Abstract
Poly‐γ‐glutamic acid (γ‐PGA) is an important natural biopolymer that is used widely in fields of foods, medicine, cosmetics, and agriculture. Several B. amyloliquefaciens LL3 mutants were constructed to improve γ‐PGA synthesis via single or multiple marker‐less in‐frame deletions of four gene clusters (itu, bae, srf, and fen) encoding antibiotic substances. γ‐PGA synthesis by the Δsrf mutant showed a slight increase (4.1 g/L) compared with that of the wild‐type strain (3.3 g/L). The ΔituΔsrf mutant showed increased γ‐PGA yield from 3.3 to 4.5 g/L, with an increase of 36.4%. The γ‐PGA yield of the ΔituΔsrfΔfen and ΔituΔsrfΔfenΔbae mutants did not show a further increase. The four gene clusters’ roles in swarming motility and biofilm formation were also studied. The Δsrf and Δbae mutant strains were both significantly defective in swarming, indicating that bacillaene and surfactin are involved in swarming motility of B. amyloliquefaciens LL3. Furthermore, Δsrf and Δitu mutant strains were obviously defective in biofilm formation; therefore, iturin and surfactin must play important roles in biofilm formation in B. amyloliquefaciens LL3.
Keywords: antibiotic substance, biofilm formation, gene marker‐less deletion, poly‐γ‐glutamic acid, swarming
1. Introduction
Bacillus amyloliquefaciens strains are ubiquitous in the soil and are great reservoirs of important natural products, such as α‐amylase, levansucrase, and fibrinolytic enzymes. Besides being powerful cell factories, B. amyloliquefaciens strains are also used as plant growth‐promoting and bio‐control bacteria, partly because of their ability to produce substances with antifungal, antibacterial, and nematocidal activities. The plant‐associated bacterium B. amyloliquefaciens FZB42, for example, has five gene clusters involved in the synthesis of lipopeptides and polyketides, which direct the synthesis of the cyclic lipopeptides surfactin, bacillomycin, fengycin, an unknown peptide, and the iron‐siderophore bacillibactin (Chen, Koumoutsi, & Scholz, 2009; Chen et al., 2009; Wu et al., 2015). Those antibiotic substances might play vital roles in Bacillus species living in the soil, possibly by promoting adaptation to fluctuating environmental conditions and suppressing competing bacteria or fungi (Rebecca et al., 2007; Susanne et al., 2014). However, when B. amyloliquefaciens cells are used as producers for desired products in the laboratory or industry, the capacity to synthesize various antibiotic substance becomes a disadvantage. It complicates the purification process and impairs the production of target products by competing for the same substrates. Those large gene clusters are also targets of genome reduction applications because they are dispensable for the cell's growth in rich media.
B. amyloliquefaciens LL3 is a glutamic acid‐independent poly‐γ‐glutamic acid (γ‐PGA)‐producing strain that was isolated from traditional fermented food. γ‐PGA is a promising biomaterial that is nonribosomally synthesized by the PgsBCA synthetase complex using l‐ and d‐glutamic acids as substrates, which exhibits outstanding qualities, such as good water solubility, biocompatibility, and biodegradability (Shih & Van, 2001). It is widely used in hydrogels, flocculants, drug delivery, cosmetics, and feed additives (Sung et al., 2005). B. amyloliquefaciens LL3 produces γ‐PGA without additional glutamic acid in the fermentation medium and thus has great potential in industrial production systems because of the lower cost and simplified process (Cao, et al., 2011). The practical use of γ‐PGA is still largely hindered by its low yield, and thus, intensive investigations have been launched to enhance its production, including optimization of fermentation conditions, modification of existing producers, and identification of new wild producers. With the availability of more and more gene manipulation methods, genome‐scale modification of existing producers becomes affordable.
In the past decades, strategies to improve γ‐PGA production were limited to optimization of medium and fermentation conditions. In the 21st century, there have been some attempts to improve the γ‐PGA yield using metabolic engineering strategies. Yeh, Wang, Lo, Chan, and Lin (2010) integrated an efficient synthetic expression control sequence (SECS) into the upstream region of the silent pgsBCA gene cluster of B. subtilis DB430 to produce γ‐PGA. Liu et al. (2011) enhanced γ‐PGA productivity by depressing exopolysaccharides production. VHb (Vitreoscilla hemoglobin) alleviates the oxygen limitation at the later stage of fermentation. The encoding gene, vgb, has been successfully expressed in a γ‐PGA‐producing strain to improve γ‐PGA production, especially under oxygen‐limited conditions (Richard & Margaritis, 2003; Zhang et al. 2013; Su et al., 2010). Heterologous expression of the γ‐PGA synthetase complex (pgsBCA) is another strategy for γ‐PGA production improvement, which has been carried out in coryneform bacteria. Corynebacterium glutamicum E12 harboring vector pMT‐HCE‐pgsBCA could express γ‐PGA synthetase genes from B. subtilis and could be considered as a host for γ‐PGA synthesis (Sung et al., 2005). Feng et al. (2015) and Feng, Gu, Sun, Han, Yang et al. (2014) enhanced γ‐PGA production of B. amyloliquefaciens LL3 by metabolically engineering its γ‐PGA synthesis‐related metabolic networks: by‐products synthesis, γ‐PGA degradation, glutamate precursor synthesis, γ‐PGA synthesis, and autoinducer synthesis. However, few reports have focused on the antibiotic substances, which may compete with γ‐PGA for similar synthesis machinery or substrates.
The whole genome of B. amyloliquefaciens LL3 was sequenced (Geng et al. 2011), and several gene clusters responsible for the synthesis of antibiotic substances were found, including the bae, srf, fen, and itu clusters, as shown in Figure S1. The bae cluster (annotated as pks in B. subtilis 168) encodes bacillaene, which was originally discovered as a bacteriostatic agent that inhibited prokaryotic protein synthesis. Surfactin, iturin A, and fengycin, encoded by the srf, itu, and fen clusters, respectively, are nonribosomally produced cyclic lipopeptides that act against phytopathogenic viruses, bacteria, fungi, and nematodes.
A transcriptional comparison between B. amyloliquefaciens LL3 (γ‐PGA+) and LL3ΔpgsBCA (γ‐PGA−) was performed using RNA‐seq (unpublished data). Interestingly, the transcript levels of the bae, srf, itu, and fen clusters experienced a sharp increase in B. amyloliquefaciens LL3 ΔpgsBCA (Table 1). Specifically, the expression levels of the first genes of the four aforementioned clusters in B. amyloliquefaciens LL3ΔpgsBCA were 14.42‐, 8.08‐, 12‐, and 9.93‐fold higher than that in B. amyloliquefaciens LL3. This suggested that the synthesis of γ‐PGA may suppress the transcription of the above four clusters. Hence, we proposed that the synthesis of the four antibiotic substances may consequently suppress the γ‐PGA synthesis directly or indirectly in turn. In addition, bacillaene, surfactin, iturin A, and fengycin as well as γ‐PGA are all nonribosomally produced. Moreover, surfactin, iturin A, and fengycin contain several glutamates or glutamines, which are the precursors of γ‐PGA (Fig. 1B). Besides, acetyl‐CoA, main precursor of bacillaene, plays important role in TCA cycle, which offers glutamate as precursor for γ‐PGA synthesis. What is more, lipopeptides or polyketides may be viewed as costly for the cells from a metabolic point of view given the big size of the corresponding operons. Therefore, those four antibiotic substances may share similar synthesis machinery and compete for common substrates with γ‐PGA. Based on these speculations, we attempted to obtain a γ‐PGA producer with higher yield and purity, using single or combined marker‐less deletions of the itu, bae, srf, and fen gene clusters (Fig. 1A). Their roles in swarming and biofilm formation were also investigated.
Table 1.
Comparison of expression level of the genes which encode the four antibiotic substance between the B. amyloliquefaciens LL3 (γ‐PGA+) and LL3 ΔpgsBCA (γ‐PGA−)
| Gene | Length | Product | Foldchange |
|---|---|---|---|
| srfA | 10755 | Nonribosomal surfactin synthetase, SrfAA | 8.08 |
| srfB | 10761 | Nonribosomal surfactin synthetase, SrfAB | 12.37 |
| srfC | 3840 | Nonribosomal surfactin synthetase C, SrfC | 12.51 |
| srfD | 732 | Nonribosomal surfactin synthetase D, SrfD | 10.46 |
| sfp | 675 | Surfactin synthetase‐activating enzyme | 1.69 |
| ituC | 7851 | Iturin A synthetase C, ItuC | 9.32 |
| ituB | 16086 | Iturin A synthetase B, ItuB | 10.52 |
| ituA | 11949 | Iturin A synthetase A, ItuA | 12.02 |
| ituD | 1203 | Malonyl‐CoA transacylase, ItuD | 1.86 |
| fenE | 3846 | Fengycin synthetase E, FenE | 11.43 |
| fenD | 7677 | Fengycin synthetase D, FenD | 9.93 |
| baeB | 693 | Polyketide biosynthesis zinc‐dependent hydrolase, BaeB | 1.07 |
| baeC | 870 | Polyketide biosynthesis malonyl‐CoA‐acyl‐carrier‐protein transacylase, BaeC | 1.94 |
| baeD | 975 | Polyketide biosynthesis acyltransferase homolog, BaeD | 3.23 |
| baeE | 2238 | Polyketide biosynthesis protein, BaeE | 4.14 |
| acpK | 249 | Polyketide biosynthesis acyl‐carrier‐protein, AcpK | 1.63 |
| baeG | 1263 | Polyketide biosynthesis 3‐hydroxy‐3‐methylglutaryl‐ACP synthase, PksG | 6.50 |
| baeH | 774 | Probable polyketide biosynthesis enoyl‐CoA hydratase, PksH | 6.17 |
| baeI | 750 | Putative polyketide biosynthesis enoyl‐CoA isomerase, PksI | 4.95 |
| baeJ | 14952 | Polyketide synthase, PksJ | 11.78 |
| baeL | 13431 | Polyketide synthase, PksL | 15.59 |
| baeL | 10542 | Polyketide synthase, PksM | 11.12 |
| baeM | 16314 | Polyketide synthase, PksN | 12.64 |
| baeR | 7446 | Polyketide synthase, PksR | 14.42 |
| baeS | 1212 | Polyketide biosynthesis cytochrome P450, PksS | 2.11 |
Figure 1.
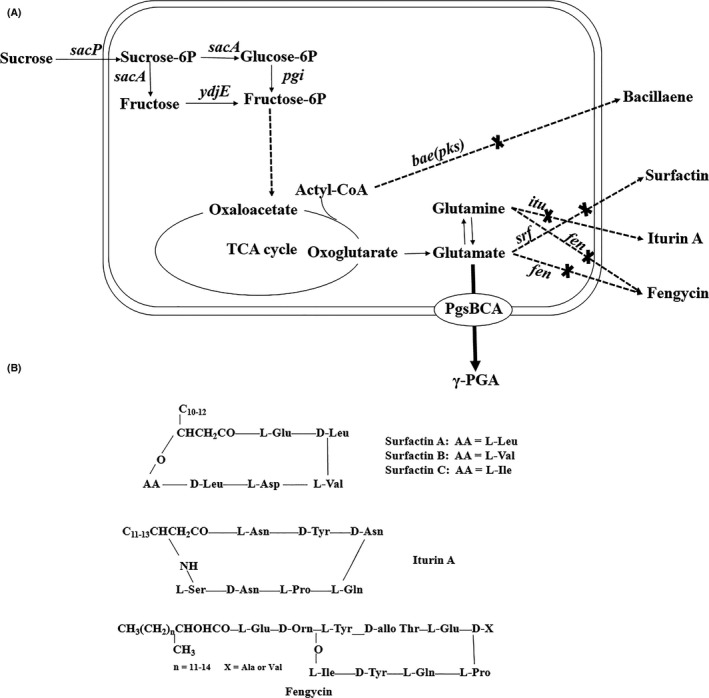
(A) Schematic of modular engineering approach in Bacillus amyloliquefaciens LL3 strain. The X marks indicate the gene deletions in the optimized pathway. (B) Condensed structural formulae of the three cyclic lipopeptides, surfactin, iturin A, and fengycin, produced by B. amyloliquefaciens LL3
2. Experimental Procedures
2.1. Strains, media, and culture conditions
E. coli DH5α was used for plasmid construction. E. coli GM2163 was used to prepare the unmethylated plasmids for subsequent use in the electroporation of B. amyloliquefaciens strains. E. coli strains were cultured in LB medium at 37°C with aeration. The B. amyloliquefaciens LL3 strain was deposited in the China Center for Type Culture Collection (CCTCC) with accession number CCTCC M 208109. B. amyloliquefaciens LL3 and its derivatives were cultured at 37°C in LB or fermentation medium for growth and γ‐PGA synthesis experiments, at 30°C when the temperature‐sensitive deletion plasmid was introduced, or at 42°C when plasmid integration/excision was performed during gene deletion. Fermentation medium for B. amyloliquefaciens LL3 and its derivatives contained sucrose 50 g/L, (NH4)2SO4 2 g/L, MgSO4 0.6 g/L, KH2PO4 6 g/L, K2HPO4 14 g/L, 2 mL mineral elements including 1 mmol/L FeSO4•4H2O, CaCl2•2H2O, MnSO4•4H2O, and ZnCl2 (pH 7.2). When required, media were supplemented with ampicillin (Ap; 100 μg/mL), chloramphenicol (Cm; 5 μg/mL), or tetracycline (Tc; 10 μg/mL).
2.2. Plasmid construction and bacterial transformation
The plasmids and primers used in this study are listed in Table 2 and Table 3. Temperature‐sensitive plasmid pKSV7 is a shuttle vector for E. coli and Bacillus, which is stable at 30°C or below and unstable at 37°C or above. Counter‐selective plasmid pKSU is a derivative of pKSV7 that carries the upp gene from B. subtilis 168. Sequences up‐ and downstream of the target gene clusters were PCR amplified and spliced in a subsequent overlapping PCR. The resulting homologous arms were digested with BamHI and SalI and ligated in the same restriction sites of pKSU to yield deletion plasmids. DNA polymerases, restriction enzymes, and T4 DNA ligase were purchased from Takara (Dalian, China). PCR, enzyme digestion, and ligation reactions were performed as recommended by the enzyme suppliers. The DNA fragments were analyzed on 0.8% agarose gels and purified using an Axygen gel DNA recovery kit (Axygen, CA, USA). Deletion plasmids were treated with Bam HI methyltransferase (New England Biolabs, MA, USA) before transformed into B. amyloliquefaciens strains.
Table 2.
Strains and plasmids used in this study
| Plasmids or Strains | Description | Source |
|---|---|---|
| Plasmids | ||
| pKSU | pKSV7 carrying the upp gene from B. subtilis 168, used for counterselection | Zhang et al. (2014) |
| pKSU‐Δsrf | pKSU carrying a mutant copy of the srf cluster | This study |
| pKSU‐Δitu | pKSU carrying a mutant copy of the itu cluster | This study |
| pKSU‐Δfen | pKSU carrying a mutant copy of the fen cluster | This study |
| Strains | ||
| B. amyloliquefaciens | ||
| LL3 | Glutamic acid‐independent poly‐γ‐glutamic acid (γ‐PGA)‐producing strain | Geng et al. (2011) |
| LL3Δupp | LL3 carrying an in‐frame deletion in the upp gene | Zhang et al. (2014) |
| LL3ΔpgsBCA | LL3 Δupp deleted for pgsBCA | Unpublished |
| LL3Δbae | LL3 Δupp deleted for its partial bae cluster | This study |
| LL3Δsrf | LL3 Δupp deleted for the srf cluster | This study |
| LL3Δitu | LL3 Δupp deleted for the itu cluster | This study |
| LL3Δfen | LL3 Δupp deleted for the fen cluster | This study |
| LL‐IS | LL3ΔuppΔitu deleted for the srf cluster | This study |
| LL‐ISF | LL‐IS deleted for the fen cluster | This study |
| LL‐ ISFB | LL‐ISF deleted for the bae cluster | This study |
| E. coli strains | ||
| DH5α | supE44 ΔlacU169(_80 lacZΔM15) recA1 endA1 hsdR17(rK− mK+) thi‐1gyrA relA1 F− Δ(lacZYA‐argF) | TransGen |
| GM2163 | F− dam‐13::Tn9 (Camr) dcm‐6 hsdR2 (rk −mk +) leuB6 hisG4 thi‐1 araC14 lacY1 galK2 galT22 xylA5 mtl‐1 rpsL136 (Strr) fhuA31 tsx‐78 glnV44 mcrA mcrB1 | Fermentas |
Table 3.
Oligonucleotide primers used in this study
| Primer names | Sequence (5′–3′)a |
|---|---|
| BaeUP‐F | CGGTCTAGAAAACTACATGTCATCTGTCATTAACG |
| BaeUP‐R | CATCGAGAAGTTCTTAAAAGATCCGGGCAGAC |
| BaeDN‐F | CCCGGATCTTTTAAGAACTTCTCGATGCCTAC |
| BaeDN‐R | TGAGTCGACGTGACGGCTTCTCTTTCAG |
| BaeOUT‐F | ATGATACCGCTCCATGTCAGCTCACTTG |
| BaeOUT‐R | CGCCGTGCTTCGTTCATCTAATTCG |
| SrfUP‐F | GCCGTCGACATGGGAATAACTTTTTATCC |
| SrfUP‐R | GGCATCGATATTGCTCCAGAGATACTGTAAAC |
| SrfDN‐F | CAGTATCTCTGGAGCAATATCGATGCCGATCG |
| SrfDN‐R | CGCGGATCCATCTTTAACCATTAAAGGAAAAG |
| SrfOUT‐F | GGAGGCTGTTTCTAAGGAAGAATTGAC |
| SrfOUT‐R | GACGTTTTATTTTGCCGGTCTGTTG |
| FenUP‐F | TGTGGATCCCTATCTTGCCCTCTGTCTTC |
| FenUP‐R | AGAAATATCCTTACGCAAACGGCAAAGTGGACC |
| FenDN‐F | TTTGCCGTTTGCGTAAGGATATTTCTGGTGCCG |
| FenDN‐R | GCAGTCGACTTGAAGAATACTGTTTATGCTT |
| FenOUT‐F | AATGGGTCAGCCGGTAGCTGGCAAG |
| FenOUT‐R | TGCGTCAAATTCAGGGGAAACATCG |
| ItuUP‐F | CGAGGATCCAAATTGAGGCAATAGGAATAG |
| ItuUP‐R | TAACAGTCAGTGTGTTGGGATCGTTTGCGGGAGAC |
| ItuDN‐F | GCAAACGATCCCAACACACTGACTGTTAAAATAGC |
| ItuDN‐R | CGAGTCGACTGGGGGCTTCACAATGATTTATGT |
| ItuOUT‐F | CGGTCATGTAGCCGATCTCACCTGG |
| ItuOUT‐R | ATTGAAATCTTCCGAATGGTGCTTG |
| qRpsU‐F | GTCGTTAGAAAAAACGAATCGCTTG |
| qRpsU‐R | TTGCGTTTTCTAGCAGCTTCTGACT |
| qPgsB‐F | TAGCCTGTGCTGCCGTACTAATCAT |
| qPgsB‐R | GTTTCCGTTTGATCGGTTTCTCCT |
Restriction sites used for the cloning of PCR amplicons are indicated in bold
Competent E. coli cells were purchased from Transgen Biotech (Beijing, China) and transformed according to the manufacturer's instructions. Deletion plasmids were transformed into B. amyloliquefaciens strains using the high osmolarity electroporation method, with modifications, as described previously (Zhang et al., 2014).
2.3. Markerless deletion of the four gene clusters
Gene deletions in this study were carried out adapting a previously reported markerless gene replacement method based on upp and will be described briefly below (Zhang et al., 2014). B. amyloliquefaciens LL3Δupp carrying an in‐frame deletion of upp and that is resistant to 1.3 mmol/L 5‐fluorouracil (5‐FU) was used as the parental strain for subsequent mutants construction. Introduction of the deletion plasmid pKSU would restore sensitivity to 5‐FU for B. amyloliquefaciens LL3 Δupp and its derivatives.
Deletion of the srf cluster will be used as an example to explain the method. The up‐ and downstream homologous arms (~1 kb each) used for srf deletion were obtained using primer pairs SrfUP‐F/SrfUP‐R and SrfDN‐F/SrfDN‐R, respectively. These two fragments were then spliced in a subsequent overlapping PCR using primer pair SrfUP‐F/SrfDN‐R. The resultant homologous arms were ligated into pKSU to yield the deletion plasmid pKSU‐Δsrf, which was then transformed into B. amyloliquefaciens LL3 Δupp in the presence of Cm at 30°C. After plasmid establishment, the recombinants were cultured at 42°C in the presence of Cm to facilitate chromosomal integration. The obtained single‐crossover recombinants were then grown in LB in the absence of Cm to facilitate plasmid excision from the genome. Cultures were diluted and plated in LB agar supplemented with 5‐FU. Deletion‐carrying strains were designated B. amyloliquefaciens LL3Δsrf and were confirmed by PCR, using primer pair SrfOUT‐F/SrfOUT‐R, and DNA sequencing. Mutants deleted for bae, itu, and fen gene clusters were similarly constructed and were designated B. amyloliquefaciens LL3Δbae, LL3Δitu, and LL3Δfen, respectively.
B. amyloliquefaciens LL3Δitu was transformed with pKSU‐Δsrf to obtain LL‐UIS (ΔsrfΔitu), which carries double deletions of the itu and srf clusters. The fen cluster was then deleted in strain LL‐IS to obtain LL‐ISF (ΔsrfΔituΔfen), which carries triple deletions. Finally, the bae cluster was accumulated in strain LL‐UISF to yield LL‐ISFB (ΔsrfΔituΔfenΔbae), which is deficient in all four antibiotic substances.
2.4. Cell growth and γ‐PGA synthesis
Fresh colonies of B. amyloliquefaciens strains were first grown overnight in test tubes containing 5 mL LB liquid and then inoculated into 100 mL fresh LB or fermentation medium in 500‐mL shake flasks to an optical density of approximately 0.05–0.1, at 600 nm (OD600). The shake flasks were then incubated at 37°C for 48 h with an agitation at 200 rpm. For growth experiments, 1‐mL culture was withdrawn periodically to determine the OD600. At the end of fermentation, the viscosity of the culture was determined using a viscosimeter (Brookfield DV‐I+, MA, USA). For dry cell weight (DCW) and γ‐PGA synthesis determination, 100 mL cultures were centrifuged at 8,000g (4°C) for 20 min. The cell pellet was washed three times with dH2O and then dried and weighed to determine the DCW. The supernatant was used to extract γ‐PGA, using an ethanol precipitation method, as previously described (Zhang et al., 2013). Experiments were independently repeated at least three times, and the means and standard deviations were calculated.
2.5. qRT‐PCR analysis of the pgsB gene
The wild‐type B. amyloliquefaciens LL3 and its derivatives were grown to mid‐log phase (approximately 20 hr) in fermentation medium. The cells were collected at 4°C and RNA was isolated using TransZolTM Up (TransGen, Beijing, China), according to the manufacturer's instructions. cDNA was reverse transcribed using a GoScriptTM Reverse Transcription System (Promega, WI, USA). Real‐time PCR analysis for the target genes was performed using the SYBR®PremixEx Taq TM II (Takara, Dalian, China). Transcript levels of the target genes were normalized against the levels of rspU (Feng, Goa, Gu, Zang, Cao et al., 2014).
2.6. Isolation of cyclic lipopeptides and HPLC‐MS analysis
Isolation of surfactins, fengycins, and iturin A and HPLC‐MS analysis were carried out using a method described previously (Luo, Liu, Zhou, Wang, & Chen, 2015). All the samples were further analyzed by matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MS) with a Shimadzu 2020 series HPLC‐MS/MS system (Shimadzu, Japan).
2.7. Swarming and biofilm formation experiments
Fresh colonies of strains to be tested were inoculated and cultivated overnight in LB medium. Then, 10‐μL culture was spotted in the middle of plates containing different agarose concentrations (0.25%, 0.5%, and 0.7%) and incubated for 24 hr.
The biofilm formation experiment was performed as described by Feng, Gu et al. (2014). Overnight cultures of the wild‐type and its derivatives were diluted to an OD600 of 1.0 in fresh LB medium. Samples of 10 μl of the diluted cells were then added to 10 ml of MSgg broth in six‐well microtiter dish. The dish was incubated for 72 hr at 30°C without stir.
3. Results
3.1. Construction of recombinant strains carrying single‐ or multiple‐gene deletions
The marker‐less gene knockout method was used to construct the gene deletion mutants, using the upp cassette and 5‐fluorouracil (5‐FU) selection (Zhang et al., 2014). The primers BaeOUT‐F/R, SrfOUT‐F/R, ItuOUT‐F/R, and FenOUT‐F/R were used to confirm the construction of gene deletion mutants. As shown in Figure 2, the single, double, triple, and quadruple mutants of the bae, srf, itu, and fen genes were successfully generated, and the single‐gene deletion mutants were designated as B. amyloliquefaciens LL3Δbae, LL3Δsrf, LL3Δitu, and LL3Δfen, respectively. The multiple‐gene deletion mutants were named LL‐IS (ΔsrfΔitu), LL‐ISF (ΔsrfΔituΔfen), and LL‐ISFB (ΔsrfΔituΔfenΔbae), respectively.
Figure 2.
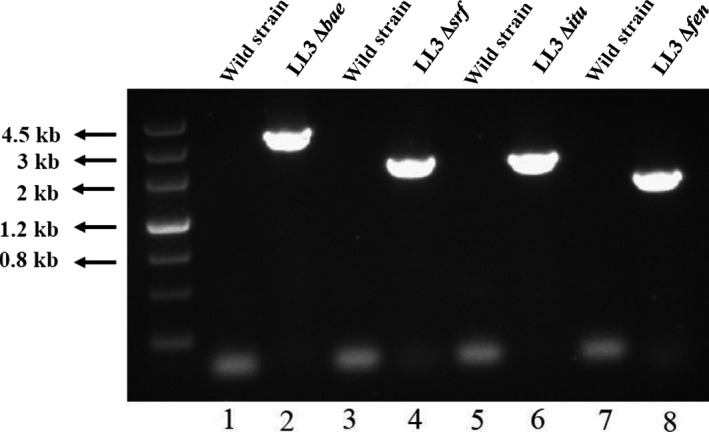
Confirmation of the deletion of the genes by agarose gel electrophoresis of PCR products with primers BaeOUT‐F/R (lanes 1 and 2), SrfOUT‐F/R (lanes 3 and 4), ItuOUT‐F/R (lanes 5 and 6), and FenOUT‐F/R (lanes 7 and 8). Chromosomal DNA of the mutant strains served as the template for PCR. Fragments of the wild‐type strain were too long to obtain PCR products
3.2. Swarming ability and biofilm formation of the mutant strains
Swarming is a social motility behavior found in Bacillus strains and is associated with biofilm development. As previously reported, surfactin plays important roles in the swarming of B. subtilis strains (Kearns et al. 2004). In the “swim plates” (LB plate with 0.25% agar), all the mutants generated colonies that spread over the plate and showed efficient swimming motility (data not shown). However, as shown in Figure 3, B. amyloliquefaciens LL3Δsrf and LL3Δbae showed obvious defects in swarming motility in the “swarm plates” (LB plate with 0.5% agar), while B. amyloliquefaciens LL3Δfen and LL3Δitu showed slight defects in swarming. This agreed with the previous reports that the srf gene cluster is involved in swarming motility.
Figure 3.
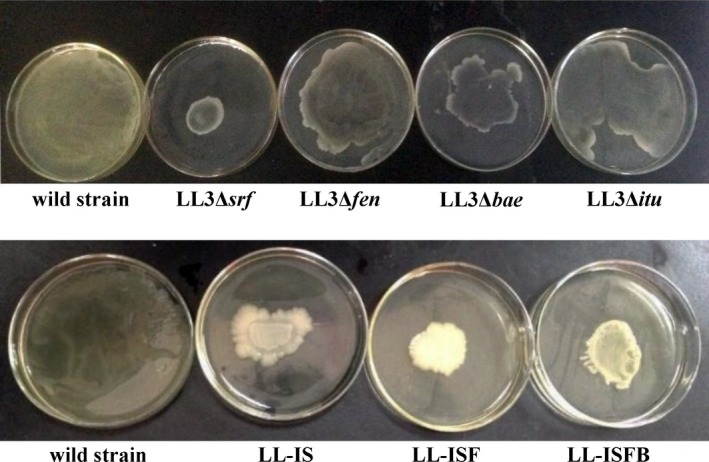
Swarming experiments of the wild‐type strain, B. amyloliquefaciens LL3Δsrf, LL3Δfen, LL3Δbae, LL3Δitu, LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔituΔsrfΔfenΔbae). Strains were observed after 24‐hr cultivation on LB medium with 0.5% agar
All the multiple‐gene mutants, B. amyloliquefaciens LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔituΔsrfΔfenΔbae), had significant defects in swarming motility (Fig. 3). LL3Δitu had a slight defect in swarming; however, when srf was deleted to construct LL‐IS (ΔituΔsrf), its swarming motility was significantly weakened, which further proved surfactin's effect on swarming.
Rahman et al. (2007)reported that the biofilm formation of transformant B. subtilis RM/iSd16 containing wild sfp, itu operon, and degQ was better than the wild‐type strain. Zeriouh, de Vicente, Perez‐Garcia, and Romero (2014) found that surfactin triggered biofilm formation of B. subtilis. In this study, the biofilm formation ability of all the mutant strains was compared with that of the wild‐type strain. B. amyloliquefaciens LL3Δbae and LL3Δfen showed similar biofilm formation compared with the wild‐type strain (Fig. 4). B. amyloliquefaciens LL3Δsrf, LL3Δitu, LL‐IS, LL‐ISF, and LL‐ISFB were significantly defective in biofilm formation (Fig. 4). This could be inferred that iturin A and surfactin play important roles in biofilm formation.
Figure 4.
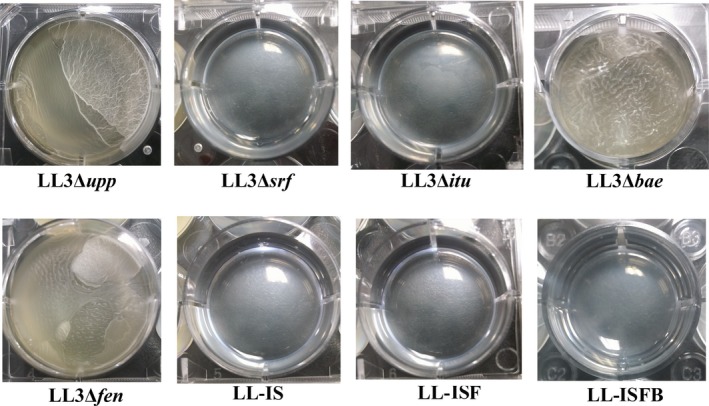
Biofilm formation of the wild‐type strain, B. amyloliquefaciens LL3Δsrf, LL3Δitu, LL3Δbae, LL3Δfen, LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔituΔsrfΔfenΔbae)
3.3. DCW, γ‐PGA synthesis and culture viscosity of the mutant strains
B. amyloliquefaciens LL3Δbae, LL3Δsrf, LL3Δitu, LL3Δfen, LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔituΔsrfΔfenΔbae) were compared with the wild‐type strain for culture viscosity, DCW, and γ‐PGA synthesis. At the end of the fermentation, surprisingly, the culture viscosity of the Δsrf, Δitu, and Δfen mutants was decreased by 46%, 20.5%, and 29%, respectively, while the Δbae mutant showed no apparent changes compared with the wild‐type strain (Fig. 5A). The DCW of the Δfen mutant experienced a slight decrease, while that of the other three mutants resembled the wild‐type strain. γ‐PGA synthesis of the Δfen, Δitu, or Δbae mutants showed no obvious changes; however, in the Δsrf mutant, the synthesis of γ‐PGA showed a slight increase (4.1 g/L) compared with that in the wild‐type strain (3.3 g/L).
Figure 5.
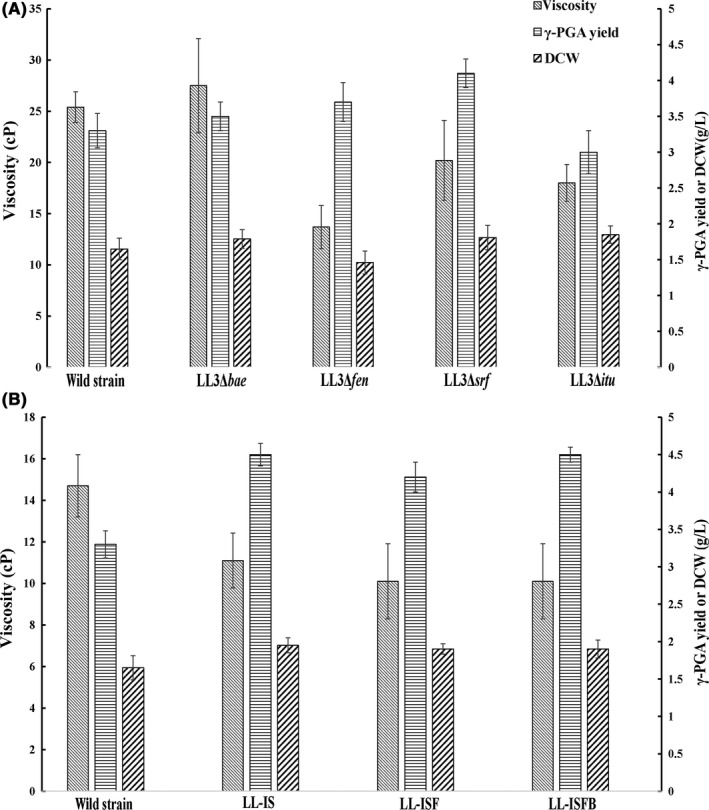
(A) Comparison of culture viscosity, DCW, and γ‐PGA yield of the wild‐type strain and the mutant strains carrying single‐gene deletion (B. amyloliquefaciens LL3Δsrf, LL3Δbae, LL3Δfen, and LL3Δitu) after 48 hr of cultivation; (B) Comparison of culture viscosity, DCW, and γ‐PGA yield of the wild‐type and mutant strains carrying multiple deletions (B. amyloliquefaciens LL‐IS, LL‐ISF, and LL‐ISFB) after 48 hr of cultivation
As shown in Figure 5B, culture viscosities of LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔbaeΔsrfΔituΔfen) were decreased by 24.6%, 31%, and 31%, respectively. The DCW of LL‐IS (ΔituΔsrf), LL‐ISF (ΔituΔsrfΔfen), and LL‐ISFB (ΔbaeΔsrfΔituΔfen) were increased by 18%, 15%, and 15%, respectively. The γ‐PGA synthesis of LL‐IS (ΔituΔsrf) increased by 36.4%, leading to a yield of 4.5 g/L, compared with 3.3 g/L in the wild‐type control, while LL‐ISF(ΔituΔsrfΔfen) showed a slight decrease compared with LL‐IS (ΔituΔsrf) and the γ‐PGA yield of LL‐ISFB (ΔbaeΔsrfΔituΔfen) resembled that of LL‐IS (ΔituΔsrf).
4. Discussion
γ‐PGA‐producing strains are generally divided into two groups according to their nutritional requirements: glutamic acid‐dependent bacteria and glutamic acid‐independent bacteria. The latter does not require additional l‐glutamate in the medium to stimulate γ‐PGA, so that their production costs are lower than the former.
B. amyloliquefaciens LL3 is a naturally isolated, Gram‐positive strain that can produce γ‐PGA without the addition of glutamic acid in the medium. It secretes various antibiotic substances to adapt to the environment, such as surfactin, iturin A, fengycin, and bacillaene. Except for bacillaene, all of them are lipopeptides. Many reports have shown that the biological control exerted by B. subtilis and related species could be attributed to nonribosomally produced cyclic lipopeptides (Ongena & Jacques, 2008; Pérez‐García, Romero, & de Vicente, 2011; Romero, de Vicente, Rakotoaly, Dufour, Veeing, Arrebola, 2007; Zeriouh et al., 2011). Lipopeptides interact with the biological membranes of microbial pathogens, inducing cell leakage and death (Romero, de Vicente, Olmos, Davila, & Pérez‐García, 2007; Zeriouh et al., 2011).
In B. amyloliquefaciens LL3, the gene cluster encoding surfactin consists of srfA, srfB, srfC, srfD, and sfp, which encode surfactin synthetase A, B, C, D and surfactin kinase, respectively. The iturin A‐encoding cluster contains ituA, ituB, ituC, and ituD. The fengycin‐encoding gene cluster comprises fenD and fenE. Bacillaene is a polyketone, which is encoded by a gene cluster comprising baeB, baeC, baeD, baeE, baeG, baeH, baeI, baeJ, baeL, baeM, baeR, and baeS. The total lengths of the four gene clusters are 28.3, 37.2, 11.5, and 72.5 kb, respectively (Fig. S1). They were all predicted as nonessential using a comparative genomics approach and comparing the B. amyloliquefaciens LL3 genome with the B. subtilis 168 genome (Database of Essential Genes, DEG, http://tubic.tju.edu.cn/deg/). The four antibiotic substances are all nonribosomally produced, like γ‐PGA. Therefore, they may share similar synthesis machinery and compete for substrates with γ‐PGA. Interestingly, a transcriptional comparison between B. amyloliquefaciens LL3 (γ‐PGA+) and LL3 ΔpgsBCA (γ‐PGA−) using RNA‐seq agreed with the above speculation (unpublished). Transcriptional levels of bae, srf, itu, and fen clusters are greatly enhanced in B. amyloliquefaciens LL3 ΔpgsBCA. Based on these results, it was decided to knock out the four gene clusters to improve γ‐PGA yield and purity.
Biofilm formation and swarming are typical characteristics of the Bacillus genus. However, few reports took B. amyloliquefaciens as object of study. At first, we detected the effects of antibiotic substances encoding gene clusters disruption on the biofilm formation and swarming ability in B. amyloliquefaciens. Ghelardi et al. (2012) showed that both SwrA and surfactin upregulate the transcription of the flagellin gene and increase bacterial swimming ability in B. subtilis. In this study, B. amyloliquefaciens LL3Δsrf and LL3Δbae showed obvious defects in swarming motility in the “swarm plates,” which correlates with previous reports that the srf gene cluster is involved in swarming motility (Kearns et al. 2004). Besides, LL3Δsrf and LL3Δitu were significantly defective in biofilm formation. This could be inferred that iturin A and surfactin play important roles in biofilm formation, which was in line with the previous study (Ghelardi et al., 2012; Luo et al. 2015).
Principally, we focused on the effects of gene clusters disruption on γ‐PGA production. Results showed that B. amyloliquefaciens LL3Δsrf was the only single‐deletion mutant strain that showed an increase in γ‐PGA production (by 24.2%) compared with the wild‐type strain. Many reports have discovered that the presence of Mn2+, a cofactor for glutamine synthetase (GS), in the medium can improve the yield of surfactin during fermentation of B. subtilis (Abdel‐Mawgoud, Aboulwafa, & Hassouna, 2008; Huang, Liu, Wang, Liu, & Lu, 2015). Glutamine synthetase is an enzyme that catalyzes L‐glutamate to glutamine and plays important roles in glutamate consumption. This is good evidence that surfactin shares same substrates with γ‐PGA. Besides, as mentioned above, surfactin upregulates the transcription of the flagellin gene (Ghelardi et al., 2012). Chan, Guttenplan, and Kearns (2014) found that defects in the flagellar motor increase synthesis of poly‐γ‐Glutamate in B. subtilis. Therefore, srf mutant may also enhance γ‐PGA synthesis indirectly.
However, B. amyloliquefaciens LL3Δbae, LL3Δfen, and LL3Δitu did not show significant increases in γ‐PGA yield. For further explanation, HPLC‐MS was used to detect whether all the strains could produce the antibiotic substances or not. As shown in Figure 6, B. amyloliquefaciens LL3Δupp could synthesize surfactin, while LL3Δsrf cannot produce surfactin anymore. It may be the main reason for the increase of γ‐PGA synthesis that surfactin competes for same substrates with γ‐PGA. In addition, iturin A and fengycin were undiscovered in the culture of LL3Δupp (Fig. S3). This can explain why LL3Δitu and LL3Δfen showed no increases in γ‐PGA synthesis.
Figure 6.
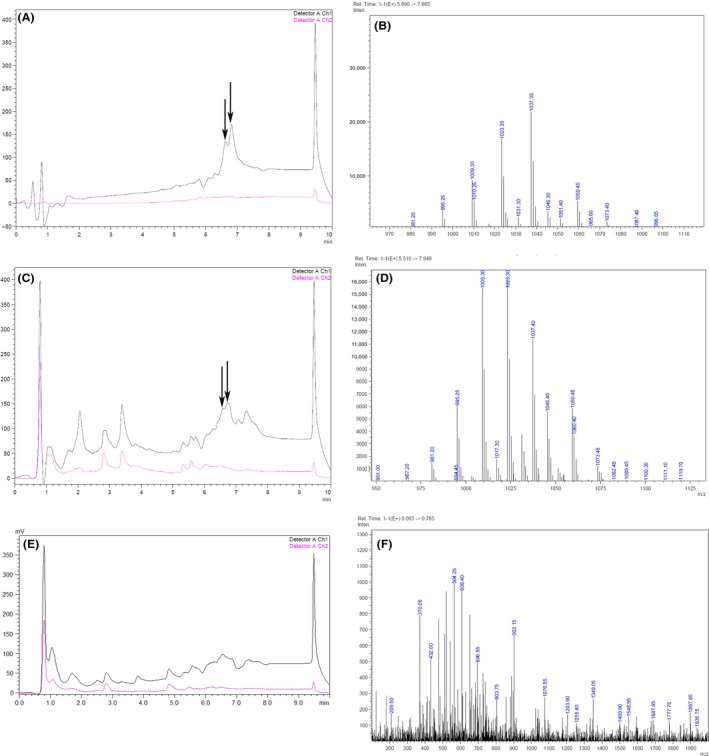
HPLC‐MS spectrograms of standard surfactin and surfactin produced by the wild strain and LL3Δsrf. (A, B) HPLC and MS spectrograms of standard surfactin. (C, D) HPLC and MS spectrograms of surfactin from the wild strain. (E, F) HPLC and MS spectrograms of the LL3Δsrf culture broth, which was disrupted in srf cluster and deficient in production of surfactin
It was further examined whether the disruption of the four gene clusters affected the expression of pgs operon. As shown in Figure S2, the pgsB expression levels of these mutant strains were comparable; thus, it can be presumed that the γ‐PGA synthesis changes in mutant strains are not likely related to the pgs operon expression level.
LL‐IS showed slight increase in γ‐PGA titer compared with LL3Δsrf although we did not discover iturin A in the culture of the wild strain. However, as mentioned above, LL3Δitu was significantly defective in biofilm formation. It is speculated that LL3Δupp might synthesize other iturin derivatives, which may also contributes to biofilm formation. Accumulation of multiple‐gene cluster deletions in one strain, LL‐ISF and LL‐ISFB did not give rise to a continuous increase in γ‐PGA yield. This may be attributed to that the wild strain might not produce fengycin or bacillaene and that the secondary metabolites might act not only as antibiotic substances but also as signal molecules affecting various cellular activities.
We previously reported that Vitreoscilla hemoglobin (VHb) alleviated the oxygen limitation leading to increased γ‐PGA production. Being too viscous to stir is an important factor of oxygen limitation during γ‐PGA fermentation. So we detected the culture viscosities of all the strains and found that culture viscosities of LL‐IS, LL‐ISF, and LL‐ISFB decreased by 24.6%, 31%, and 31%, respectively, compared with the wild‐type strain. This may be related to surfactin and iturin playing important roles in biofilm formation. Lower viscosity is very beneficial for industrial production.
Unfortunately, the four gene clusters are very large such that it would be difficult to construct the corresponding complementary strains to confirm the four products’ roles in cell motility, biofilm formation, and γ‐PGA synthesis, although our results are mainly in accordance with the previous reports.
In summary, this study attempted to uncover the effects of deletions of four gene clusters encoding antibiotic substances on γ‐PGA synthesis. Their effects on swarming and biofilm formation in B. amyloliquefaciens LL3 were also studied. The γ‐PGA yield of LL‐IS (ΔituΔsrf) (4.5 g/L) increased by 36.4% compared with the wild‐type strain (3.3 g/L), and the culture viscosity decreased by 24.6%, which is favorable for industrial production.
Funding Information
This work was supported by the National Key Basic Research Program of China (“973″ ‐Program) (2012CB725204), the National Key Technology Support Program (2015BAD16B04), the Natural Science Foundation of China (grant nos. 31470213 and 31170030), and the Project of Tianjin, China (13JCZDJC27800, 14ZCZDSF00009, and 15ZCZDNC00450).
Conflict of Interest
None declared.
Supporting information
Acknowledgments
This work was supported by the National Key Basic Research Program of China (“973″ ‐Program) (2012CB725204), the National Key Technology Support Program (2015BAD16B04), the Natural Science Foundation of China (grant nos. 31470213 and 31170030), and the Project of Tianjin, China (13JCZDJC27800, 14ZCZDSF00009, and 15ZCZDNC00450).
Gao, W. , Liu, F. , Zhang, W. , Quan, Y. , Dang, Y. , Feng, J. , Gu, Y. , Wang, S. , Song, C. and Yang, C. Mutations in genes encoding antibiotic substances increase the synthesis of poly‐γ‐glutamic acid in Bacillus amyloliquefaciens LL3. MicrobiologyOpen. 2017;6: e00398. https://doi.org/10.1002/mbo3.398
Contributor Information
Cunjiang Song, Email: songcj@nankai.edu.cn.
Chao Yang, Email: yang_chao2008@hotmail.com.
References
- Abdel‐Mawgoud, A. M. , Aboulwafa, M. M. , & Hassouna, N. A. H. (2008). Optimization of surfactin production by Bacillus subtilis isolate BS5. Applied Biochemistry and Biotechnology, 150, 305–325. [DOI] [PubMed] [Google Scholar]
- Cao, M. F. , Geng, W. T. , Liu, L. , Song, C. J. , Xie, H. , Guo, W. B. , … Wang, S. F. (2011). Glutamic acid independent production of poly‐γ‐glutamic acid by LL3 and cloning of pgsBCA genes. Bioresource Technology, 102, 4251–4257. [DOI] [PubMed] [Google Scholar]
- Chan, J. M. , Guttenplan, B. S. , & Kearns, B. D. (2014). Defects in the flagellar motor increase synthesis of poly‐γ‐Glutamate in Bacillus subtilis . Journal of Bacteriology, 196, 740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. H. , Koumoutsi, A. , & Scholz, R. (2009). More than anticipated ‐ production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. Journal of Molecular Microbiology and Biotechnology, 16, 14–24. [DOI] [PubMed] [Google Scholar]
- Chen, X. H. , Koumoutsia, A. , Scholza, R. , Schneiderb, K. , Vaterb, J. , Süssmuthb, R. , … Borrissa, R. (2009). Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. Journal of Biotechnology, 140, 27–37. [DOI] [PubMed] [Google Scholar]
- Feng, J. , Gao, W. X. , Gu, Y. Y. , Zhang, W. , Cao, M. F. , Song, C. J. , … Wang, S. F. (2014). a) Functions of poly‐gamma‐glutamic acid (γ‐PGA) degradation genes in γ‐PGA synthesis and cell morphology maintenance. Applied Microbiology and Biotechnology, 98, 6397–6407. [DOI] [PubMed] [Google Scholar]
- Feng, J. , Gu, Y. Y. , Quan, Y. F. , Cao, M. F. , Gao, W. X. , Zhang, W. , … Song, C. J. (2015). Improved poly‐γ‐glutamic acid production in Bacillus amyloliquefaciens by modular pathway engineering. Metabolic Engineering, 32, 106–115. [DOI] [PubMed] [Google Scholar]
- Feng, J. , Gu, Y. Y. , Sun, Y. , Han, L. F. , Yang, C. , Zhang, W. , … Wang, S. F. (2014). Metabolic engineering of Bacillus amyloliquefaciens for poly‐gamma‐glutamic acid (γ‐PGA) overproduction. Microbial Biotechnology, 7, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, W.T ., Cao, M.F ., Song, C.J ., Xie, H ., Liu, L ., Yang, C .,… Wang, S.F . (2011). Complete genome sequence of Bacillus amyloliquefaciens LL3, which exhibits glutamic acid‐independent production of poly‐γ‐glutamic acid. J Bacteriol 193: 3393–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardi, E. , Salvetti, S. , Ceragioli, M. , Gueye, S. A. , Celandroni, F. , & Senesib, S. (2012). Contribution of surfactin and SwrA to flagellin expression, swimming, and surface motility in Bacillus subtilis[J]. Applied and Environment Microbiology, 78, 6540–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X. F. , Liu, J. N. , Wang, Y. H. , Liu, J. , & Lu, L. J. (2015). The positive effects of Mn2+on nitrogen use and surfactin production by Bacillus subtilis ATCC 21332. Biotechnology and Biotechnological Equipment, 29, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns, D. B ., Chu, F ., Rudner, R . & Losick, R . (2004). Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol 52,357–369. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Ma, X. , Wang, Y. , Liu, F. , Qia, J. Q. , Li, X. Z. , … Zhou, T. (2011). Depressed biofilm production in Bacillus amyloliquefaciens C06 causes γ‐poly glutamic acid (γ‐PGA) overproduction. Current Microbiology, 62, 235–241. [DOI] [PubMed] [Google Scholar]
- Luo, C. P. , Liu, X. H. , Zhou, H. F. , Wang, X. Y. , & Chen, Z. Y. (2015). Nonribosomal peptide synthase gene clusters for lipopeptide biosynthesis in Bacillus subtilis 916 and their phenotypic functions. Applied and Environment Microbiology, 81, 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongena, M. , & Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends in Microbiology, 16, 115–125. [DOI] [PubMed] [Google Scholar]
- Pérez‐García, A. , Romero, D. , & de Vicente, A. (2011). Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Current Opinion in Biotechnology, 22, 187–193. [DOI] [PubMed] [Google Scholar]
- Rahman, M.S ., Ano, T ., Shoda, M . (2007). Biofilm fermentation of iturin A by a recombinant strain of Bacillus subtilis 168. J Biotechnol, 127(3), 503–7 [DOI] [PubMed] [Google Scholar]
- Richard, A. , & Margaritis, A. (2003). Rheology, oxygen transfer, and molecular weight characteristics of poly‐(glutamic acid) fermentation by Bacillus subtilis . Biotechnology and Bioengineering, 82, 299–305. [DOI] [PubMed] [Google Scholar]
- Romero, D. , de Vicente, A. , Olmos, J. L. , Davila, J. C. , & Pérez‐García, A. (2007). Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaerafusca . Journal of Applied Microbiology, 103, 969–976. [DOI] [PubMed] [Google Scholar]
- Romero, D. , de Vicente, A. , Rakotoaly, R. H. , Dufour, S. E. , Veening, J. W. , Arrebola, E. ,…Pérez‐García A. (2007). The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca . Molecular Plant‐Microbe Interactions, 20, 430–440. [DOI] [PubMed] [Google Scholar]
- Shih, I. L. , & Van, Y. T. (2001). The production of poly‐(γ‐glutamic acid) from microorganisms and its various applications. Bioresource Technology, 79, 207–225. [DOI] [PubMed] [Google Scholar]
- Su, Y. S. , Li, X. , Liu, Q. Z. , Hou, Z. W. , Zhu, X. Q. , Guo, X. P. , & Ling, P. X. (2010). Improved poly‐γ‐glutamic acid production by chromosomal integration of the Vitreoscilla hemoglobin gene (vgb) in Bacillus subtilis . Bioresource Technology, 101, 4733–4736. [DOI] [PubMed] [Google Scholar]
- Sung, M. H. , Park, C. , Kim, C. J. , Poo, H. , Soda, K. , & Ashiuchi, M. (2005). Natural and edible biopolymer poly‐γ‐glutamic acid: synthesis, production, and applications. Chemical Record, 5, 352–366. [DOI] [PubMed] [Google Scholar]
- Susanne, M. , Sarah, N. , Christopher, H. , Paul, D. S. , Daniel, B. K. , & John, R. K. (2014). Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus . Applied and Environment Microbiology, 80, 5603–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. M. , Wu, H. J. , Chen, L. N. , Yu, X. F. , Borriss, F. , & Gao, X. W. (2015). Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Scientific Reports, 5, 12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, C. M. , Wang, J. P. , Lo, S. C. , Chan, W. C. , & Lin, M. Y. (2010). Chromosomal integration of a synthetic expression control sequence achieves poly‐γ‐glutamate production in a Bacillus subtilis strain. Biotechnology progress, 24, 1001–1007. [DOI] [PubMed] [Google Scholar]
- Zeriouh, H. , de Vicente, A. , Perez‐Garcia, A. , & Romero, D. (2014). Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environmental Microbiology, 16, 2196–2211. [DOI] [PubMed] [Google Scholar]
- Zeriouh, H. , Romero, D. , Garcia‐Gutierrez, L. , Cazorla, F. M. , de Vicente, A. , & Pérez‐García, A. (2011). The iturin‐like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Molecular Plant‐Microbe Interactions, 24, 1540–1552. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Gao, W. X. , Feng, J. , Zhang, C. , He, Y. L. , Cao, M. F. , … Wang, S. F. (2014). A markerless gene replacement method for B. amyloliquefaciens LL3 and its use in genome reduction and improvement of poly‐γ‐glutamic acid production. Applied Microbiology and Biotechnology, 98, 8963–8973. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Xie, H. , He, Y. L. , Feng, J. , Gao, W. X. , Gu, Y. Y. , … Song, C. J. (2013). Chromosome integration of the Vitreoscilla hemoglobin gene (vgb) mediated by temperature‐sensitive plasmid enhances γ‐PGA production in Bacillus amyloliquefaciens . FEMS Microbiology Letters, 343, 127–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


