may be important for their degradation. When the microbiome was characterized by segment of the intestinal tract, isolates spanned three genera: Lactobacillus, Clostridium, and Enterococcus. Over half the isolates exhibited oxalate-degrading capacity in vitro, and Lactobacillus isolates contained the Oxc gene. Oxalobacter spp. were also identified throughout the intestinal tract, but were much less abundant and were more concentrated in the more distal regions (cecum and large intestine). Other oxalate-degrading genera (especially Lactobacilli spp.) were more concentrated in the foregut, the point where oxalate first enters the gastrointestinal tract. The authors hypothesized that each gut region supplied a niche for diverse functional taxa and communities of microorganisms that can function together to degrade and detoxify a large oxalate load as it is made bioavailable by digestive processes. The analogies, or lack thereof, in humans remain to be determined.
Conclusions
Diet and gastrointestinal function play a key role in determining the composition of the urine. It also seems quite likely that the gastrointestinal microbiome would great influence how key components of the diet are metabolized and absorbed. Key urinary parameters that the microbiome might influence include oxalate and citrate. Indeed, the intestinal microbiome contains numerous obligate and generalized oxalate degraders. Evidence suggests that the fecal content of O. formigenes, the best studied oxalate-degrader, varies depending on stone risk and urinary oxalate excretion. However, to date trials with probiotics designed to degrade oxalate, including those containing Oxalobacter, Lactobacillus, and/or Bifidobacterium spp., have all been disappointing. Thus, although the intestinal microbiome likely plays a role to modify urinary stone risk, whether we can develop tools to manipulate it and decrease this risk remains to be determined.
Acknowledgements
Funding: Studies were partially supported by (I) the Rare Kidney Stone Consortium (U54KD083908), a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS). This consortium is funded through a collaboration between NCATS, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); (II) Mayo Clinic O’Brien Urology Research Center, DK100227; (III) NIH grant AT R21AT2534; (IV) V.S.L. Pharmaceuticals Inc., Gaithersburg, MD; and (V) the Mayo Foundation. The funding sources had no role in the study design, conduct, or reporting.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Lieske JC, Rule AD, Krambeck AE, et al. Stone composition as a function of age and sex. Clin J Am Soc Nephrol 2014;9:2141-6. 10.2215/CJN.05660614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Krambeck AE, Williams JC, Jr, et al. Distinguishing characteristics of idiopathic calcium oxalate kidney stone formers with low amounts of Randall's plaque. Clin J Am Soc Nephrol 2014;9:1757-63. 10.2215/CJN.01490214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evan AP, Coe FL, Lingeman JE, et al. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. Anat Rec (Hoboken) 2007;290:1315-23. 10.1002/ar.20580 [DOI] [PubMed] [Google Scholar]
- 4.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int 2007;71:795-801. 10.1038/sj.ki.5002113 [DOI] [PubMed] [Google Scholar]
- 5.Evan AP, Lingeman JE, Coe FL, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int 2009;76:1081-8. 10.1038/ki.2009.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asplin JR, Parks JH, Chen MS, et al. Reduced crystallization inhibition by urine from men with nephrolithiasis. Kidney Int 1999;56:1505-16. 10.1046/j.1523-1755.1999.00682.x [DOI] [PubMed] [Google Scholar]
- 7.Asplin JR, Parks JH, Nakagawa Y, et al. Reduced crystallization inhibition by urine from women with nephrolithiasis. Kidney Int 2002;61:1821-9. 10.1046/j.1523-1755.2002.00307.x [DOI] [PubMed] [Google Scholar]
- 8.Lieske JC, Norris R, Swift H, et al. Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 1997;52:1291-301. 10.1038/ki.1997.454 [DOI] [PubMed] [Google Scholar]
- 9.Moe OW, Bonny O. Genetic hypercalciuria. J Am Soc Nephrol 2005;16:729-45. 10.1681/ASN.2004100888 [DOI] [PubMed] [Google Scholar]
- 10.Pak CY. A critical evaluation of treatment of calcium stones. Adv Exp Med Biol 1980;128:451-65. 10.1007/978-1-4615-9167-2_50 [DOI] [PubMed] [Google Scholar]
- 11.Lieske JC, Turner ST, Edeh SN, et al. Heritability of dietary traits that contribute to nephrolithiasis in a cohort of adult sibships. J Nephrol 2016;29:45-51. 10.1007/s40620-015-0204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edvardsson VO, Goldfarb DS, Lieske JC, et al. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol 2013;28:1923-42. 10.1007/s00467-012-2329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 2001;59:270-6. 10.1046/j.1523-1755.2001.00488.x [DOI] [PubMed] [Google Scholar]
- 14.Hesse A, Schneeberger W, Engfeld S, et al. Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J Am Soc Nephrol 1999;10 Suppl 14:S329-33. [PubMed] [Google Scholar]
- 15.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 2005;33:1-16. 10.1007/s00240-004-0445-3 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Asplin JR, Evan AP, et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 2006;38:474-8. 10.1038/ng1762 [DOI] [PubMed] [Google Scholar]
- 17.Freel RW, Hatch M, Green M, et al. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 2006;290:G719-28. 10.1152/ajpgi.00481.2005 [DOI] [PubMed] [Google Scholar]
- 18.Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 2006;69:691-8. 10.1038/sj.ki.5000162 [DOI] [PubMed] [Google Scholar]
- 19.Worcester EM, Evan AP, Coe FL, et al. A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type I. Am J Physiol Renal Physiol 2013;305:F1574-84. 10.1152/ajprenal.00382.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stauffer JQ. Hyperoxaluria and intestinal disease. The role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis 1977;22:921-8. 10.1007/BF01076170 [DOI] [PubMed] [Google Scholar]
- 21.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997;126:497-504. 10.7326/0003-4819-126-7-199704010-00001 [DOI] [PubMed] [Google Scholar]
- 22.Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol 2014;192:316-24. 10.1016/j.juro.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 23.Ryall RL. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J Urol 1997;15:155-64. 10.1007/BF02201852 [DOI] [PubMed] [Google Scholar]
- 24.Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol 2009;11:134-44. [PMC free article] [PubMed] [Google Scholar]
- 25.Sakhaee K, Williams RH, Oh MS, et al. Alkali absorption and citrate excretion in calcium nephrolithiasis. J Bone Miner Res 1993;8:789-94. 10.1002/jbmr.5650080703 [DOI] [PubMed] [Google Scholar]
- 26.Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010;78:1178-85. 10.1038/ki.2010.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraz RR, Marques NC, Froeder L, et al. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol Res 2009;37:95-100. 10.1007/s00240-009-0177-5 [DOI] [PubMed] [Google Scholar]
- 28.Lieske JC, Goldfarb DS, De Simone C, et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 2005;68:1244-9. 10.1111/j.1523-1755.2005.00520.x [DOI] [PubMed] [Google Scholar]
- 29.Argenzio RA, Liacos JA, Allison MJ. Intestinal oxalate-degrading bacteria reduce oxalate absorption and toxicity in guinea pigs. J Nutr 1988;118:787-92. [DOI] [PubMed] [Google Scholar]
- 30.Campieri C, Campieri M, Bertuzzi V, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 2001;60:1097-105. 10.1046/j.1523-1755.2001.0600031097.x [DOI] [PubMed] [Google Scholar]
- 31.Goldfarb DS, Modersitzki F, Asplin JR. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin J Am Soc Nephrol 2007;2:745-9. 10.2215/CJN.00600207 [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Knight J, Easter LH, et al. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 2011;186:135-9. 10.1016/j.juro.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 2008;19:1197-203. 10.1681/ASN.2007101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siener R, Bangen U, Sidhu H, et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 2013;83:1144-9. 10.1038/ki.2013.104 [DOI] [PubMed] [Google Scholar]
- 35.Hatch M, Gjymishka A, Salido EC, et al. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 2011;300:G461-9. 10.1152/ajpgi.00434.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppe B, Beck B, Gatter N, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 2006;70:1305-11. 10.1038/sj.ki.5001707 [DOI] [PubMed] [Google Scholar]
- 37.Mogna L, Pane M, Nicola S, et al. Screening of different probiotic strains for their in vitro ability to metabolise oxalates: any prospective use in humans? J Clin Gastroenterol 2014;48 Suppl 1:S91-5. 10.1097/MCG.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 38.Miller AW, Dearing D. The metabolic and ecological interactions of oxalate-degrading bacteria in the Mammalian gut. Pathogens 2013;2:636-52. 10.3390/pathogens2040636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AW, Kohl KD, Dearing MD. The gastrointestinal tract of the white-throated Woodrat (Neotoma albigula) harbors distinct consortia of oxalate-degrading bacteria. Appl Environ Microbiol 2014;80:1595-601. 10.1128/AEM.03742-13 [DOI] [PMC free article] [PubMed] [Google Scholar]