Abstract
BACKGROUND/OBJECTIVES
Stress-induced cognitive impairment is related to the suppression of hippocampal neurogenesis that results from an increase of oxidative stress. Therefore, the aim of this study was to investigate the effects of administration of a blueberry drink, having a high antioxidant power, on the cognitive performance of adult rats exposed to chronic mild stress.
MATERIALS/METHODS
Twelve-week-old male Sprague-Dawley rats (n = 48) were randomly divided into four groups: control (CO), stress (ST), control + 5% blueberry drink (CO + B), and stress + 5% blueberry drink (ST + B). After eight weeks, the cognitive performance was assessed using a multiple T-maze water test. Levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and ascorbic acid were measured in the brain, and catecholamine concentrations were measured in plasma.
RESULTS
The brain weights of the rats from the ST and ST + B groups were significantly lower than those of the rats from the CO and CO + B groups. The cognitive performance of the ST group was impaired when compared to that of the CO group. This impairment was significantly improved by the blueberry drink supplementation (P < 0.05). The brain SOD and CAT concentrations were not influenced by the stress or by the blueberry drink. However, the brain levels of GPx and ascorbic acid were significantly lower in the ST group than those in the CO group and were increased by the blueberry drink supplementation. The plasma catecholamine concentrations were affected by chronic mild stress and by the blueberry drink. The plasma norepinephrine and dopamine concentrations were decreased by the chronic stress and improved by the blueberry drink supplementation. The plasma epinephrine level was only influenced by the stress.
CONCLUSION
These findings suggest that the blueberry drink may protect against the cognitive impairment induced by chronic mild stress.
Keywords: Blueberry, cognitive impairment, chronic stress, antioxidant, catecholamine
INTRODUCTION
Stress is defined as any situation capable of perturbing physiological or psychological homeostasis [1]. The response to stress involves the activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, and psychological stress appears to be a more potent activator of the HPA axis. The HPA system, which releases glucocorticoids, and the sympathetic adrenomedullary system, which releases catecholamines, act as integrated units controlling stress responses [2]. It has been shown that psychological stress can induce dysfunctions in the nervous system, including cognitive impairment, anxiety, and insomnia [3]. Recent studies have shown that both intense acute and chronic stress can have detrimental neurocognitive effects [4,5], and repeated stress induces neurochemical and neuroanatomical changes in the brain, mainly in the HPA axis [6]. Memory and learning performances were also observed to decrease under chronic stress [7].
Oxidative stress, reflecting the accumulation of oxygencontaining free radicals, increases with aging and may play a critical role in age-related functional deficits of the brain [8,9]. The brain is particularly prone to oxidative damage owing to its high rate of oxygen consumption [10]. Therefore, it has been proposed that strategies improving the antioxidant defenses may prevent some of the effects of aging [11]. Research suggests that polyphenolic compounds contained in colorful fruits and vegetables exhibit potent antioxidant activity that reduces the age-related sensitivity to oxidative stress [12] and may be involved in protecting cognitive functions [13]. A large number of dietary supplements using flavonoid-rich foods have demonstrated beneficial effects on memory and learning in both animals and humans [14,15,16]. In particular, blueberries (fruits of Vaccinium uliginosum L. and various other Vaccinium species) are one of several fruits with high levels of polyphenolic flavonoids and anthocyanins, which have a high antioxidant power [17]. Blueberry supplementation was effective in reversing cognitive declines in aged rats [18]. In aged rats fed blueberries for eight weeks, unmetabolized forms of anthocyanins were found in the cerebellum, hippocampus, cortex, and striatum [19]. Twelve-week blueberry supplementation in aged rats improved age-related deficits in cognitive and motor functioning [20].
Recently, the majority of studies have focused on the cognitive effects of flavonoid-rich foods in aged animals [13,21] and transgenic mouse models of Alzheimer's disease [20]. Only a few studies have investigated cognitive functions in young/adult rodents, and recent data suggest that flavonoids are capable of inducing cognitive improvements in young and healthy animals [22,23]. Dietary blueberry supplementation prevented deficits in the learning performance in adult rats and protected against neuronal loss induced by injections of kainic acid to the hippocampus [24] or by 56Fe particle irradiation [25]. It also improved the performance in memory tasks with protective effect on DNA in the hippocampus and cerebral cortex [26]. However, the effect of blueberries on the cognitive dysfunction induced by chronic mild stress has not been studied yet.
The aim of this study was to investigate the effects of eight-week administration of a blueberry drink on the cognitive performance in 12-week-old rats exposed to chronic mild stress. Loss of cognitive abilities during aging is a complex process that starts to become evident during middle age in rats (12-24-month-old), even in the absence of a specific neurodegenerative disease [27]. In the present study, a chronic mild stress model [28,29] was used to provide a realistic simulation of the stresses of daily life. After the rearing stage, the cognitive function of rats was assessed using a multiple T-maze water test. The levels of antioxidants (superoxide dismutase, catalase, glutathione peroxidase, and ascorbic acid) were measured in the brain, and catecholamine levels were measured in plasma.
MATERIALS AND METHODS
Materials
A freeze-dried 100% wild blueberry powder was purchased from Bactolac Pharmaceutical, Inc. (Hauppauge, NY, USA). A blueberry drink was prepared by adding the blueberry powder to fresh tap water (PurePlus, Inc., Incheon, Korea). Assay kits for catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The standard of ascorbic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA), and all reagents used in ascorbic acid analysis were high-performance liquid chromatography (HPLC) grade. Catecholamines were measured using the 3-CAT plasma enzyme-linked immunosorbent assay (ELISA) kit from Labor Diagnostika (Nord, Nordhorn, Germany).
Animals and experimental design
Twelve-week-old male Sprague-Dawley rats (Hanlim Experimental Animal Laboratory, Seoul, Korea) weighing 470 ± 10 g were fed a laboratory diet. The animals were kept individually and housed in a temperature-controlled room (24 ± 1℃) with a relative humidity of 50-60% and a 12/12 h light/dark cycle (lights on at 6:00 a.m.). The rats had ad libitum access to food in the form of dry pellets (Purina, Nestle Purina PetCare Korea, Ltd., Seoul, Korea) and fresh tap water. The study protocol was approved by the Committee on Animal Welfare Regulations of Chung-Ang University (IRB 14-70).
After acclimation to laboratory conditions for one week, the rats were randomly divided into four groups (n = 12 per group): a control group (CO, no chronic mild stress/tap water), a blueberry drink only group (CO + B, no chronic mild stress/5% blueberry drink), a stress only group (ST, chronic mild tress/tap water), and a stress + blueberry drink group (ST + B, chronic mild stress/5% blueberry drink). To prepare a 5% blueberry drink, 5 g of the blueberry powder was mixed daily with 100 mL of tap water and then stirred with a magnetic stir bar under protection from light until completely dissolved. The blueberry drink was provided to the CO + B and ST + B groups instead of the regular drinking water, and the water bottles for the groups were wrapped in aluminum foil to protect from light in order to prevent the destruction of bioactive compounds.
Feed and water consumption was recorded daily, and the rats were weighed weekly. A feed efficiency ratio (FER) was also calculated. All animals were maintained under the appropriate experimental conditions for eight weeks before performing the multiple T-maze water test.
Chronic mild stress model
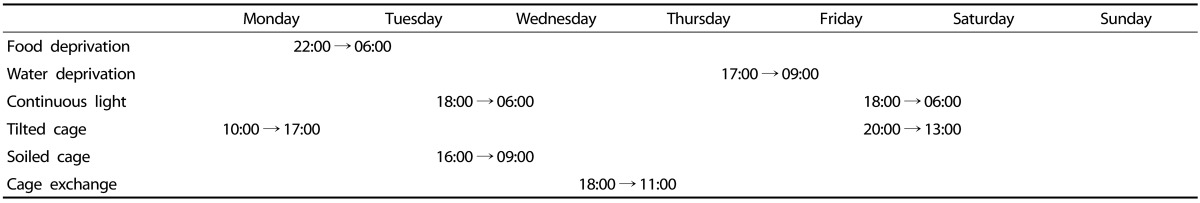
The chronic mild stress model was slightly modified from those described previously for rats by Willner et al. [28] and for mice by Monleon et al. [29]. Briefly, the rats were subjected to the following stressors (one or two in any 24-h period): one period of food deprivation (8 h), one period of water deprivation (16 h), two periods of overnight illumination (12 h), one period of cage exchange (17 h), two periods of a 45° cage tilt (7 h/17 h), one period of soiled bedding (200 mL of water per cage; 17 h), or one period of no stress (24 h). Table 1 shows the timing and length of all the stressors used in the chronic mild stress protocol. All the individual stressors used have been classified as "mild" according to the Animals (Scientific Procedures) Act of 1986 (UK legislation) [28]. The stressors were scheduled throughout the eight weeks before the water maze test.
Table 1. Time and length of activities used in the chronic mild stress procedure.
Multiple T-maze water test
The multiple T-maze water test was based on the method of Ishizaki [30]. The apparatus (130 cm long, 130 cm wide, and 30 cm deep) was designed with both the T-maze and a straightaway course. Warm water (18 ± 1℃) was poured into the maze to a depth of 20 cm so that the rats were able to swim, and the temperature of the room was kept at 18 ± 1℃. The maze testing began two months after the beginning of the experiment. The rats were trained to swim over three trials on one day in the straightaway course and then underwent three trials per day for two days with the T-maze. The time the rats took to finish the T-maze and the number of errors they made when they entered the blind alley of the T-maze were measured.
Sample collection
After completing the multiple T-maze water test, the rats were anesthetized with diethyl ether, and blood was collected from the heart and immediately placed on ice. The whole intact brain was then carefully removed and placed in an ice-chilled Petri dish. The brain was weighed and washed with isotonic saline. The blood was collected into ethylenediaminetetraacetic acidcoated tubes and centrifuged at 3,000 rpm for 15 min at 4℃. The plasma was transferred into 1.5-mL microtubes for catecholamine analysis. All samples were stored at -80℃ until analysis.
Analysis of antioxidant enzyme activities and ascorbic acid in brain tissue
SOD, CAT, and GPx activities in the brain were measured using their respective assay kits. The total ascorbic acid in the brain was measured using an HPLC method [31,32].
Determination of plasma catecholamines
Plasma norepinephrine, epinephrine, and dopamine concentrations were quantified using the 3-CAT plasma ELISA kit.
Statistical analysis
Statistical analysis was performed using the SPSS for Windows 19.0 software (SPSS, Inc., Chicago, IL, USA). The effects of the chronic mild stress, blueberry drink, and their interaction were tested using two-way analysis of variance (ANOVA), followed by Fisher's least significant difference (LSD) test. Results were considered statistically significant at P < 0.05. Data are presented as the mean ± standard error of the mean (SE).
RESULTS
Body weight, brain weight, and water and food intake
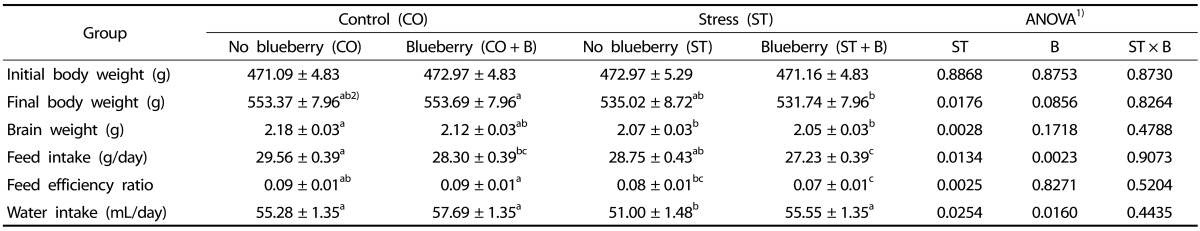
There were no significant differences in the final body and brain weights at the end of the experiment between the CO and CO + B groups (P ≥ 0.05). However, the brain weights of the rats from the ST and ST + B groups were significantly lower than those of the rats from the CO and CO + B groups (Table 2). No significant interactions were observed between the stress and blueberry drink regarding the final body and brain weights. The feed intake and water intake were both affected by the stress and blueberry drink. The ST + B group had the lowest feed intake as well as the lowest FER. The water intake was significantly affected by the chronic mild stress and blueberry drink. The water intake in the ST group was significantly lower than that in the other groups. There were no significant interactions between the stress and blueberry drink regarding the feed intake, FER, and water intake. The rats consumed the blueberry powder at approximately 5.5 g/kg of body weight daily.
Table 2. Effects of the blueberry drink on body weight, brain weight, and water and food intake in adult rats under chronic mild stress conditions.
Data are expressed as the mean ± SE of 12 animals per group.
1) Significant differences between the chronic mild stress (ST) and blueberry drink (B) or interaction between these factors (ST × B) were tested by two-way ANOVA and expressed as P-values, followed by Fisher's LSD test.
2) Mean values with different superscript letters are significantly different (P < 0.05).
Mean time and errors in the multiple T-maze water test
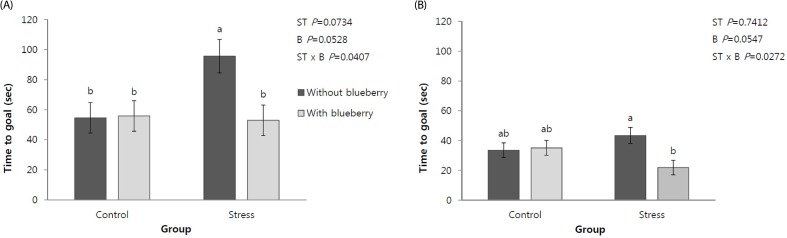
Fig. 1 shows the effects of the chronic mild stress and blueberry drink on the mean time needed to reach the goal in the multiple T-maze water test over two days. The time to reach the goal was significantly affected by the chronic mild stress but not by the blueberry drink, and there was a significant interaction between the stress and blueberry drink regarding the time spent to reach the goal (P < 0.05). On the first day of the test, the mean time to reach the goal was significantly longer in the ST group than in the other groups. However, the time for the ST + B group was similar to that of the CO and CO+B groups. On the second day of the test, the ST + B group showed the shortest time spent to reach the goal among the groups, and the time was significantly shorter than that of the ST group.
Fig. 1. Effects of the blueberry drink on the mean time to reach the goal in the multiple T-maze water test on two days in adult rats under chronic mild stress conditions.
(A) mean time to reach the goal on the first day of the multiple T-maze water test and (B) mean time to reach the goal on the second day of the multiple T-maze water test. The data are expressed as the mean ± SE of 12 animals per group. Significant differences between the stress (ST) and blueberry drink (B) or interaction between these factors (ST × B) were tested by two-way ANOVA and expressed as P-values, followed by Fisher's LSD test. Different letters represent statistical differences between the means (P < 0.05).
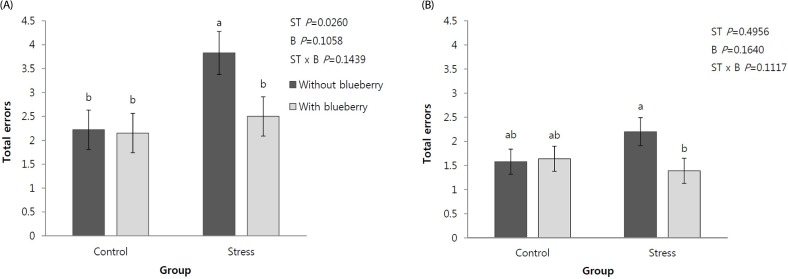
The numbers of errors that the rats made when they entered the blind alley of the multiple T-maze are shown in Fig. 2. The number of errors was only affected by the stress on the first day. No interactions between the stress and blueberry drink were observed with regard to the total number of errors in the multiple T-maze water test. The number of errors in the ST group was significantly higher compared to those in the other groups, similar to the mean time required to reach the goal on the first day of the test. Under chronic mild stress, the blueberry drink significantly decreased the total number of errors.
Fig. 2. Effects of the blueberry drink on the total number of errors in the multiple T-maze water test on two days in adult rats under chronic mild stress conditions.
The data are expressed as the mean ± SE of 12 animals per group. Significant differences between the stress (ST) and blueberry drink (B) or interaction between these factors (ST × B) were tested by two-way ANOVA and expressed as P-values, followed by Fisher's LSD test. Different letters represent statistical differences between the means (P < 0.05).
Brain SOD, CAT, GPx, and ascorbic acid concentrations
The effects of the chronic mild stress and blueberry drink on the brain SOD, CAT, GPx, and ascorbic acid levels are shown in Table 3. The brain SOD and CAT concentrations were not affected by the stress or by the blueberry drink. The GPx and ascorbic acid concentrations were significantly increased by the blueberry drink supplementation. The chronic mild stress only affected the brain ascorbic acid level. The concentrations of GPx and ascorbic acid in the ST group were significantly lower than those in the ST + B group (P < 0.05).
Table 3. Effects of the blueberry drink on brain superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and ascorbic acid levels in adult rats under chronic mild stress conditions.
Data are expressed as the mean ± SE of 12 animals per group.
1) Significant differences between the stress (ST) and blueberry drink (B) or interaction between these factors (ST × B) were tested by two-way ANOVA and expressed as P-values, followed by Fisher's LSD test.
2) Mean values with different superscript letters are significantly different (P < 0.05).
Plasma catecholamine concentrations
The plasma norepinephrine levels were affected by the blueberry drink. The concentration in the ST group (167.28 ± 68.71 pg/mL) was significantly increased by the blueberry drink supplementation (425.09 ± 64.78 pg/mL in the ST + B group) (Table 4). The plasma epinephrine and dopamine values were affected by the chronic mild stress, and both concentrations in the ST group were significantly lower than those in the ST + B group. The plasma dopamine concentration was increased by the blueberry drink supplementation from 364.57 pg/mL in the ST group to 765.80 pg/mL in the ST + B group. No significant interactions between the chronic mild stress and blueberry drink were observed with regard to the plasma catecholamine concentrations.
Table 4. Effects of the blueberry drink on plasma norepinephrine, epinephrine, and dopamine levels in adult rats under chronic mild stress conditions.
Data are expressed as the mean ± SE of 12 animals per group.
1) Significant differences between the stress (ST) and blueberry drink (B) or interaction between these factors (ST × B) were tested by two-way ANOVA and expressed as P-values, followed by Fisher's LSD test.
2) Mean values with different superscript letters are significantly different (P < 0.05).
DISCUSSION
Recently, there has been an increase in diseases related to psychological stress, and thus, more attention has been given to the prevention of stress-induced injuries. Stress is associated with the activation of the HPA axis. The hippocampus is critical for the learning, consolidation, and retrieval of declarative memories. It is also important for the formation of spatial memory [33]. Thus, the stress-induced impairment of learning and memory is related to the suppression of hippocampal neurogenesis, and one of the reasons why chronic stress suppresses hippocampal neurogenesis is that it increases the oxidative stress [34]. Research has focused on whether ingestion of antioxidants leads to the reduction of oxidative stress and to the improvement in impaired cognitive function. Therefore, this study investigated the antioxidant activities and neuroprotective effects of a blueberry drink on the cognitive impairment induced by chronic mild stress in Sprague-Dawley adult rats.
Exposure to stress situations can influence the feeding behavior, which results in changes of body weight of rats [35]. In this study, the ST and ST + B groups showed a reduced feed intake and a decreased body weight. The blueberry drink did not restore the feed intake, nor did it ameliorate the body weight loss caused by the chronic stress. The water intake in the ST group was significantly lower than that in the other groups, which might have resulted from water deprivation (16 h/week) under the mild stress conditions. However, the liquid intake in the ST + B group did not differ from that in the CO and CO + B groups; therefore, the blueberry drink in the current study improved liquid drinking by the stressed animals. Thus, similar amounts of the blueberry powder (5.76 and 5.55 g/kg of body weight) were consumed by the CO + B and ST + B groups, respectively, in this study, although the ST + B group had a period of liquid deprivation during mild stress.
Various studies have used the multiple T-maze test for analyzing spatial memory in mice and rats [36,37]. The advantage of the multiple T-maze test is that it uses a more complex choice system, with multiple choice points, and thus, can be used to test both reference and working memories [38]. Elizalde et al. [39] showed that the mice exposed to chronic mild stress showed long-lasting behavioral effects and recognition memory deficits as demonstrated by a significant increase in the swimming latency time compared to that in controls. Chen et al. [1] showed that the total time and number of mistakes in stressed groups in a water maze test increased in comparison to those in a control group. In this study, the adult rats from the ST group showed a significant increase in the mean time needed to reach the goal and in the number of errors, which indicates that the chronic mild stress used in this study induced cognitive impairment.
Studies have suggested that polyphenolic compounds may be involved in protecting cognitive functioning through their antioxidant activities [13]. Positive effects of polyphenolic consumption include direct effects on signaling, shown to enhance neuronal communication, the ability to buffer against excess calcium, the enhancement of neuroprotective adaptations, and the reduction of stress signals [40]. The major classes of berry phenolic compounds include flavonoids such as flavonols, flavanols, and anthocyanins, condensed tannins, hydrolysable tannins, stilbenoids, and phenolic acids [41]. It appears that berry fruits mediate signaling pathways involved in inflammation and cell survival, in addition to enhancing neuroplasticity, neurotransmission, and calcium buffering, all of which lead to attenuation of age- and pathologic-related deficits in behavior [10]. In this study, the cognitive impairment induced in adult rats by the chronic mild stress was improved by the supplementation of the blueberry drink. The mean time to reach the goal and the number of errors in the multiple T-maze test were significantly lower in the ST + B group than those in the ST group and similar to the results shown by the CO group. Thus, our findings indicate that dietary supplementation with a blueberry drink may protect and increase the spatial working memory and learning ability of adult rats exposed to chronic psychological stress. This is similar to the results of other studies that reported protective effects of blueberries on cognitive impairment induced by injections of kainic acid to the hippocampus [24] and by 56Fe particle irradiation [25].
The metalloproteins SOD, CAT, and GPx provide the first line of antioxidant defense against reactive oxygen species through enzyme-catalyzed dismutation of O2- to H2O2, which is further reduced to oxygen and water [42]. Chronic stress has been shown to increase the vulnerability of the brain, particularly the hippocampus, by altering the antioxidant capacity through the mediation of glucocorticoids. Papandreou et al. [43] have shown that after short-term supplementation with blueberry powder, a significant antioxidant enhancement was observed in adult mouse brains. In the current study, although no effects were observed on SOD and CAT, the brain GPx level was significantly lowered by the chronic mild stress and restored by the supplementation of the blueberry drink. Ascorbic acid can act as an antioxidant and oxygen radical scavenger, and has been detected in high amounts in brain tissue. Moreover, it has been shown that ascorbic acid acts as a neuromodulator of both glutamate- and dopamine-mediated neurotransmission and is an essential co-factor in the synthesis of norepinephrine and many neuropeptides [44]. It has been shown that short- and long-term supplementation with ascorbic acid results in positive effects on memory and passive avoidance learning in rats [45]. In our study, the concentration of ascorbic acid in the brains of the rats from the ST group was significantly lower than in those from the other groups; however, dietary supplementation with the blueberry drink significantly improved the ascorbic acid content in the brain.
It is well known that the sympathetic nervous system is closely involved in the regulation of the stress response. Psychological stress activates the sympathetic nervous system, and, in turn, catecholamines are released from the sympathetic nerve terminal and the adrenal medulla. Catecholamines include norepinephrine, epinephrine, and dopamine, which are involved in the modulation of the body's cognitive and emotional state and other psychoactivities. Norepinephrine and dopamine levels decrease upon experiencing psychological stress [1]. In the current study, the levels of norepinephrine and dopamine in the plasma of the ST group were lower than those in the plasma of the CO group, and these deficits were significantly ameliorated by the blueberry supplementation. Furthermore, the catecholamine levels in the CO + B group were slightly higher than those in the CO group. A significant decrease in the GPx level was detected in the ST group in this study; therefore, chronic mild stress may produce oxidants [35]. Increases in oxidative stress and hydrogen peroxide attenuated the dopamine release in the dorsal striatum and decreased the basal dopamine levels [46]. Thus, the supplementation of blueberry, having a high antioxidant power, possibly ameliorated the oxidative damage induced by the stress, which resulted in a significant improvement of the dopamine level in the ST + B group in this study.
Based on the compositional analysis, 1 g of the blueberry powder in this study contained 19.96 mg of total polyphenols in gallic acid equivalents (data not shown). The approximately 5.5 g/kg of body weight of the blueberry powder consumed daily by the rats equated to the amount of polyphenols in 53 g of fresh whole blueberries, based on the polyphenol content [47]. The amount of raw blueberries given to the animals (53 g) would correspond to approximately 515 g of raw blueberries for a human adult weighing 60 kg, using the formula for a human equivalent dose based on the body surface area [48]. Further, the amount of the blueberry powder, 5.5 g/kg of body weight of the rats, would correspond to 0.88 g/kg of body weight of human adults according to the equation [48]. Future studies should investigate different concentrations of blueberry drinks to maximize the protective effects of blueberry on the cognitive impairment caused by chronic mild stress.
In conclusion, our findings suggest that blueberry supplementation may protect against the cognitive impairment induced by chronic psychological stress. These results may be due to the antioxidant and neuroprotective effects of a blueberry drink, resulting from the high concentrations of polyphenols and flavonoids found in blueberry. Based on these findings, blueberry appears to have potential benefits in terms of prevention of a cognitive decline during stress, and these effects may extend to the cognitive decline associated with other neuropathic diseases.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Chen WQ, Zhao XL, Hou Y, Li ST, Hong Y, Wang DL, Cheng YY. Protective effects of green tea polyphenols on cognitive impairments induced by psychological stress in rats. Behav Brain Res. 2009;202:71–76. doi: 10.1016/j.bbr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Mori A. Stress, aging, and brain oxidative damage. Neurochem Res. 1999;24:1479–1497. doi: 10.1023/a:1022597010078. [DOI] [PubMed] [Google Scholar]
- 3.Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, Costlow C, Irwin MR. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447–457. doi: 10.1097/00006842-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 5.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work. Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Oh JK, Kim YS, Park HJ, Lim EM, Pyun KH, Shim I. Antidepressant effects of Soyo-san on immobilization stress in ovariectomized female rats. Biol Pharm Bull. 2007;30:1422–1426. doi: 10.1248/bpb.30.1422. [DOI] [PubMed] [Google Scholar]
- 7.Abidin I, Yargiçoglu P, Agar A, Gümüslü S, Aydin S, Oztürk O, Sahin E. The effect of chronic restraint stress on spatial learning and memory: relation to oxidant stress. Int J Neurosci. 2004;114:683–699. doi: 10.1080/00207450490430543. [DOI] [PubMed] [Google Scholar]
- 8.Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 2009;34:670–678. doi: 10.1007/s11064-008-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Unno K, Tahara S, Shimada A, Chiba Y, Hoshino M, Kaneko T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell. 2008;7:459–469. doi: 10.1111/j.1474-9726.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 10.Shukitt-Hale B. Blueberries and neuronal aging. Gerontology. 2012;58:518–523. doi: 10.1159/000341101. [DOI] [PubMed] [Google Scholar]
- 11.Shukitt-Hale B, Lau FC, Joseph JA. Berry fruit supplementation and the aging brain. J Agric Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Rendeiro C, Vauzour D, Kean RJ, Butler LT, Rattray M, Spencer JP, Williams CM. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology (Berl) 2012;223:319–330. doi: 10.1007/s00213-012-2719-8. [DOI] [PubMed] [Google Scholar]
- 15.Krikorian R, Shidler MD, Nash TA, Kalt W, Vinqvist-Tymchuk MR, Shukitt-Hale B, Joseph JA. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beking K, Vieira A. Flavonoid intake and disability-adjusted life years due to Alzheimer's and related dementias: a population-based study involving twenty-three developed countries. Public Health Nutr. 2010;13:1403–1409. doi: 10.1017/S1368980009992990. [DOI] [PubMed] [Google Scholar]
- 17.Prior RL, Cao G, Prior RL, Cao G. Analysis of botanicals and dietary supplements for antioxidant capacity: a review. J AOAC Int. 2000;83:950–956. [PubMed] [Google Scholar]
- 18.Malin DH, Lee DR, Goyarzu P, Chang YH, Ennis LJ, Beckett E, Shukitt-Hale B, Joseph JA. Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition. 2011;27:338–342. doi: 10.1016/j.nut.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 20.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 22.Hajipour S, Sarkaki A, Mohammad S, Mansouri T, Pilevarian A, RafieiRad M. Motor and cognitive deficits due to permanent cerebral hypoperfusion/ischemia improve by pomegranate seed extract in rats. Pak J Biol Sci. 2014;17:991–998. doi: 10.3923/pjbs.2014.991.998. [DOI] [PubMed] [Google Scholar]
- 23.Shif O, Gillette K, Damkaoutis CM, Carrano C, Robbins SJ, Hoffman JR. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol Biochem Behav. 2006;84:17–25. doi: 10.1016/j.pbb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Duffy KB, Spangler EL, Devan BD, Guo Z, Bowker JL, Janas AM, Hagepanos A, Minor RK, DeCabo R, Mouton PR, Shukitt-Hale B, Joseph JA, Ingram DK. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2008;29:1680–1689. doi: 10.1016/j.neurobiolaging.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Barros D, Amaral OB, Izquierdo I, Geracitano L, do Carmo, Henriques AT, Ramirez MR. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav. 2006;84:229–234. doi: 10.1016/j.pbb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Kluger A, Gianutsos JG, Golomb J, Ferris SH, George AE, Franssen E, Reisberg B. Patterns of motor impairement in normal aging, mild cognitive decline, and early Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 1997;52B:P28–P39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- 28.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 29.Monleon S, D'Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 30.Ishizaki O. Learning behavior of rats on the water maze (author's transl) Jikken Dobutsu. 1978;27:9–12. doi: 10.1538/expanim1978.27.1_9. [DOI] [PubMed] [Google Scholar]
- 31.van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Lüscher TF. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem Biophys Res Commun. 2003;303:483–487. doi: 10.1016/s0006-291x(03)00360-7. [DOI] [PubMed] [Google Scholar]
- 32.Kand'ár R, Záková P. Determination of ascorbic acid in human plasma with a view to stability using HPLC with UV detection. J Sep Sci. 2008;31:3503–3508. doi: 10.1002/jssc.200800303. [DOI] [PubMed] [Google Scholar]
- 33.Huang TT, Leu D, Zou Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch Biochem Biophys. 2015;576:2–7. doi: 10.1016/j.abb.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto J, Hosung L, Uwaya A, Isami F, Ohno M, Mikami T. Morinda citrifolia fruit reduces stress-induced impairment of cognitive function accompanied by vasculature improvement in mice. Physiol Behav. 2010;101:211–217. doi: 10.1016/j.physbeh.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Dal-Pizzol F, Gavioli EC, Quevedo J. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 2009;54:358–362. doi: 10.1016/j.neuint.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Kunesová G, Hlavácek J, Patočka J, Evangelou A, Zikos C, Benaki D, Paravatou-Petsotas M, Pelecanou M, Livaniou E, Slaninova J. The multiple T-maze in vivo testing of the neuroprotective effect of humanin analogues. Peptides. 2008;29:1982–1987. doi: 10.1016/j.peptides.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Sato N, Murakami Y, Nakano T, Sugawara M, Kawakami H, Idota T, Nakajima I. Effects of dietary nucleotides on lipid metabolism and learning ability of rats. Biosci Biotechnol Biochem. 1995;59:1267–1271. doi: 10.1271/bbb.59.1267. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elizalde N, Gil-Bea FJ, Ramírez MJ, Aisa B, Lasheras B, Del Rio J, Tordera RM. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- 40.Lau FC, Shukitt-Hale B, Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26(Suppl 1):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 42.Salminen LE, Paul RH. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: a theoretical review. Rev Neurosci. 2014;25:805–819. doi: 10.1515/revneuro-2014-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198:352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Shahidi S, Komaki A, Mahmoodi M, Atrvash N, Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76:109–113. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Chen WQ, Zhao XL, Wang DL, Li ST, Hou Y, Hong Y, Cheng YY. Effects of epigallocatechin-3-gallate on behavioral impairments induced by psychological stress in rats. Exp Biol Med (Maywood) 2010;235:577–583. doi: 10.1258/ebm.2010.009329. [DOI] [PubMed] [Google Scholar]
- 46.Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016;2016:9730467. doi: 10.1155/2016/9730467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunea A, Rugina DO, Pintea AM, Sconta Z, Bunea CI, Socaciu C. Comparative polyphenolic content and antioxidant activities of some wild and cultivated blueberries from Romania. Not Bot Horti Agrobo. 2011;39:70–76. [Google Scholar]
- 48.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]