Abstract
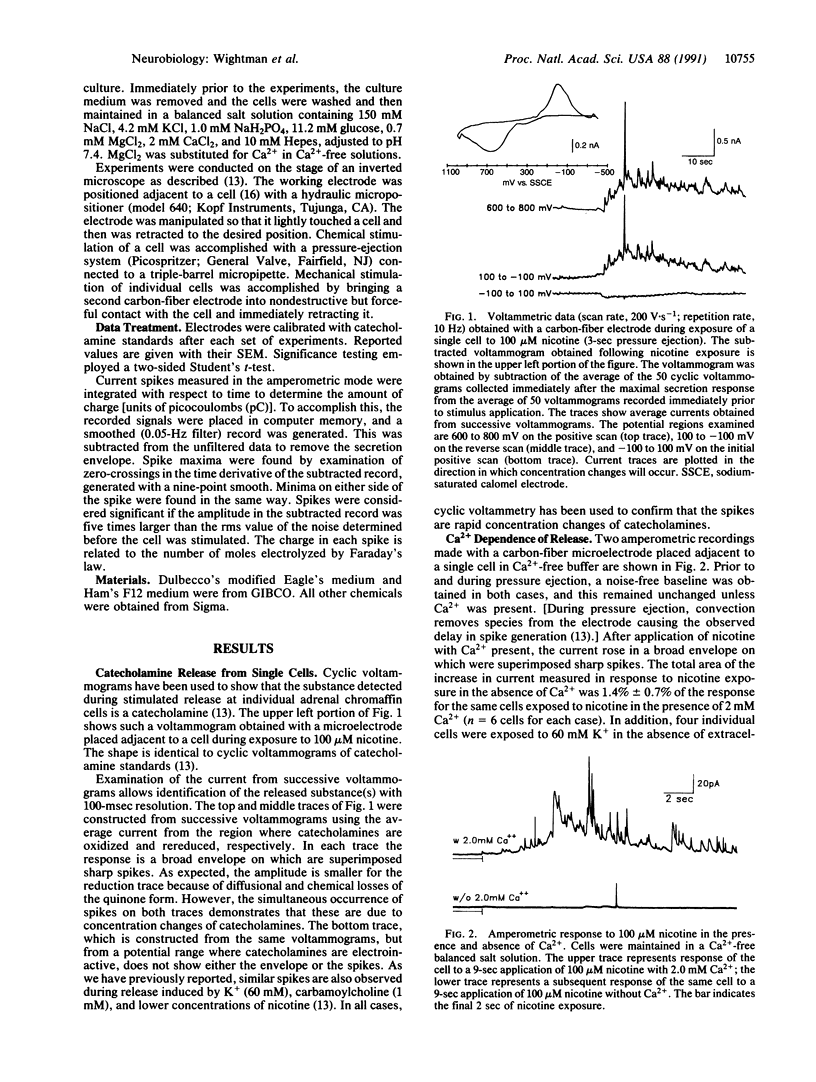
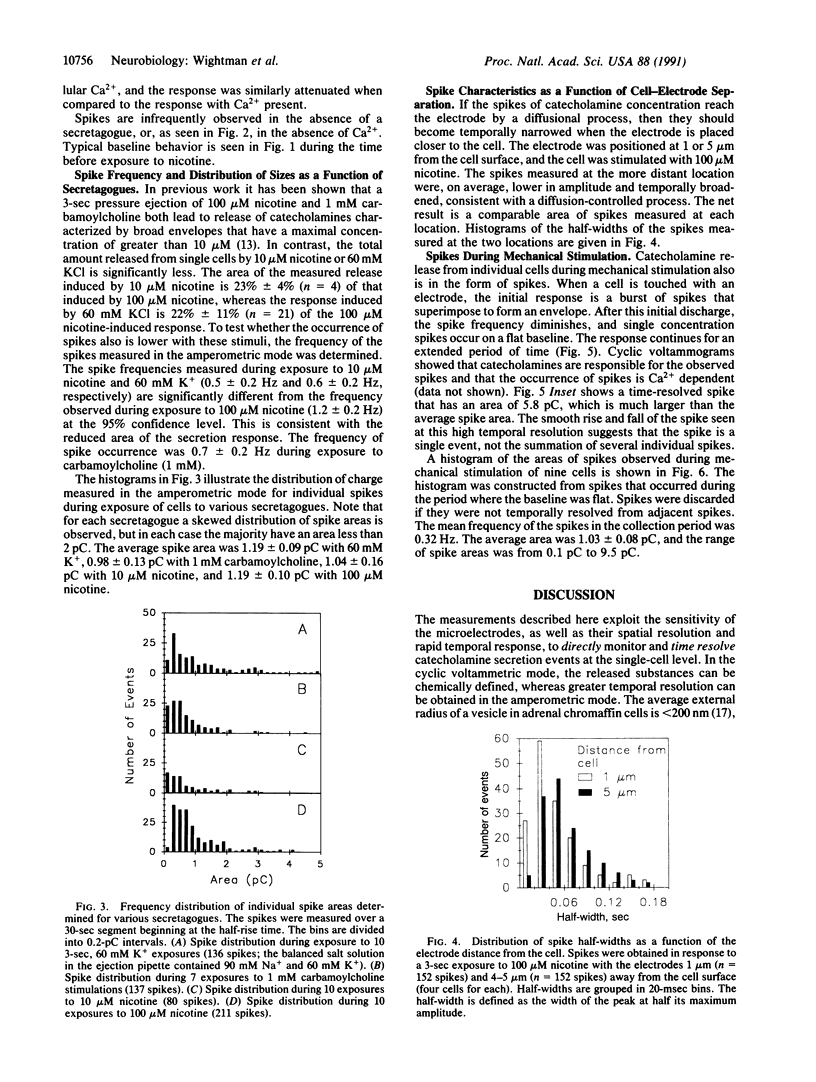
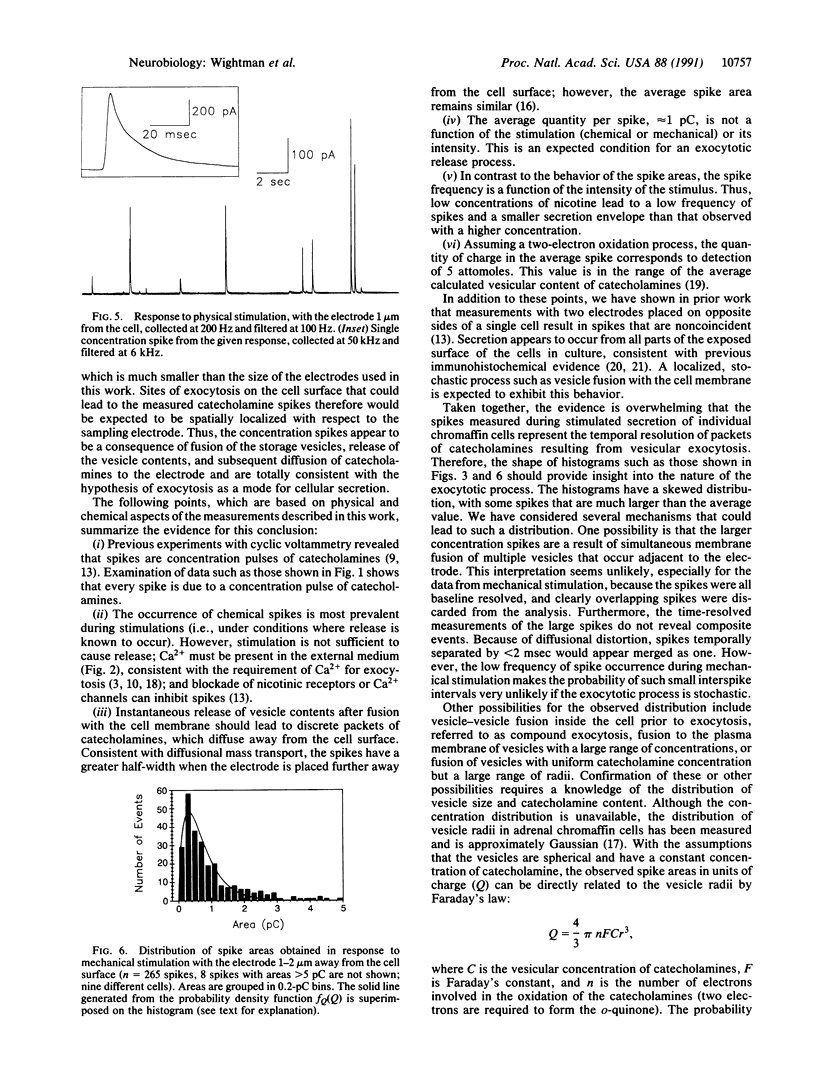
Secretion of catecholamines from single bovine chromaffin cells in culture was elicited by brief pressure ejections from a micropipette containing nicotine, carbamoylcholine, or potassium ions or by mechanical stimulation. Release was monitored electrochemically with a carbon-fiber microelectrode placed adjacent to the cell. Cyclic voltammetry was used to identify secreted species, whereas constant potential amperometry was used for improved temporal resolution (millisecond range) of catecholamine detection. During secretion, brief current spikes were observed, which were shown to be due to detection of catecholamines by electrooxidation. The spikes have the physical characteristics of multimolecular packets of catecholamines released at random times and locations from the surface of the single cell. The half-width of the spikes was found to increase with an increase in cell-electrode spacing. The properties of the catecholamine spikes correlate well with expectations based on secretion from individual storage vesicles. Spikes do not occur in the absence of Ca2+ in the buffer, and the majority of spikes are found to be distributed between 0.2 and 2 picocoulombs, corresponding to 1-10 attomoles of catecholamine detected. The frequency of the spikes increases with the intensity of the stimulus, but the average quantity of catecholamine in each spike is independent of the stimulus. Thus, these measurements represent time-resolved observation of quantal secretion of catecholamines and provide direct evidence for the exocytotic hypothesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur J. E., Kristensen E. W., May L. J., Wiedemann D. J., Wightman R. M. Fast-scan voltammetry of biogenic amines. Anal Chem. 1988 Jul 1;60(13):1268–1272. doi: 10.1021/ac00164a006. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Coupland R. E. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 1968 Jan 27;217(5126):384–388. doi: 10.1038/217384a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Gundersen C. B., Meriney S. D., Young S. H. Direct measurement of ACh release from exposed frog nerve terminals: constraints on interpretation of non-quantal release. J Physiol. 1989 Dec;419:225–251. doi: 10.1113/jphysiol.1989.sp017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARP N. A. Further observations on the state of the catechol amines stored in the adrenal medullary granules. Acta Physiol Scand. 1959 Nov 15;47:271–279. doi: 10.1111/j.1748-1716.1960.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Ciesielski-Treska J., Aunis D. A phase-contrast and immunofluorescence study of adrenal medullary chromaffin cells in culture: neurite formation, actin and chromaffin granule distribution. Cell Tissue Res. 1981;218(2):331–343. doi: 10.1007/BF00210348. [DOI] [PubMed] [Google Scholar]
- Heuser J. E. Review of electron microscopic evidence favouring vesicle exocytosis as the structural basis for quantal release during synaptic transmission. Q J Exp Physiol. 1989 Dec;74(7):1051–1069. doi: 10.1113/expphysiol.1989.sp003333. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Frye R. A. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982 Sep;39(3):635–646. doi: 10.1111/j.1471-4159.1982.tb07940.x. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983 Oct 13;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe K. T., Jankowski J. A., Wightman R. M. Etched carbon-fiber electrodes as amperometric detectors of catecholamine secretion from isolated biological cells. Anal Chem. 1991 Aug 1;63(15):1589–1594. doi: 10.1021/ac00015a017. [DOI] [PubMed] [Google Scholar]
- Leszczyszyn D. J., Jankowski J. A., Viveros O. H., Diliberto E. J., Jr, Near J. A., Wightman R. M. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem. 1990 Sep 5;265(25):14736–14737. [PubMed] [Google Scholar]
- Leszczyszyn D. J., Jankowski J. A., Viveros O. H., Diliberto E. J., Jr, Near J. A., Wightman R. M. Secretion of catecholamines from individual adrenal medullary chromaffin cells. J Neurochem. 1991 Jun;56(6):1855–1863. doi: 10.1111/j.1471-4159.1991.tb03441.x. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Burridge K., Wilson S. P., Kirshner N. Visualization of the exocytosis/endocytosis secretory cycle in cultured adrenal chromaffin cells. J Cell Biol. 1983 Dec;97(6):1906–1917. doi: 10.1083/jcb.97.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F. E., Schäfer T., Tapparelli C., Grob M., Karli U. O., Heumann R., Thoenen H., Bookman R. J., Burger M. M. Inhibition of exocytosis by intracellularly applied antibodies against a chromaffin granule-binding protein. Nature. 1989 Jun 29;339(6227):709–712. doi: 10.1038/339709a0. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Fesce R., Grohovaz F., Haimann C., Hurlbut W. P., Iezzi N., Torri Tarelli F., Villa A., Ceccarelli B. Neurotransmitter release and synaptic vesicle recycling. Neuroscience. 1990;35(3):477–489. doi: 10.1016/0306-4522(90)90323-v. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Kirshner N. Quantal secretion from adrenal medulla: all-or-none release of storage vesicle content. Science. 1969 Aug 29;165(3896):911–913. doi: 10.1126/science.165.3896.911. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Viveros O. H. Primary culture of adrenal medullary chromaffin cells in a chemically defined medium. Exp Cell Res. 1981 May;133(1):159–169. doi: 10.1016/0014-4827(81)90366-9. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]



