Abstract
Background
Phenotypes often differ even within family members carrying the same SCN5A mutation. We aimed to evaluate the genetic modifiers in a family with Brugada syndrome (BrS) and sick sinus syndrome (SSS) with an SCN5A mutation that causes the truncated alpha-subunit of cardiac Na channel protein.
Methods
To detect the genetic modifiers, we performed targeted panel sequencing of the coding region of 46 genes that are related to primary arrhythmia syndrome, by using a bench-top, next generation sequencer. Phenotype–genotype relationships were evaluated among the family members.
Results
Index proband was a 13-year old (yo) boy with cardiac conduction defect as well as BrS. Genetic analysis revealed that he and his three asymptomatic family members carried a novel nonsense mutation: SCN5A-Q779X. Both genotype-positive mother and sister exhibited coved type ST elevation and his sister had SSS, whereas his elder brother exhibited saddleback type ST elevation induced by pilsicainide administration. We detected four non-synonymous variants (DSG2-R773K, SCN1B-L210P, -S248R, and -R250T) in the proband, his mother and his sister, but not in his brother.
Conclusion
Phenotypic differences between the proband and his brother carrying the same nonsense SCN5A mutation could be explained by modifiers such as SCN1B, and DSG2 gene variants.
Keywords: SCN5A, Overlap syndrome, Children, Pilsicainide challenge test
1. Introduction
Loss-of-function mutations in SCN5A, that encodes the alpha-subunit of the cardiac Na channel, lead to variable phenotypes, viz. Brugada syndrome (BrS), progressive cardiac conduction disease, atrial fibrillation, and sick sinus syndrome (SSS) [1]. In young children carrying SCN5A mutations, reportedly, cardiac conduction disturbance appears as the most common manifestation of disease, with both atrial and ventricular arrhythmia [2]. The phenotype of the patients with the same SCN5A mutations is variable because various modifiers might modulate it. We describe a proband, and his family members with a novel nonsense SCN5A mutation, who displayed variable phenotypes, and searched for the genetic modifiers that might affect the phenotype.
2. Material and methods
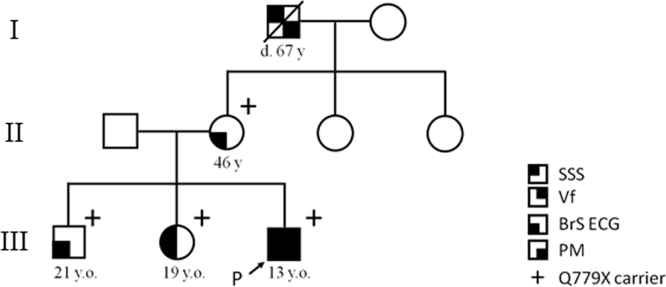
Gene analysis was performed for the proband, his parents, and two siblings as summarized in the family tree (Fig. 1) on obtaining the consents of the family members. Genomic DNA was isolated from blood lymphocytes, and screened for the open reading frame of KCNQ1, KCNH2, KCNE1-3, KCNE5, KCNJ2, and SCN5A. Genetic screening was performed using denaturing high-performance liquid chromatography (dHPLC WAVE system, Transgenomic, Omaha, NE, USA). For abnormal screening patterns, direct sequencing was performed using an automated sequencer (ABI PRISM, 3100x, Applied Biosystems, Foster City, CA, USA). For detection of the genetic modifiers, we performed targeted panel sequencing for coding region of 46 genes (Supplementary information) that were related to primary arrhythmia syndrome for proband and 4 family members, using a bench-top next generation sequencer, the MiSeq (Illumina, San Diego, CA, USA). We then evaluated the detected variants by frequently referring to the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/snp/), Human Genetic Variation Browser that is a Japanese SNP database http://www.genome.med.kyoto-u.ac.jp/SnpDB/), and the 1000 genomes database (http://www.1000genomes.org/home). The pathogenicity of the variants was estimated by three different types of prediction software: (1) combined annotation dependent depletion (CADD) (http://cadd.gs.washington.edu/), (2) PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and (3) SIFT (http://sift.jcvi.org/).
Fig. 1.
Pedigree of the family. Phenotypic traits are designated within pedigree symbols. NA: not available for clinical and genetic information.
3. Results
3.1. Case presentation
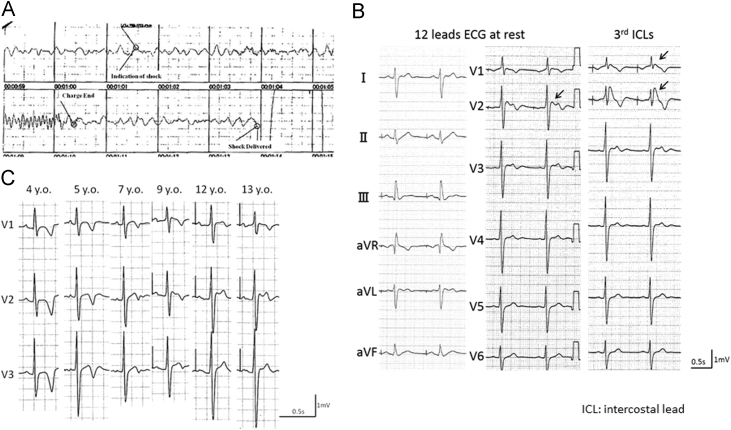
Index proband was a 13-year-old (yo) boy who was suspected to have a sino-atrial block because of an auscultation abnormality at the age of three (family tree in Fig. 1). At age four, he presented with pre-syncope and underwent electrophysiological test. His PR interval was 179 ms, QRS was 107 ms, and QT interval was 394 ms (QTcF 451 ms). The sinus node recovery time was significantly prolonged to 7,268 ms, and HV interval to 70 ms. The atrio-ventricular nodal effective refractory period was 360 ms. Against a diagnosis of SSS a dual chamber pacemaker was implanted. At the age of 13, he had cardio-pulmonary arrest on running. The electrocardiogram (ECG) monitored by an autonomic external defibrillator (AED) showed ventricular fibrillation (VF) (Fig. 2A), which was successfully restored to sinus rhythm. On complete recovery, his resting ECG in V2 showed ST elevation of a saddleback type at the 4th, and coved type at the 3rd intercostal leads sites (Fig. 2B). During atrial pacing at 60 bpm, his PR interval prolonged to 262 ms, QRS interval was 125 ms, and QTc was 436 ms (Table 1). Fig. 2C depicts temporal changes in the ECG (V1-V3) recorded from the age 4 to 13. At age 4, we failed to find any Brugada pattern, while the J point gradually elevated from the age of 9. An echocardiographic examination revealed no structural abnormalities. The signal averaged electrocardiogram (SAECG) revealed positive late potentials, and microvolt-T wave alternans (μV-TWA) was negative. He, therefore, was diagnosed with BrS. His pacemaker was replaced with an implantable cardioverter defibrillator (ICD).
Fig. 2.
Clinical characteristics of proband. A: Ventricular fibrillation recorded by an automated external defibrillator in the thirteen year old B: Twelve leads ECG, arrows present Brugada ECG C: Temporal changes of ECG in 4th intercostal leads.
Table 1.
Summary of electrophysiological property of the pedigree.
| Age | symptom | Rest ECG | 3rd ICLs | Pilsicainide administration | HR | PR interval | QRS interval | QTc Bazett’s | |
|---|---|---|---|---|---|---|---|---|---|
| Proband | 13 y.o. | SSS, VF | Saddle back | Coved | n.d. | 60 bpm atrial pacing | 262 ms | 125 ms | 436 ms |
| Mother | 46 y.o. | None | Normal | Coved | n.d. | 68 bpm | 215 ms | 128 ms | 445 ms |
| Sister | 19 y.o. | None | Normal | Normal | Coved | 57 bpm (44 bpm) | 188 ms (214 ms) | 112 ms (148 ms) | 424 ms (418 ms) |
| Brother | 21 y.o. | None | Normal | Normal | Saddle back | 68 bpm (75 bpm) | 185 (216 ms) | 134 ms (134 ms) | 427 ms (401 ms) |
The values in the pilsicainide administration are given in parentheses.
ICL: intercostal lead, y.o.: years old, SSS: sick sinus syndrome, VF ventricular fibrillation, n.d.: not done.
3.2. Phenotypes of the family members
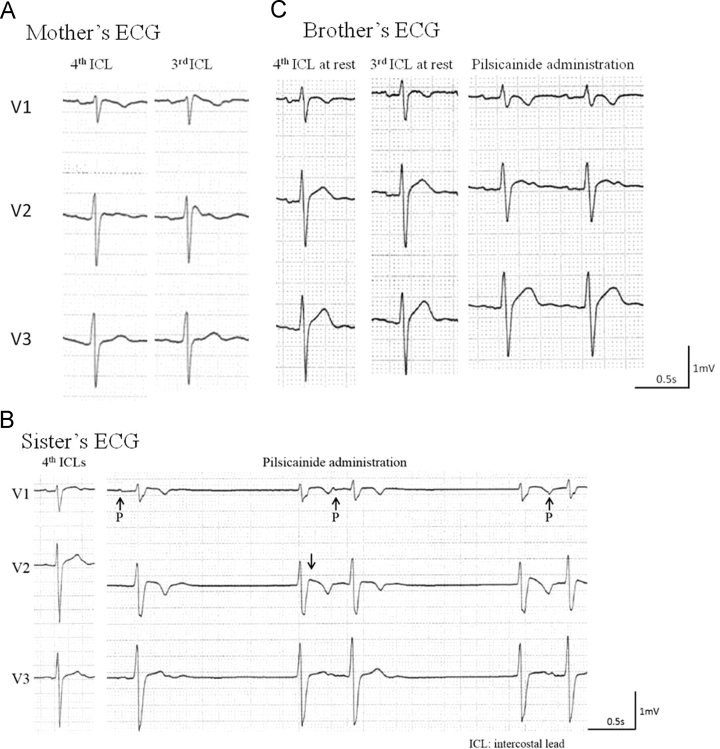
The proband had asymptomatic parents and two siblings as shown in the family tree in Fig. 1. All except for his father had abnormal ECG. His mother (46-yo) had a coved type ST elevation in the 3rd precordial leads (Fig. 3A). His elder sister (19-yo) had SSS, and a coved type Brugada ECG during a pilsicainide (class Ic antiarrhythmia drug) challenge test (Fig. 3B), and his elder brother (21-yo) had a saddle back Brugada ECG during pilsicainide administration (Fig. 3C). We injected 1 mg/kg of pilsicainide for 10 minutes. The prolongation of the PR interval was detected in his mother at rest, and in his sister and brother on pilsicainide administration (Table 1). They were all positive for SAECG and negative for μV-TWA. His maternal grandfather, who died of cancer, had received a pacemaker for SSS. We obtained his ECG recording that showed sinus rhythm at 70 bpm, a PR interval of 190 ms, QRS duration of 106 ms, and no Brugada ECG pattern (data not shown).
Fig. 3.
Family׳s ECG in right precordial leads. A: Mother׳s ECG, left: 4th intercostal leads, right: 3rd intercostal leads Coved type ECG was exhibited in 3rd intercostal leads. B: Elder sister׳s ECG, left: rest ECG, right: 3rd ICL lead on pilsicainide administration SSS and coved type ECG was exhibited during pilsicainide administration C: Elder brother׳s ECG, left: rest ECG, middle: 3rd ICL leads, right: 3rd ICL lead on pilsicainide administration Saddle back like ECG was exhibited during pilsicainide administration.
3.3. Genetic analyses
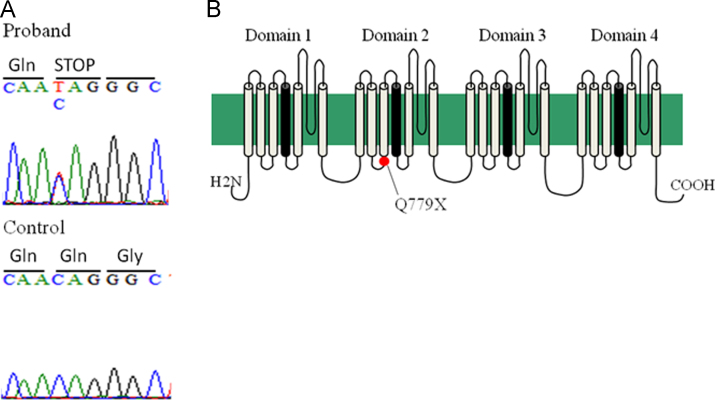
We identified a SCN5A mutation in the proband, and the three family members except for his father. The mutation, a single-base substitution at nucleotide 2335 (c.2335C>T), is located in the cytoplasmic loop of the Na channel alpha-subunit and changed the codon CAG (glutamine: Q) to an in-frame stop codon, TAG, at position 779, Q779X (Fig. 4). A premature stop codon in this position results in nonsense mediated decay (NMD), leading to decay of mRNA [3]. Therefore, the expression level of mRNA and protein of cardiac Na channel is presumably low in SCN5A-Q779X carriers.
Fig. 4.
Genetic analysis of the proband. A: Electropherograms of SCN5A gene showing a mutation, p.Q779X (c.2335C>T) in the proband. B: Topology of sodium channel, voltage-gated, type V, alpha subunit encoded by SCN5A. Q779 is located in the cytoplasmic loop connecting segments 3 and 4 of domain 2.
To clarify the phenotypic variation among the mutant SCN5A carriers in the index family, we performed a targeted panel sequence using MiSeq, for the mutation carriers. In the analysis, we further detected four non-synonymous variants of arrhythmia-related genes in the proband, his mother and his sister, but not in his brother exhibiting a milder phenotype. The first one was desmoglein 2 (DSG2)-R773K (rs2278792) and the others were SCN1B L210P (rs55742440,), S248R (rs67701503), and R250T (rs67486287). DSG2 is one of the desmosomal genes whose mutations cause arrhythmogenic right ventricular cardiomyopathy. SCN1B encodes an ancillary beta-subunit of the cardiac Na+ channels and affects the cardiac conduction, and was reported as a causative gene for BrS. Table 2 summarizes Minor allele frequencies (MAF) and prediction scores for the pathogenicity of each variant. None of them seemed to be the main cause of the disease because their MAFs of them were greater than 0.1, and were predicted to be of benign or mild pathogenicity.
Table 2.
MAF and prediction scores of each variation.
| Gene | Variation | rsID | NCBI MAF | JP MAF | CADD | PolyPhen2 | SIFT |
|---|---|---|---|---|---|---|---|
| DSG2 | R773K | rs2278792 | 0.24 | 0.488 | 11.3 | 0.014 | 0.29 |
| SCN1B | L210P | rs55742440 | 0.3776 | 0.273 | 8.3 | 0 | 0.09 |
| S248R | rs67701503 | 0.1274 | 0.178 | 14.1 | 0.504 | 0 | |
| R250T | rs67486287 | 0.1192 | 0.178 | 11.8 | 0.402 | 0 |
CADD: combined annotation dependent depletion, MAF: minor allele frequency.
4. Discussion
We identified a novel, heterozygous nonsense SCN5A mutation, Q779X, in a family with overlapped phenotype with progressive conduction defects and BrS. The proband suffered from SSS at the age of three, and subsequent ventricular fibrillation with a coved type Brugada ECG pattern at age of 13 was detected. Mild prolongation of the HV intervals had been observed at the age of four, indicating the presence of an extensive conduction delay. At 9 years of age, a right precordial ECG abnormality became gradually significant.
SSS is complicated in children with a left isomerism heart [4], or on surgical intervention for congenital heart disease [5]. It is also seen in channelopathies such as long QT syndrome, BrS [6], or as a part of a progressive cardiac conduction disease [7]. SCN5A mutations are known to be associated with all these phenotypes. Loss-of-function SCN5A mutants have been shown to be related to BrS as well as profound bradyarrhythmias [8]. In children with these mutations, prolonged cardiac conduction intervals have been reported to emerge as the most common manifestation of the disease, and are accompanied with both atrial and ventricular arrhythmias [2]. Three genotype-positive children in our family shared similar clinical characteristics.
The intravenous challenge test with a Na+ channel blocker was conducted in two of our genotype-positive members, and the drug produced PR prolongation (Table 1 and Fig. 3). The PR prolongation, which results from common loss-of-function SCN5A mutation, was enhanced by a Na+ channel blocker in our family members.
The heterozygous nonsense SCN5A mutation of the family was at amino acid 779 that lies in the middle of the cytoplasmic loop of domain 2 (Fig. 4, topography). A missense SCN5A mutation at the same position, Q779K, was previously reported for a Japanese pedigree of long QT syndrome, which harbored a compound heterozygous mutation, E284K, in KCNQ1 [9]. A change in the residual charge from neutral (Q: glutamine) to basic (K: lysine) may cause a gain-of-function outcome. In contrast, the nonsense mutation at the same codon leads to NMD [3], thereby exerting severe loss-of-function effects.
The index boy presented progressive conduction defects and VF during earlier stage of his life. His mother and two siblings had similar ECG abnormalities at rest and when challenged with Na-channel blockers, but remained asymptomatic in spite of the same nonsense SCN5A mutation. This variation in phenotypic severity could be partially explained by the gender difference, because BrS is predominantly noted in males as the disease has an autosomal-dominant mode of transmission [10].
The reason for the variation in phenotypic severity between the index boy and his elder brother is unknown. Both had the same SCN5A mutation, however, the proband had additional DSG2 and SCN1B variants. These variants could be modifiers for the phenotype. DSG2 is located on chromosome 18, and encodes a calcium-binding transmembrane glycoprotein component of desmosomes, desmoglein 2. Few mutations in DSG2 have been shown to decrease the Na+ current, leading to conduction disturbances, and are associated with arrhythmogenic right ventricular dysplasia [11]. The SCN1B gene encodes a beta-1 subunit of the sodium channel that is known to modulate the sodium channel through a non-covalent interaction with the alpha-subunit encoded by SCN5A. MAF for each variant was not low, indicating that they were not pathogenic mutations; although, the pathogenicity estimation scores of SCN1B-S248R and R250T were relatively high (Table 2). Therefore, the phenotype variation among the SCN5A-Q779X carriers could be partially explained by modifier effects of these variants, in addition to the gender difference.
We could only once record the ECGs of family members; therefore, we might have failed to record coved type Brugada pattern ECG, which might have been recorded during follow-up. We did not perform functional analysis of genetic modifiers to confirm the effect on the phenotype. Additionally, we only screened 46 genes related with cardiac arrhythmias, and did not screen introns that might affect the transcription or expression. Thus, we might have overlooked mutation in other genes or in the intronic regions that could be related to the phenotype.
5. Conclusion
SCN5A-positive proband and the female members of the family with overlap syndrome phenotype carried additional variants in SCN1B and DSG2. In contrast, these variants were absent in their SCN5A-positive male family member who showed a milder phenotype. Therefore, in addition to the gender difference, SCN1B and DSG2 variants could be modifiers of BrS and SSS phenotypic expression, and our results may help explain the enigma of variable phenotypes for those with SCN5A mutations.
Conflict of interest
All authors declare no conflict of interests associated with this study.
Footnotes
Grant: This study was supported by research grant from the Ministry of Education, Culture, Science, and Technology of Japan; health science research grant from the Ministry of Health, Labour and Welfare of Japan for Clinical Research on Measures for Intractable Diseases (to S.O., T.M. and M.H.).
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2016.05.007.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Wilde A.A., Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res. 2011;108:884–897. doi: 10.1161/CIRCRESAHA.110.238469. [DOI] [PubMed] [Google Scholar]
- 2.Chockalingam P., Wilde A.A. Loss-of-function sodium channel mutations in infancy: a pattern unfolds. Circulation. 2012;125:6–8. doi: 10.1161/CIRCULATIONAHA.111.071837. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook J.A., Neu-Yilik G., Hentze M.W. Nonsense-mediated decay approaches the clinic. Nat Genet. 2004;36:801–808. doi: 10.1038/ng1403. [DOI] [PubMed] [Google Scholar]
- 4.Momma K., Takao A., Shibata T. Characteristics and natural history of abnormal atrial rhythms in left isomerism. Am J Cardiol. 1990;65:231–236. doi: 10.1016/0002-9149(90)90090-n. [DOI] [PubMed] [Google Scholar]
- 5.Gillette P.C., Shannon C., Garson A., Jr. Pacemaker treatment of sick sinus syndrome in children. J Am Coll Cardiol. 1983;1:1325–1329. doi: 10.1016/s0735-1097(83)80147-8. [DOI] [PubMed] [Google Scholar]
- 6.Kyndt F., Probst V., Potet F. Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation. 2001;104:3081–3086. doi: 10.1161/hc5001.100834. [DOI] [PubMed] [Google Scholar]
- 7.Holst A.G., Liang B., Jespersen T. Sick sinus syndrome, progressive cardiac conduction disease, atrial flutter and ventricular tachycardia caused by a novel SCN5A mutation. Cardiology. 2010;115:311–316. doi: 10.1159/000312747. [DOI] [PubMed] [Google Scholar]
- 8.Makiyama T., Akao M., Tsuji K. High risk for bradyarrhythmic complications in patients with Brugada syndrome caused by SCN5A gene mutations. J Am Coll Cardiol. 2005;46:2100–2106. doi: 10.1016/j.jacc.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Itoh H., Shimizu W., Hayashi K. Long QT syndrome with compound mutations is associated with a more severe phenotype: a Japanese multicenter study. Heart Rhythm. 2010;7:1411–1418. doi: 10.1016/j.hrthm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu W., Matsuo K., Kokubo Y. Sex hormone and gender difference—role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–421. doi: 10.1111/j.1540-8167.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo S., Lodder E.M., Verkerk A.O. Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res. 2012;95:409–418. doi: 10.1093/cvr/cvs219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material