1. Introduction
Fatty acids are major constituents of cellular membranes acting as a substrate reservoir for the formation of bioactive lipid metabolites and influencing the environment of membrane proteins (Tokuyama and Nakamoto, 2011). Many metabolites are formed by enzymatic metabolism in what is known as the arachidonic acid cascade. Arachidonic acid (ARA) is a long chain omega-6 polyunsaturated fatty acid (PUFA) and a substrate of cyclooxygenase and lipoxygenase enzymes. These enzymes form prostaglandins and leukotrienes respectively, the majority of which have proinflammatory activity (Funk, 2001; Haeggstrom and Funk, 2011). Cytochrome P450 (CYP450) monoepoxygenase metabolism of PUFAs is a third, less studied pathway of the ARA cascade (Capdevila et al., 1992). CYP450 activity forms regioisomers of epoxy fatty acids (EpFAs) from the parent PUFAs (Spector, 2009). However, EpFAs are quickly transformed by the soluble epoxide hydrolase (sEH) enzyme which metabolizes them to their corresponding vicinal diols (Spector et al., 2004). The EpFA metabolites of ARA, epoxyeicosatrienoic acids (EETs), are antihyperalgesic in acute inflammatory pain and chronic neuropathy models (Inceoglu et al., 2006; Inceoglu et al., 2008; Inceoglu et al., 2012). The diol products of EETs metabolism, dihydroxyeicosatrienoic acids (DiHETs), lack this activity in numerous in vivo models (Zeldin, 2001). Small molecule inhibitors of the sEH enzyme (sEHI) have been developed and optimized for in vivo administration. The sEHI block the metabolism of EpFA into diols and stabilize the concentrations of these beneficial bioactive mediators (Inceoglu et al., 2012). The cascade was named for ARA metabolism but it is now clear that other PUFA including omega-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are also substrates for these enzymes (Schmitz and Ecker, 2008). DHA and EPA are metabolized via CYPP450s into multiple regioisomers of epoxydocosapentaenoic acids (EDPs) and eicosatetraenoic acids (EEQs), respectively, which are further metabolized by sEH into their corresponding diols (Fer et al., 2008; Konkel and Schunck, 2011; Morisseau et al., 2010).
DHA is the predominant fatty acid in the central nervous system (CNS) with concentrations reaching 20–50% of fatty acids in the brain (Burdge, 2004). DHA is essential to CNS development and normal brain functioning in adults (Uauy and Mena, 2001). DHA also effects nociceptive signaling by lowering pain in inflammatory conditions including rheumatoid arthritis (Kremer, 2000) and inflammatory bowel disease (Belluzzi et al., 2000). Additionally, DHA blocked acetic acid writhing and formalin induced pain in murine models (Nakamoto et al., 2010; Nobre et al., 2013). A recent study suggests DHA indirectly affects opioid signaling via modulating the release of endogenous opioid peptides (Nakamoto et al., 2011). CYPP450 metabolites have also demonstrated activity via opioid system mechanisms (Conroy et al., 2010; Conroy et al., 2013). Importantly, the EpFA of DHA and EPA were ≥100 fold more potent than their parent PUFAs suggesting the antihyperalgesia of the PUFAs is likely due to their epoxide metabolites (Morisseau et al., 2010). Here, we tested our primary hypothesis that epoxidized metabolites are mediating analgesia in neuropathic pain models and a secondary hypothesis that this action involves but is not restricted to opioid system signaling.
2. Materials and Methods
2.1 Animals
All procedures and animal care included in these experiments adhered to the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and were performed in accordance with protocols approved by the Animal Use and Care Committee (IACUC) of the University of California, Davis. Great care was taken to minimize suffering of the animals and to reduce the number of animals used. Experiments on sEH knockout mice used mice on a 129X1/SvJ C57BL/6 background, backcrossed over ten generations with targeted disruption of the EPHX2 gene and maintained at the facilities of the University of California, Davis (Sinal et al., 2000). The EPHX2 strain of mice was maintained as heterozygous animals with knockout and control animals obtained from a backcross followed by genotyping. All other experiments on wild type mice used groups of male C57BL/6 mice (20–22 grams) purchased from Charles River Laboratories. Both wild type and sEH null mice were housed under standard conditions (25°C) in a fixed 12-h light/dark cycle with ad libitum food and water.
2.2 Chemicals
The sEH inhibitor t-TUCB: trans-4-[4-(3-trifluoromethoxyphenyl-l-ureido)-cyclohexyloxy]-benzoic acid (also referred to as UC1728) and TPPU: 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (UC1770) were synthesized in house and characterized as previously described (Hwang et al., 2007; Liu et al., 2009; Rose et al., 2010). t-TUCB and TPPU were prepared immediately before use in PEG400 and subcutaneously administered to mice in a 50 µl volume. The EDP mixture was a regioisomeric mixture of the methyl esters of epoxidized DHA synthesized in house per previously described methods (Morisseau et al., 2010). The EDP mixture was formulated in 7% ethyl alcohol in miglyol and injected subcutaneously and the ratio of regioisomers given in the Supplemental Material (Fig. S1) was analyzed per previously published methods (Yang et al., 2009). Celecoxib was formulated in PEG400 and naloxone (Sigma-Aldrich) was formulated in saline and both injected subcutaneously in a 50 µl volume.
2.4 Behavioral Assays
All groups of mice were allowed to acclimate for a minimum of 3 days and then were assessed for their naïve mechanical threshold baseline scores. Streptozocin treatment with 150 mg/kg i.p. streptozocin (Davidson et al., 2009) results in robust hyperglycemia in mice. Several overt phenotypic phenomena of streptozocin induced diabetes were observed in the mice but not quantified including polyuria, poor grooming and ataxia. All included groups of mice were assessed phenotypically and for allodynia with an electronic aesthesiometer in the von Frey assay. Allodynia is the sensing of pain from innocuous stimuli that is a hallmark of diabetic neuropathy and is assessed in in the model with grams of force to elicit a hind paw withdrawal. The diabetic state was then further confirmed with blood glucose monitoring. The glucose levels averaged from 378 mg/dl among included groups, however all included mice had levels >250 mg/dl and were considered diabetic. The sEH knockout mice become equally hyperglycemic with average blood glucose of >270 mg/dl. After the induction of diabetes all groups were assessed for their diabetic mechanical withdrawal thresholds. Allodynia was indicated by a > 30% decrease in withdrawal compared to pre-diabetic baseline values and was assessed and quantified in the mice using an electronic von Frey aesthesiometer (IITC, Woodland Hills, CA). For the von Frey assay mice were first acclimated to the clear acrylic chambers on a steel mesh floor. Then the mouse hind paw was probed through the mesh with a rigid tip probe to measure the grams of force required to elicit a hind paw withdrawal. Mechanical withdrawal thresholds (MWT) were measured a minimum of 3 times per mouse in all groups and calculated as the mean ± S.E.M. for a group of mice tested on the same day under the same conditions. The mean MWT for groups are reported as pre-streptozocin baseline scores (Naive) and post streptozocin painful baseline scores (Diabetic) prior to the start of treatment in the CPP in the figures. MWTs for the mice averaged post-diabetes averaged a 45% decline in threshold baseline indicating allodynia. Similar results were observed for the sEH null mice groups with decreases in MWT (51% decline).
The conditioned place preference (CPP) assay used an apparatus as previously described (Wagner et al., 2014b). Briefly, the apparatus is a 30×16×20 cm rectangular acrylic box with distinct visual patterns and a floor with tactilely distinct sides of equal size. The CPP assay was conducted 10 days post streptozocin administration and after diabetic baseline allodynia was assessed with the von Frey assay. For the CPP, mice are habituated to the open box for 30 minute sessions at the same time of day on 2 consecutive days. Then on day 1 pre-conditioning preference is assessed by placing mice in the apparatus with access to both chambers and observing them for 30 minutes. This is followed by 3 conditioning days where the vehicle is counterbalanced daily with the compounds each for 30 minute intervals. For conditioning mice receive vehicle and are immediately isolated to one chamber in the morning. At least four hours after the vehicle, the same mouse is treated with sEH inhibitor or drug and immediately isolated to the counterbalanced chamber. On the next (5th) day, mice are tested drug-free for their preference with access to both chambers and observed for 30 minutes. The recorded test periods are quantified with tracking software and the results are calculated as test minus preconditioning time in the drug paired chamber. The results are represented in the figures as the change in time (Time (seconds), y axis) in the drug paired chamber. Increased time spent in a chamber (e.g. increased time in the drug-paired chamber) indicated preference for that chamber. The vehicle control group received PEG400 counterbalanced to the non-preferred chamber at least 4 hrs after saline injection and placement in the preferred chamber. Earlier tests revealed no difference in PEG400, PEG300 or saline injections compared to naïve mice.
2.5 Statistics
The nociceptive assays were evaluated with a one way analysis of variance or t-test where appropriate. ANOVA Post-hoc analysis included Holms-Sidak and Dunn’s analysis as indicated with p<0.05 considered statistically significant. Statistics were computed using commercial statistical software (Systat Software, Inc., Chicago IL).
3. Results
3.1 EDPs mediate analgesia in neuropathic pain
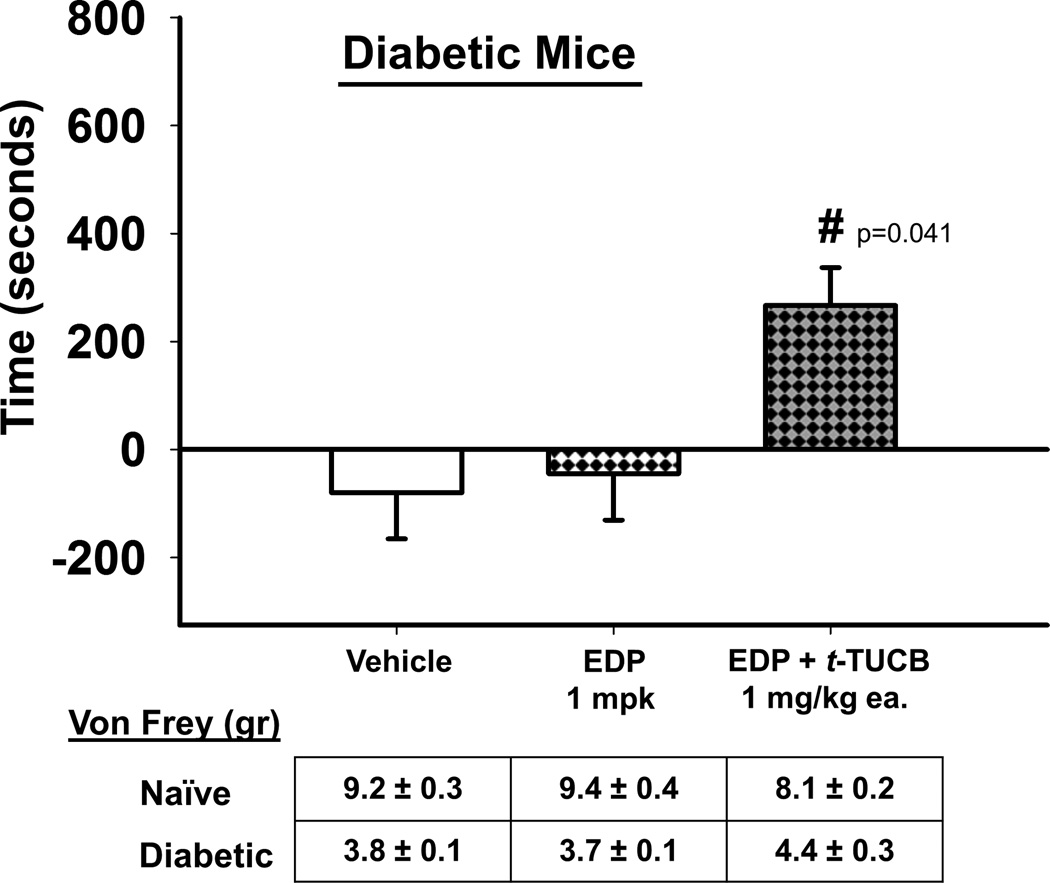
Given published reports that DHA can reduce pain (Nakamoto et al., 2011; Nakamoto et al., 2010) as well as published (Morisseau et al., 2010; Wagner et al., 2014a) and unpublished studies from this laboratory in other pain models we tested the hypothesis that this analgesic activity was due at least in part to the action of epoxidized DHA metabolites, the EDPs. For this we used an exogenous administration of a regioisomeric mixture of EDPs and assessed it for the ability to induce a CPP response in neuropathic mice (Fig. 1). The EDPs are rapidly metabolized in vivo and did not induce a CPP response as a single administration. Therefore we combined EDPs with a low dose of the t-TUCB which was previously determined to have no significant effect as a single administration in this system (Wagner et al., 2014b). The combination of exogenous EDPs with 1 mg/kg t-TUCB significantly induced a CPP response when compared to the EDP alone and vehicle (One Way ANOVA, Holms-Sidak method, p=0.041, n=4–7). Although the EDPs are rapidly transformed, the absence of CPP response induced by a low 1mg/kg dose of t-TUCB (Fig. S3A) when compared to the EDP + t-TUCB combination at the same dose suggests the EDPs are mediating the analgesia and importantly in a chronic pain model. The EDPs lacked significant effect in naïve mice at the same 1 mg/kg dose (Fig. S3B).
Figure 1.
EDPs, the omega-3 DHA derived epoxidized metabolites, mediate analgesia in a model of diabetic neuropathic pain. Exogenous EDPs 1 mg/kg combined with an ineffective low dose of t-TUCB 1 mg/kg to block their rapid degradation by the sEH enzyme were significantly effective against neuropathic pain (t-TUCB 1 mg/kg alone appears in supplemental material). In the CPP assay this is indicated by increased time (seconds, y axis) spent in the drug paired chamber (One Way ANOVA, p= 0.041). Prior to the CPP assay diabetic mice were assessed for phenotypic allodynia indicating diabetic neuropathy in the von Frey assay. The results depicted below the graph are the grams of force to elicit a hind paw withdrawal (von Frey (gr)) of pre-streptozocin baseline scores (Naïve) and post streptozocin painful baseline scores (Diabetic) prior to the start of treatment in the CPP assay.
3.2 Antagonism of the Mu-opioid receptor blocks EDP and sEHI mediated analgesia in the CPP assay
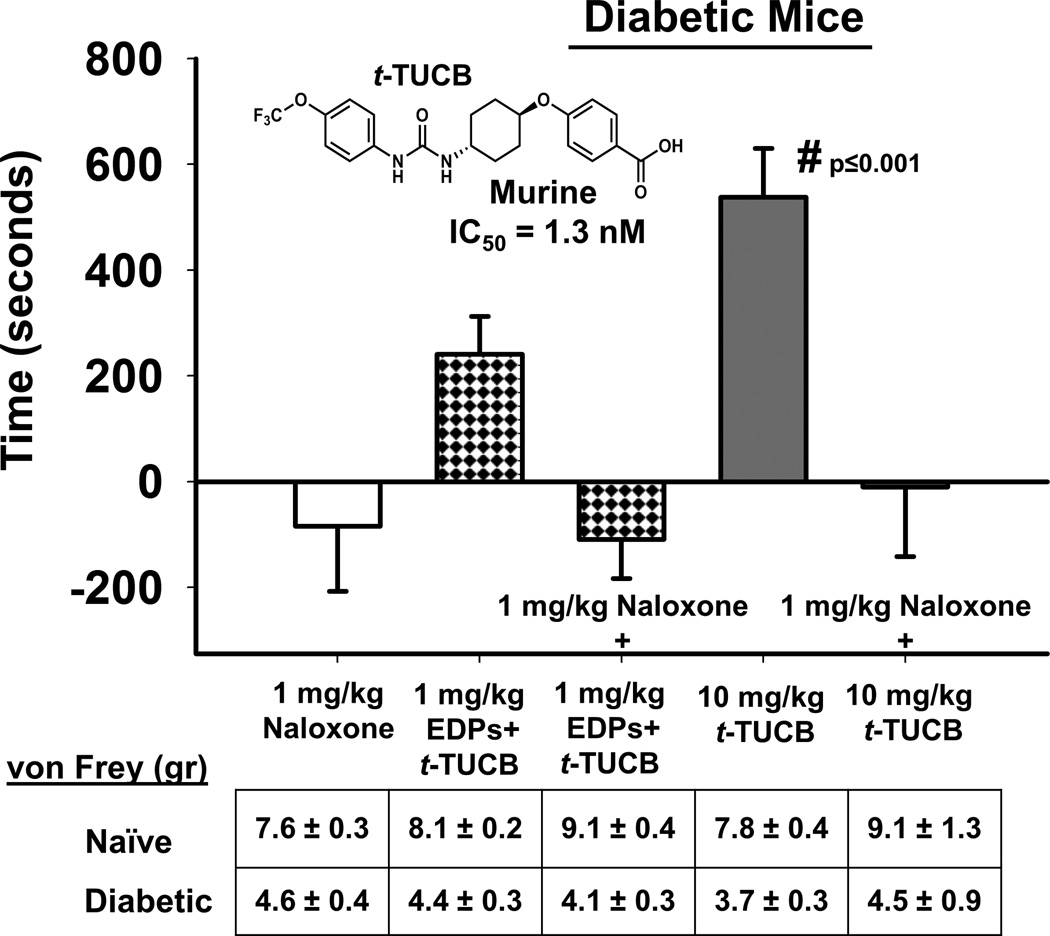
When the mu-opioid receptor antagonist naloxone was tested as a single administration in diabetic neuropathic mice there was no significant effect of the compound. The administration of EDP + t-TUCB (repeated from Fig. 1) produced a CPP response indicating pain relief. This was blocked by the co-administration of naloxone. To support this result we also tested t-TUCB in neuropathic mice, the sEHI at 10mg/kg was significantly analgesic against the induced diabetic neuropathy (Fig. 2). This robust analgesia was equally antagonized by co-administration of naloxone returning scores to control levels (One Way ANOVA, Holms-Sidak method, p=0.001, n=4–8).
Figure 2.
Analgesia mediated by EDPs and sEHI is blocked mu-opioid antagonism. The analgesia mediated by 1 mg/kg EDPs co-administered with low dose 1mg/kg t-TUCB was blocked by naloxone (1 mg/kg) a mu-opioid receptor antagonist. The sEH inhibitor t-TUCB blocks the degradation of multiple classes of EpFA in vivo and a single administration of 10 mg/kg dose effectively induced a CPP response indicating pain relief (One Way ANOVA, p≤0.001). In the CPP assay this is indicated by increased time (seconds, y axis) spent in the drug paired chamber. The efficacy of 10 mg/kg t-TUCB was also blocked by naloxone. There was no significant effect of the naloxone in control neuropathic mice. The diabetic mice were assessed for phenotypic allodynia indicating diabetic neuropathy in the von Frey assay prior to the CPP assay. The results depicted below the graph are the grams of force to elicit a hind paw withdrawal (von Frey (gr)) of pre-streptozocin baseline scores (Naïve) and post streptozocin painful baseline scores (Diabetic) prior to the start of treatment in the CPP assay.
3.3 sEH null mice with induced diabetic neuropathy do not respond to sEHI
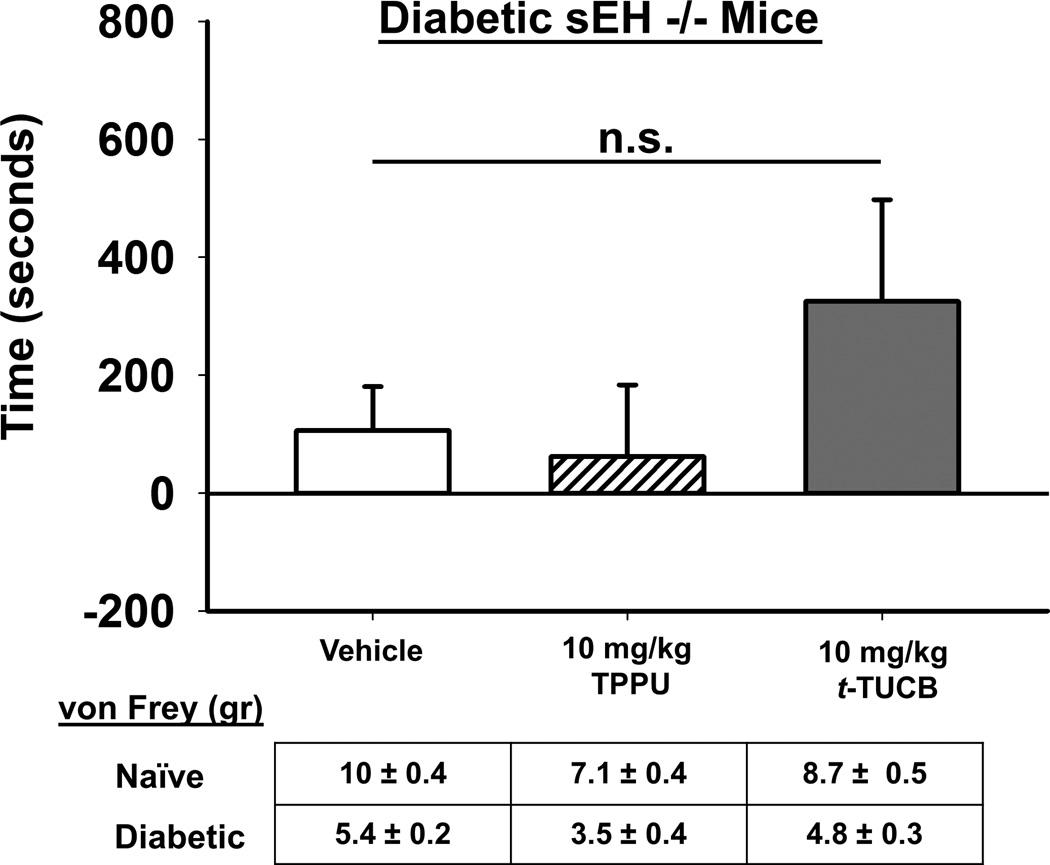
We then tested both t-TUCB and a structurally different sEHI that has the same pharmacophore TPPU in sEH null mice that were rendered diabetic with streptozocin similar to the wild type mice (Fig.3). The same dose of the t-TUCB that elicited a robust CPP in wild type neuropathic mice did not elicit a significant response in sEH null diabetic mice (One Way ANOVA, p=0.333, n=7–8). There was a positive trend with t-TUCB but this was not result in a statistically significant. The second sEHI TPPU was without any effect. The results of sEHI administration in the diabetic null mice supports both that the sEHI mediated analgesia occurs via targeting the sEH enzyme and the efficacy is not related to reward based mechanisms.
Figure 3.
Inhibitors of sEH do not significantly induce a CPP response in diabetic sEH null mice. In sEH null mice induced with diabetic neuropathy neither t-TUCB nor TPPU, a structurally distinct but similarly potent sEHI, have a significant effect in the CPP assay (One Way ANOVA, p=0.333). In the CPP assay this is measured by increased time (seconds, y axis) spent in the drug paired chamber. This is in contrast to the robust effect of t-TUCB in wild type diabetic mice (Fig 2) indicating that targeting the sEH enzyme mediates the sEHI efficacy. The diabetic mice were assessed for phenotypic allodynia indicating diabetic neuropathy in the von Frey assay prior to the CPP assay. The results depicted below the graph are the grams of force to elicit a hind paw withdrawal (von Frey (gr)) of pre-streptozocin baseline scores (Naïve) and post streptozocin painful baseline scores (Diabetic) prior to the start of treatment in the CPP assay.
3.4 sEHI mediate effective analgesia in neuropathic pain compared to coxibs
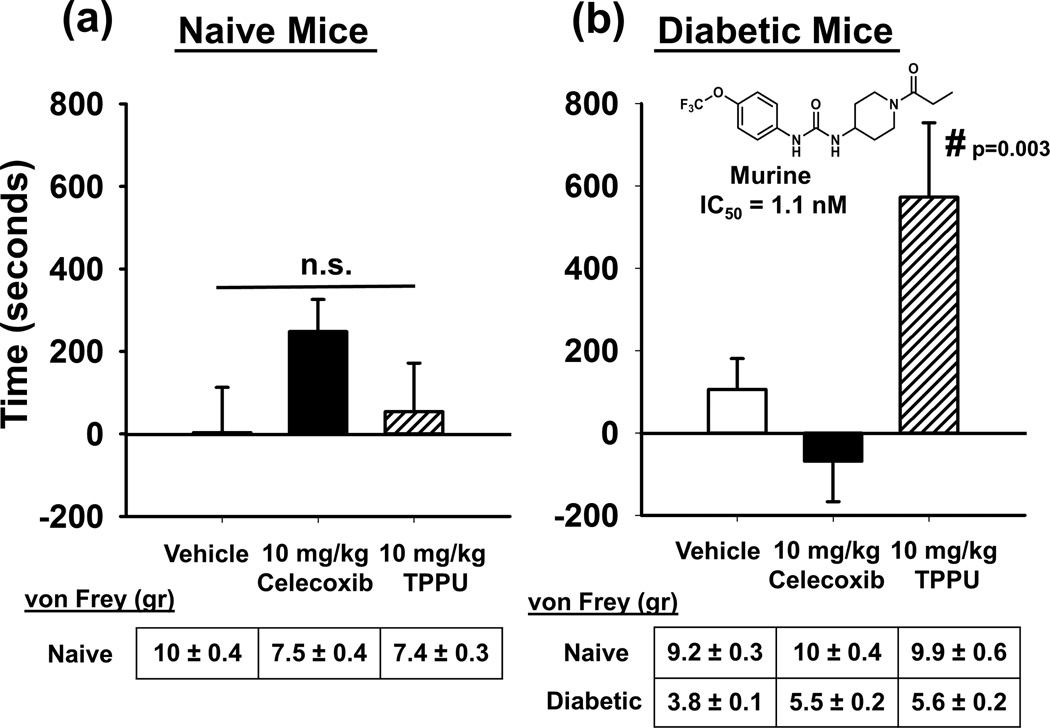
We used the structurally distinct sEHI TPPU in the same testing paradigm to interrogate the analgesia mediated by t-TUCB and to compare with celecoxib (Fig. 4). TPPU at the same 10 mg/kg dose used in diabetic sEH null mice had no effect in naïve mice (One Way ANOVA, p=0.132, n=6–8). Celecoxib at the same dose seemed to induce a CPP but the effects were not statistically significant. This corresponds to literature describing other drugs of this class inducing a CPP response (Fattore et al., 2000). The current results in diabetic mice support an often observed lack of efficacy of non-steroidal anti-inflammatory drugs on neuropathic pain in humans and animal models (Gore et al., 2007; Mendlik and Uritsky, 2015; Wagner et al., 2013). DHA has recently demonstrated effects in diabetic pain models often attributed to its antiinflammatory activity (Heng et al., 2015; Li et al., 2015). However, if chronic neuropathy were treated by antiinflammatory action alone, we would have expected celecoxib to have an antihyperalgesic effect which was not the case, although both the EDPs and the sEHI were efficacious against the neuropathic pain. However, when TPPU was tested in diabetic neuropathic mice there was a robust and significant effect indicating analgesia (One Way ANOVA, Holms-Sidak method, p=0.003, n=6–8). In earlier studies, TPPU in mice displayed favorable pharmacokinetics properties in mice although it took several days to reach a steady state concentration via drinking water at a much lower dose than used here (Ulu et al., 2012). The results obtained with these experiments using the CPP testing paradigm for both sEHI demonstrate the analgesia is due to targeting and inhibiting the sEH enzyme.
Figure 4.
The sEHI effectively mediates analgesia in neuropathic pain and outperforms celecoxib. (a) When the sEHI TPPU at 10mg/kg was tested in naïve mice it had no significant effect in the CPP assay. The same 10mg/kg dose of celecoxib seemed to induce a response but it was not significant (One Way ANOVA, p=0.132). In the CPP assay this is indicated by increased time (seconds, y axis) spent in the drug paired chamber. (b) When assessed in diabetic neuropathic mice the 10mg/kg TPPU showed a significant and robust response and celecoxib was without effect (One Way ANOVA, p=0.003). The diabetic mice were assessed for phenotypic allodynia indicating diabetic neuropathy in the von Frey assay. The results depicted below the graph (where applicable) are the grams of force to elicit a hind paw withdrawal (von Frey (gr)) of pre-streptozocin baseline scores (Naïve) and post streptozocin painful baseline scores (Diabetic) prior to the start of treatment in the CPP assay.
4. Discussion
With these experiments we tested several hypotheses regarding EpFA mediated analgesia against chronic pain. We used the CPP assay which is more able to assess the tonicity of chronic pain (Davoody et al., 2011; Felice et al., 2013) and therefore the results may be more translatable to human clinical conditions. The CPP is also operant and eliminates expectation bias (Navratilova and Porreca, 2014) and misinterpretation of motor skill decrement and sedation as analgesia as with evoked responses. Furthermore, it enables examination of rewarding effects (abuse potential) in control animals.
We first tested the hypothesis that EpFAs mediate analgesia and are responsible for the effects of parent PUFAs by administering EDPs to neuropathic mice. There is considerable evidence describing the health benefits and biological functions of DHA including antinociceptive effects. DHA attenuates loss of nerve conduction velocity and nerve blood flow in diabetic rats (Pitel et al., 2007), acetic acid writhing and formalin induced pain in mice (Nakamoto et al., 2010) and spinal cord injury pain in rats (Figueroa et al., 2013). Inhibiting sEH in vivo has been successful in reducing diabetic neuropathy in rats indicative of EpFA activity (Inceoglu et al., 2012; Wagner et al., 2013). The EpFAs also previously blocked allodynia in neuropathic mice demonstrating direct application of the metabolites is antinociceptive (Wagner et al., 2014b). Interestingly, EDPs appear to be a more efficacious class of EpFA in modeled inflammatory pain and most of the EDPs, with the exception of the 19,20 EDP regioisomer, are also preferred substrates of sEH (Morisseau et al., 2010). Here we demonstrated that EDPs are analgesic against the tonic pain of diabetic neuropathy. The low dose t-TUCB combined with the EDPs to block their transformation to diol metabolites was ineffective as a single administration (Fig. S3A) indicating EDPs mediate the analgesia. We tested several doses and routes of administration for EDPs or enriched DHA oil (Fig. S3B) and found stabilizing EDPs with sEHI the most effective approach. There was a more robust CPP response to the sEHI than the EDPs alone suggesting that EDPs are rapidly metabolized and absent sEHI stabilization have limited effect in this assay (Fig. S3B). The results indicate that sEH inhibition may be a better pharmaceutical strategy than EDPs because sEHI stabilize multiple classes of EpFAs all of which may contribute to the analgesic response.
Mu-opioid receptors (MOR) are the target of endogenous opioid peptides including beta-endorphin, Met- and Leu-enkephalin, and to a limited extent dynorphin (Pasternak and Pan, 2013). They are also the primary route of analgesia produced by opioids such as morphine. Recently, DHA mediated analgesia was reversed by MOR antagonists in the late stage formalin test in mouse (Nakamoto et al., 2010) and in diabetic neuropathy in rat (Heng et al., 2015; Nakamoto et al., 2010). However, DHA was found not to bind opioid receptors directly but to influence beta-endorphin release (Nakamoto et al., 2011). Given the lack of mechanistic knowledge regarding the antinociceptive activity of PUFAs it remains possible that EpFA are mediating these effects. Evidence supporting this hypothesis includes transgenic mice with neuron specific deletion of CYP450 reductase (CYP450r) which among many effects blocks EpFA formation. Mutant CYP450r mice responded normally to painful stimuli but had deficits in antinociceptive responses to morphine (Conroy et al., 2010). In the same study intracerebroventricular administration of CYP450 inhibitors blocked morphine analgesia. More recently, site specific MOR antagonism in wildtype versus CYP450r mutants revealed attenuation of pain responses in the deficient mice (Hough et al., 2015). Furthermore, sEH inhibition increases endogenous EpFAs and is antinociceptive (Inceoglu et al., 2012; Wagner et al., 2013). Based on this evidence we tested if MOR antagonism blocks analgesia produced by CYP450 derived EDPs or sEHI which stabilize them. The MOR antagonist naloxone had no significant effect in control mice though aversion has been observed by others (Sakoori and Murphy, 2008). However, naloxone blocked the analgesia from the EDP + t-TUCB combination and also the robust CPP response to 10 mg/kg t-TUCB (Fig. 2). Naloxone is not completely selective, but its action here does suggest there is an effect at least in part via opioid signaling. The value of opioids for chronic pain conditions is debated (Dellemijn, 1999). Despite this, opioids are still sometimes prescribed for chronic pain and it is more often safety issues that limit their use (Moulin et al., 2007). Given the analgesia mediated by the EDPs and sEHI and the antagonism by naloxone, it might be anticipated that that the sEHI elicit side effects similar to MOR agonists. Our experiments demonstrate EpFA are analgesic in the absence of rewarding side effects (abuse potential). The lack of addictive behavior with sEHI discussed below and the high efficacy of sEHI in neuropathic pain models suggests that sEHI are not acting simply as opioids. Additionally, experiments with CYP450r deficient mice demonstrated EpFA mediated analgesia lacked MOR agonist side effects of respiratory depression, constipation, and hyperlocomotion (Hough et al., 2014).
We explored the different CPP results between sEHI and EDPs by examining if the sEHI outcome was compound related or elicited narcotic side effects by testing a second potent sEHI in the model (Fig. 4A&B). TPPU 10mg/kg lacked effect in control mice but was robustly effective against neuropathic pain. We also tested both inhibitors in sEH null mice induced with diabetic neuropathy and neither sEHI elicited a significant effect (Fig. 3). A lack of rewarding effect was previously demonstrated in non-diabetic sEH null mice (Wagner et al., 2014b). The absence of reward of two structurally different sEHI and the absence of analgesic responses in neuropathic sEH null mice supports the previous observations that inhibiting the sEH target mediates the analgesia while lacking off-target side effects. The lack of reward is interesting given the pronounced analgesia sEHI elicit as well as its antagonism with naloxone. Notably, there is evidence of cyclooxygenase and lipoxygenase inhibitors modulating opioid signaling though neither have strong reward side effects (Vaughan et al., 1997). Thus, the action of CYP450 products is distinct from MOR agonists because both the analgesic and side effects of opioids result from binding the receptor (Kieffer, 2000).
There are alternative mechanisms of action for the analgesia mediated by EpFAs such as the descending modulation of pain. EpFAs in the ventrolateral periaqueductal gray (PAG) region modulate the analgesic actions of morphine and endogenous opioid peptides that act on the descending analgesic pathway (Conroy et al., 2010). There is GABA modulation by EpFA and sEHI (Inceoglu et al., 2013) and therefore possible GABA interaction with the descending modulation of pain. GABA interneurons in the PAG or raphe magnus modulate the enkephalin sensitive neurons in these areas which may be subject to opioid receptor antagonism (Garcia-Larrea and Peyron, 2007). Additional evidence points to CYP450 inhibitors active at a point of convergence for opioid and non-opioid analgesics downstream of opioid receptors (Heinricher et al., 2010). Super-perfusion of ARA to rat PAG decreased GABAergic postsynaptic currents (Vaughan et al., 1997). This effect was hypothesized to depend on ARA metabolites, in this case lipoxygenase derived, but CYP450 metabolites were not investigated. Interestingly, the ARA effect was potentiated by the cyclooxygenase inhibitor indomethacin which provides a possible explanation for the antihyperalgesic synergy of co-administration of sEHI with coxibs in vivo (Schmelzer et al., 2006).
The recent demonstration that EpFA modulate endoplasmic reticulum stress (ER stress) is another mechanism of antinociception in chronic pain states. ER stress and the unfolded protein response are regulatory mechanisms leading to apoptosis and cell death when overwhelmed. In the peripheral nervous system of diabetic rats sEHI lowered activated ER stress responses including decreasing cleaved activating transcription factor 6 (ATF6) expression and ATF4 mRNA in the sciatic nerves (Inceoglu et al., 2015). sEHI also blocked phosphorylation of the three major ER stress sensors PERK, eIF2α, and IRE1α. sEHI downregulated ATF4 and ATF6 mRNA in hepatic fibrosis (Harris et al., 2015) and deletion of sEH is correlated with decreased mitogen activated protein kinases p38 and c-jun NH2-terminal kinase (JNK) activation in acute pancreatitis (Bettaieb et al., 2014). JNK and p38 are stress activated and have roles in the generation of pain sensitivity (Ji et al., 2009; Purves et al., 2001). Given these several modes of action, more evidence is required to determine if modulating opioid signaling is a primary mechanism of EpFA mediated analgesia. However, it does suggest spinal/supraspinal modulation of nociception by EpFA in chronic pain states.
Interestingly, there was an apparent induced CPP with t-TUCB over TPPU in neuropathic sEH null mice. Recently we reported that t-TUCB is a far more potent inhibitor of fatty acid amide hydrolase (FAAH) than TPPU (Sasso et al., 2015). Nevertheless, t-TUCB had no effect when tested in non-diabetic sEH null mice (Wagner et al., 2014b) which concurs with evidence that the sEHI are not active in the absence of pain (Inceoglu et al., 2006; Wagner et al., 2013). Ultimately these results in diabetic mice (wild type and sEH null) support that the analgesia is dependent on EpFA and sEHI, unlike opioids, lack reward and are highly effective in neuropathic pain models.
Supplementary Material
Significance.
EDPs and sEHI mediate analgesia in modeled chronic pain and this analgesia is blocked by naloxone. However, unlike opioids, sEHI are highly effective in neuropathic pain models and importantly lack the rewarding effects of opioids.
Acknowledgments
Bo Wang developed and provided the tracking software used to measure the CPP assay results.
Funding Sources: This study was partially supported by grants from the National Institute of Health NIEHS Grant R01 ES002710, NIEHS Superfund Research Program P42 ES004699, NINDS U54 NS079202-01, a NIH/NIEHS 1K99ES024806-01 (to K.S.S.L.) and NIH 5T32DC008072-05 and 5T32L086350-08 (to K.W.). Partial support from NIEHS SBIR R44ES025598 and NINDS Blueprint to the Clinic Grant UH2NS094258. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation. The enriched DHA oil used for the study was a generous gift from Solutex NA LLC, Spain.
Footnotes
Conflict of interest: The University of California holds patents on the sEH inhibitors used in this study as well as their use to treat inflammation, inflammatory pain, and neuropathic pain. BD Hammock is a founder and K Wagner is an employee of EicOsis L.L.C., a startup company advancing sEH inhibitors into the clinic.
Author Contributions: The authors have all contributed significantly and reviewed the manuscript, K Wagner designed and conducted the behavioral experiments, SKK Lee and J Yang provided the pharmacokinetic and mass spectrometry analysis and BD Hammock reviewed and contributed to the manuscript and all agree that it is ready for submission.
References
- Belluzzi A, Boschi S, Brignola C, Munarini A, Cariani G, Miglio F. Polyunsaturated fatty acids and inflammatory bowel disease. The American journal of clinical nutrition. 2000;71:339S–342S. doi: 10.1093/ajcn/71.1.339s. [DOI] [PubMed] [Google Scholar]
- Bettaieb A, Chahed S, Tabet G, Yang J, Morisseau C, Griffey S, Hammock BD, Haj FG. Effects of Soluble Epoxide Hydrolase Deficiency on Acute Pancreatitis in Mice. PLoS ONE. 2014;9:e113019. doi: 10.1371/journal.pone.0113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004;7:137–144. doi: 10.1097/00075197-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, et al. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci. 2010;13:284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JL, Nalwalk JW, Phillips JG, Hough LB. CC12, a P450/epoxygenase inhibitor, acts in the rat rostral, ventromedial medulla to attenuate morphine antinociception. Brain Research. 2013;1499:1–11. doi: 10.1016/j.brainres.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E, Coppey L, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek M. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Experimental diabetes research 2009. 2009 doi: 10.1155/2009/431980. Article ID 431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. The journal of pain : official journal of the American Pain Society. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Melis M, Diana M, Fratta W, Gessa G. The cyclo-oxygenase inhibitor nimesulide induces conditioned place preference in rats. Eur J Pharmacol. 2000;406:75–77. doi: 10.1016/s0014-2999(00)00665-8. [DOI] [PubMed] [Google Scholar]
- Felice MD, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Annals of neurology. 2013;74:257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fer M, Corcos L, Dreano Y, Plee-Gautier E, Salaun JP, Berthou F, Amet Y. Cytochromes P450 from family 4 are the main omega hydroxylating enzymes in humans: CYP4F3B is the prominent player in PUFA metabolism. Journal of lipid research. 2008;49:2379–2389. doi: 10.1194/jlr.M800199-JLR200. [DOI] [PubMed] [Google Scholar]
- Figueroa JD, Cordero K, Serrano-Illan M, Almeyda A, Baldeosingh K, Almaguel FG, Leon MD. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage. 2007;37(Suppl 1):S71–S79. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Gore M, Dukes E, Rowbotham DJ, Tai KS, Leslie D. Clinical characteristics and pain management among patients with painful peripheral neuropathic disorders in general practice settings. Eur J Pain. 2007;11:652–664. doi: 10.1016/j.ejpain.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Harris TR, Bettaieb A, Kodani S, Dong H, Myers R, Chiamvimonvat N, Haj FG, Hammock BD. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–111. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol. 2010;104:3222–3230. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L-J, Qi R, Yang R-H, Xu G-Z. Docosahexaenoic acid inhibits mechanical allodynia and thermal hyperalgesia in diabetic rats by decreasing the excitability of DRG neurons. Experimental Neurology. 2015;271:291–300. doi: 10.1016/j.expneurol.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Cleary RA, Phillips JG, Fang C, Yang W, Ding X. Deficits in Neuronal Cytochrome P450 Activity Attenuate Opioid Analgesia but not Opioid Side Effects. European Journal of Pharmacology. 2014;0:255–262. doi: 10.1016/j.ejphar.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Yang W, Ding X. Neuronal cytochrome P450 activity and opioid analgesia: relevant sites and mechanisms. Brain Res. 2015;1616:10–18. doi: 10.1016/j.brainres.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci U S A. 2012;109:11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, Hammock BD. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS ONE. 2013;8:e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL. Opioid receptors: from genes to mice. J Pain. 2000;1:45–50. doi: 10.1054/jpai.2000.9823. [DOI] [PubMed] [Google Scholar]
- Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochimica et biophysica acta. 2011;1814:210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. The American journal of clinical nutrition. 2000;71:349S–351S. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- Li MY, Wang YY, Cao R, Hou XH, Zhang L, Yang RH, Wang F. Dietary fish oil inhibits mechanical allodynia and thermal hyperalgesia in diabetic rats by blocking nuclear factor-kappaB-mediated inflammatory pathways. The Journal of Nutritional Biochemistry. 2015;26:1147–1155. doi: 10.1016/j.jnutbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009;156:284–296. doi: 10.1111/j.1476-5381.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlik MT, Uritsky TJ. Treatment of Neuropathic Pain. Curr Treat Options Neurol. 2015;17:50. doi: 10.1007/s11940-015-0381-2. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto K, Nishinaka T, Ambo A, Mankura M, Kasuya F, Tokuyama S. Possible involvement of beta-endorphin in docosahexaenoic acid-induced antinociception. European Journal of Pharmacology. 2011;666:100–104. doi: 10.1016/j.ejphar.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Nishinaka T, Mankura M, Fujita-Hamabe W, Tokuyama S. Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biological & pharmaceutical bulletin. 2010;33:1070–1072. doi: 10.1248/bpb.33.1070. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17:1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre ME, Correia AO, Borges Mde B, Sampaio TM, Chakraborty SA, Goncalves Dde O, Brito GA, Leal LK, Felipe CF, Lucetti DL, et al. Eicosapentaenoic acid and docosahexaenoic acid exert anti-inflammatory and antinociceptive effects in rodents at low doses. Nutrition research. 2013;33:422–433. doi: 10.1016/j.nutres.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel S, Raccah D, Gerbi A, Pieroni G, Vague P, Coste TC. At low doses, a gamma-linolenic acid-lipoic acid conjugate is more effective than docosahexaenoic acid-enriched phospholipids in preventing neuropathy in diabetic rats. The Journal of nutrition. 2007;137:368–372. doi: 10.1093/jn/137.2.368. [DOI] [PubMed] [Google Scholar]
- Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Expression of morphine-conditioned place preference is more vulnerable than naloxone-conditioned place aversion to disruption by nociceptin in mice. Neuroscience Letters. 2008;443:108–112. doi: 10.1016/j.neulet.2008.07.043. [DOI] [PubMed] [Google Scholar]
- Sasso O, Wagner K, Morisseau C, Inceoglu B, Hammock BD, Piomelli D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol Res. 2015;97:7–15. doi: 10.1016/j.phrs.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Progress in lipid research. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50 Suppl:S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biological & pharmaceutical bulletin. 2011;34:1174–1178. doi: 10.1248/bpb.34.1174. [DOI] [PubMed] [Google Scholar]
- Uauy R, Mena P. Lipids and neurodevelopement. Nutrition reviews. 2001;59:S34–S46. doi: 10.1111/j.1753-4887.2001.tb05500.x. discussion S46-38. [DOI] [PubMed] [Google Scholar]
- Ulu A, Appt SE, Morisseau C, Hwang SH, Jones PD, Rose TE, Dong H, Lango J, Yang J, Tsai HJ, et al. Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. British Journal of Pharmacology. 2012;165:1401–1412. doi: 10.1111/j.1476-5381.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Wagner K, Inceoglu B, Dong H, Yang J, Hwang SH, Jones P, Morisseau C, Hammock BD. Comparative efficacy of 3 soluble epoxide hydrolase inhibitors in rat neuropathic and inflammatory pain models. Eur J Pharmacol. 2013;700:93–101. doi: 10.1016/j.ejphar.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014a doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014b;15:907–914. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.