Abstract
BACKGROUND/OBJECTIVES
The present study investigated the hypothesis that a highly pure linear β-1,3-glucan produced by Agrobacterium sp. R259 enhances human natural killer (NK) cell activity and suppresses pro-inflammatory cytokines.
SUBJECTS/METHODS
In an eight-week, double-blind, randomized, placebo-controlled clinical trial, 83 healthy adults with white blood cell counts of 4,000-8,000 cells/µL were participated and randomly assigned to take two capsules per day containing either 350 mg β-1,3-glucan or placebo. Six participants withdrew their study consent or were excluded due to NK cell activity levels outside the normal range. NK cell activity and serum levels of immunoglobulin G (IgG) and cytokines, such as interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-6, IL-10, IL-12 and tumor necrosis factor (TNF)-α were measured.
RESULTS
NK cell activity and the serum levels of IL-10 were significantly higher from baseline to week 8 in the β-glucan group compared with the placebo group (P = 0.048, P = 0.029). Consumption of β-1,3-glucan also significantly increased NK cell activity compared with placebo after adjusting for smoking and stress status (P = 0.009). In particular, the effect of β-1,3-glucan on NK cell activity was greater in participants with severe stress than in those experiencing mild stress. However, the administration β-1,3-glucan did not significantly modulate the levels of IFN-γ, IL-2, IL-4, IL-6, IL-12, TNF-α and IgG compared with the placebo.
CONCLUSION
The results showed that supplementation with bacterial β-1,3-glucan significantly increased NK cell activity without causing any adverse effects. Additionally, the beneficial effect of β-1,3-glucan on NK cell activity was greater in participants experiencing severe stress.
Keywords: Glucans, natural killer cells, interleukin-10, cytokines, clinical trial
INTRODUCTION
Natural β-D-glucose polysaccharides, the β-glucans, which occur in the cell walls of cereal, fungi, yeast and bacteria, have been reported to be a modulator of immune responses [1]. Variant forms of β-glucans from different sources have been identified, such as bacterial β-glucan structured with only a β-1,3 linkage, yeast and fungal β-glucans containing a β-1,3 backbone with 1,6 side branches, and cereal β-glucan with β-1,3 and β-1,4 backbones [2].
β-Glucans have been shown to activate pattern recognition receptors expressed on immune cells, such as macrophages, dendritic cells, neutrophils and lymphocytes [1,3]. Additionally, the enhancement of natural killer (NK) cell activity by β-glucan has been reported to play an important role in immune potency and to have anti-carcinogenic effects in in vitro and in vivo studies [4,5]. The immune stimulation and antitumoral activities of these β-glucans have been thought to be caused only by the β-1,3-glucans [6]. However, several types of β-1,3-glucans from different sources have been shown to result in a variety of different immune responses.
The majority of previous studies have reported on the effects of yeast or fungal β-1,3/1,6-glucan on immune responses [7,8,9,10]. Yeast-derived β-1,3/1,6-glucans increased the serum concentration of interleukin (IL)-10 in overweight humans [7]. Mushroom-derived β-1,3/1,6-glucans also enhanced IL-8 synthesis in both peripheral blood mononuclear cells (PBMC) and polymorphonuclear leukocyte cultures from human volunteers [8], increased the number of circulating NK cells in athletes [9], and increased NK cell activity in healthy adults [10]. However, β-1,3/1,4-glucans from oats did not alter NK cell activity or the levels of plasma cytokines in athletes [11].
Purified linear β-1,3-glucans extracted from the mushroom Cordyceps militaris were reported to have anti-inflammatory properties in human monocytic cells; however, the yield of β-1,3-glucan was only 1.8% in that study [12]. Bacterial linear β-1,3-glucan has advantages in terms of cost, yield and efficiency, compared with β-glucans derived from mushrooms, plants, yeasts and fungi [13]. The linear β-1,3-glucan (curdlan) produced by Agrobacterium species inhibited cancer progression through immune modulation in a mouse model of breast cancer [14], and promoted myeloid-derived suppressor cells to differentiate into a more mature state in tumor-bearing mice [15].
In addition, bacterial linear β-1,3-glucans produced by Agrobacterium sp. R259, a mutant strain, increased interferon (IFN)-γ production in PBMCs of mice [16], and reversed the functional defects of NK cells and excessive immunoglobulin (Ig) A production in mice with inflammatory bowel syndrome [17]. However, no clinical study has investigated the effects of linear β-1,3-glucan produced by Agrobacterium sp. R259 on NK cell activity and cytokine concentrations. Therefore, the present study investigated the hypothesis that a highly pure β-1,3-glucan of low molecular weight (around 17 kDa) produced by Agrobacterium sp. R259 enhances NK cell activity and suppresses pro-inflammatory cytokines in healthy adults.
SUBJECTS AND METHODS
Study materials
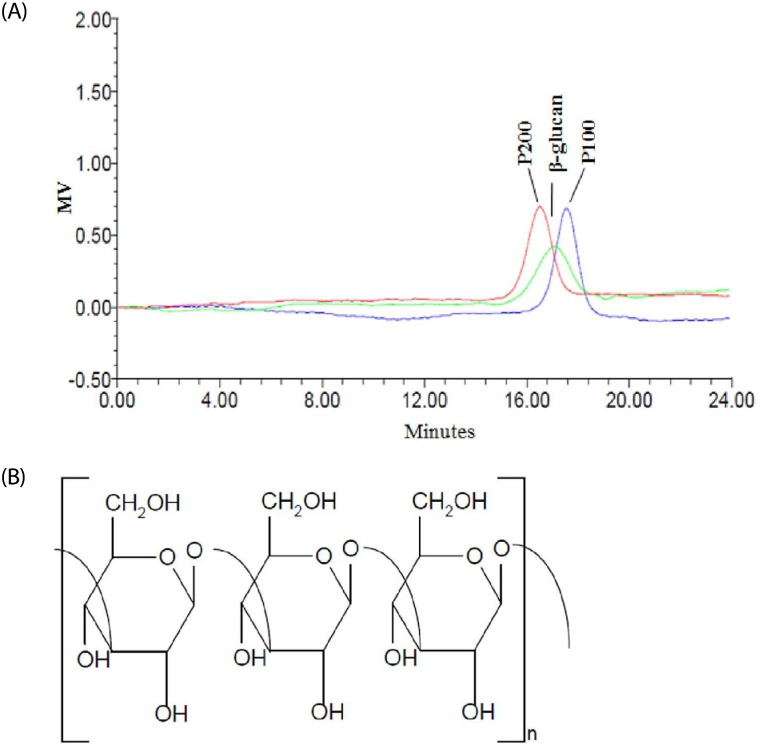
The β-1,3-glucan was prepared by fermentation using Agrobacterium sp. R259 (Korean Culture Type Collection, Daejeon, Korea; KCTC 10197BP) in sugar medium (Naturence Co., Ltd., Gongju City, Korea). After fermentation, β-1,3-glucan was separated by centrifugation at 8,000 × g for 30 min, precipitated, washed, and spray-dried to obtain purified β-glucan (85%). The estimated molecular size of the β-1,3-glucan was approximately 17~25 kDa, as determined by HPLC analysis (Fig. 1). The endotoxin level of the β-1,3-glucan was determined using a Limulus Amebocyte Lysate test kit (Pyrogent Plus, Lonza, Walkersville, MD, USA), and found to be < 0.07 EU/mL. According to prior studies [16,17] on immune responses to bacterial β-1,3-glucan, the optimal daily dose of β-1,3-glucan was 350 mg. Each capsule of β-1,3-glucan contained 206.0 mg β-1,3-glucan powder (as 175.0 mg β-1,3-glucan), 64.0 mg cellulose, 22.0 mg dextrin, 3.8 mg magnesium stearate, and 4.2 mg silicon dioxide; each placebo capsule contained 180.0 mg cellulose, 112.8 mg dextrin, 3.0 mg magnesium stearate, and 4.2 mg silicon dioxide.
Fig. 1. HPLC chromatogram (A) and structure of β-1,3-glucan (B).
Participants
The study participants were recruited via poster and newspaper advertisements during September 2014. The inclusion criteria for participants were as follows: age between 25 and 70 years old, a white blood cell (WBC) count between 4,000–8,000 cells/µL, no chronic disease such as hypertension, diabetes and thyroid disease, no medical history of myocardial infarction or cerebrovascular disease, not pregnant or lactating. The exclusion criteria were as follows: any infectious disease, an immune or psychiatric disorder, intake any medication or supplements regularly within the previous 3 months, a creatinine level ≥ 2 times the normal upper limit, and an aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level ≥ 3 times the normal upper limit. The perception of stress was investigated whether in the habitual stress status of the subject. This self-reporting of stress using a four-step scale (never, mild, severe and very severe) was used previously by Fries et al. [18]. One hundred and fifty-six volunteers were screened, and eighty-three healthy adults were the participants who met the study criteria. All participants were in accordance with the declaration of Helsinki, and gave a written informed consent, which was approved by the Institutional Review Board of Hanyang University (HYI-14-084-1).
Study design
This study was designed as an eight-week, double-blind, randomized controlled clinical trial. Four visits were required of participants, including 1 for screening and 3 for the intervention study. Participants were interviewed to obtain socio-demographic data and medical history, including age, marital status, and family history of immune disorders, smoking habits, alcohol consumption, physical activity, stress status, supplement usage, and medication. Two capsules of β-glucan or placebo containing cellulose were provided daily. This study was registered with the Clinical Research Information Service (KCT0001868).
The three scheduled visits for this clinical trial were conducted at the following times after the initial screening: visit 1 (baseline, 7-10 days post-screening); visit 2 (week 4); and visit 3 (week 8). Fasting blood and urine samples were collected and stored at −20℃ until final analysis for study outcomes and a safety assessment test. At weeks 4 and 8, compliance was monitored by counting the number of remaining capsules.
Participants were informed not to change their usual dietary habit and lifestyle, and not to take any health products containing β-glucan during the study. To investigate whether their dietary habits changed, participants were asked about their previous day's food intake using a 24-hr dietary recall at visits 2 to 4. These dietary records were analyzed with Can-pro 4.0 (Computer Aided Analysis Program 4.0 for professionals, Korean Society of Nutrition, Seoul, Korea).
Clinical and biochemical measurements
Height was measured using a stadiometer at the baseline visit, and weight was measured with participants wearing light clothing and without shoes using an Inbody 720 (Biospace Corporation, Seoul, Korea). Body mass index (BMI, kg/m2) was calculated as weight divided by the square of the body height.
NK cell activity and cytokines were measured at weeks 0, 4, and 8. PBMCs were isolated by density gradient separation, resuspended in phosphate-buffered saline, and determined using trypan blue solution. NK cell activity was calculated from the results of nonradioactive cytotoxicity assay kits (Promega Inc., Madison, WI, USA). Effector cells (PBMCs) were seeded in 96-well plates, with 50 µL of target cells (2×105/mL, K562 cells, Korean Cell Line Bank, Seoul, Korea), and incubated for 4 hours at 37℃. The ratio of effector:target cells was 100:1, and each assay was performed in triplicate. The cytotoxic activity of cells was determined using an iMark TM microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490 nm. Cytotoxicity was calculated using the following formula:
| Cytotoxicity (%) = [(experimental-effector spontaneous-target spontaneous)/(target maximum-target spontaneous)]×100. |
The serum levels of cytokines, including IFN-γ, IL-2, IL-4, IL-6, IL-10, IL-12 and tumor necrosis factor (TNF)-α were measured using the Luminex system (Bio-Plex 200, Bio-Rad, Korea) on multi-analyte panels with a Bio-Plex cytokine assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer's protocol. Data analysis was performed with Bio-plex Manager 6.1 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Serum immunoglobulin G (IgG) was measured using a turbidimetry immunoassay system (Cobas C501, Roche, Japan) with an IGG-2 immunoassay kit (Roche Diagnostics GmbH, Mannheim, Germany), and the degree of the turbidity caused by the aggregate was determined measuring absorbance at 340 nm.
Safety assessment
General blood parameters, blood pressure, pulse rate and body temperature were measured during each study visit, and adverse events and drug usage were also recorded during the study. Hematology lab tests, including red blood cell (RBC) count, WBC count, hemoglobin, hematocrit, platelet and differential leukocyte count, were determined by a Coulter STKS hemocytometer (Beckman Coulter Inc., Fullerton, CA, USA). Serum were analyzed with a Hitachi 7150 automated analyzer (Hitachi Ltd., Tokyo, Japan) for blood chemistry assays, and urine were examined with a Clinitek Atlas automated urine chemistry analyzer (Siemens Healthcare Diagnostics, NY, USA). Blood pressure and pulse rate were measured by an Omron HEM-7051 device (Omron healthcare, Kyoto, Japan), and body temperature was measured using an infrared thermometer (Thermoscan IRT-4020, Braun Corporation, Kronberg, Germany).
Randomization
Participants were randomly assigned to an experimental group to receive either β-glucan or the placebo using a computer-generated randomization program. The sequentially numbered packs with either β-glucan or placebo were randomly assigned to participants at their first visit. The identity codes concealed in sequentially numbered envelopes were managed by the study investigators, and monitored by clinical research associates (Neonutra Corporation, Seoul, Korea). All study investigators and participants remained blinded to the identity codes during the study.
Statistical analysis
The primary outcome of this study was the changes in NK cell activity between the β-glucan and placebo groups after 8 weeks. The power calculation was based on the results of previous studies [10]. In order to achieve a statistical power of 80% (P < 0.05, two-tailed test), the required sample size was calculated to be 32 in each group. The final required sample size was calculated to be 40 in each group, allowing for the predicted drop-out rate of 20% during the study [19,20].
Statistical analyses were conducted using SAS software, version 9.2 (SAS, Inc., Cary, North Carolina, USA). The final results from study participants were evaluated using full analysis set (FAS). Participants were included if they completed the 8-week trial and had taken β-glucan or placebo at least once. The continuous variables of the baseline characteristics between the β-glucan and placebo groups were compared using an independent t-test; categorical variables were compared using a Chi-square test or Fisher's exact test. NK cell activity and cytokine concentrations between the two groups from week 0 to week 4 and week 8 were compared using an ANCOVA test after adjusting for smoking and stress status. Statistical significance was considered at P < 0.05. All data are presented as means ± SDs for continuous variables or as n (%) for categorical variables.
RESULTS
Participant characteristics
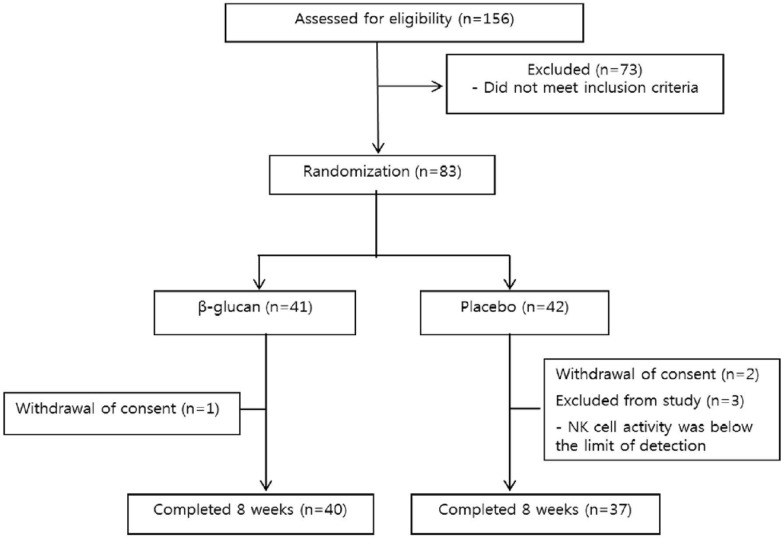
Among the eighty-three males and females recruited, forty-one participants were randomly assigned to the β-glucan group, and forty-two were assigned to the placebo group (Fig. 2). One participant in the β-glucan group and two in the placebo group withdrew their study consent, and three participants in the placebo group were excluded due to NK cell activity outside the normal range. Compliance was not significantly different between the β-glucan and placebo groups (94.47 ± 7.65% vs. 92.40 ± 10.65%, P = 0.333). Nutrients intake during this study were not observed to be significantly different between the two groups, with the exception of baseline carbohydrate intake (Supplemental Table 1). However, the carbohydrate intake between two groups was not any longer significantly different at week 8.
Fig. 2. Schematic diagram of study design.
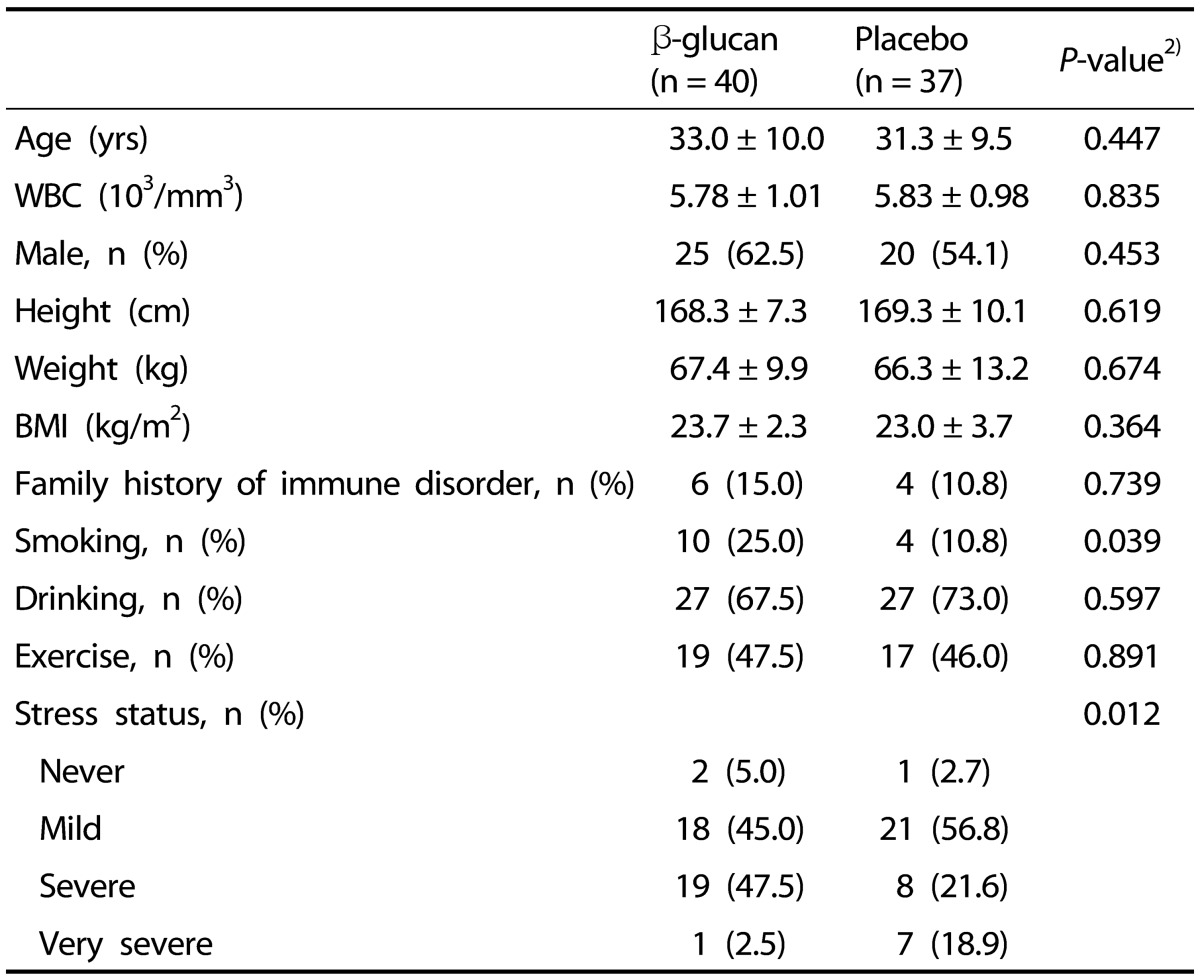
The general characteristics of the participants are shown in Table 1. Baseline characteristics, such as age, WBC counts, sex, BMI, presence of family history related with immune disorders, drinking and exercising were not significantly different between the groups. However, there were a higher proportion of current smokers and more participants reporting very severe stress in the β-glucan group as compared with the placebo group.
Table 1. Baseline characteristics of β-glucan and placebo groups1).
1) Values are means ± SDs or numbers of participants (percentage distribution).
2) P-values were determined by the independent t-test or the Chi-square test or Fisher's exact test between β-glucan and placebo groups.
WBC, white blood cell; BMI, body mass index.
NK cell activity and cytokine concentrations in the β-glucan and placebo groups
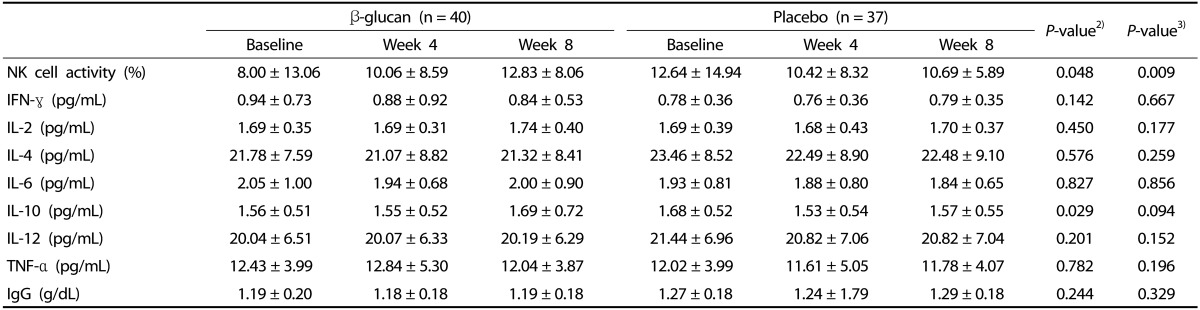
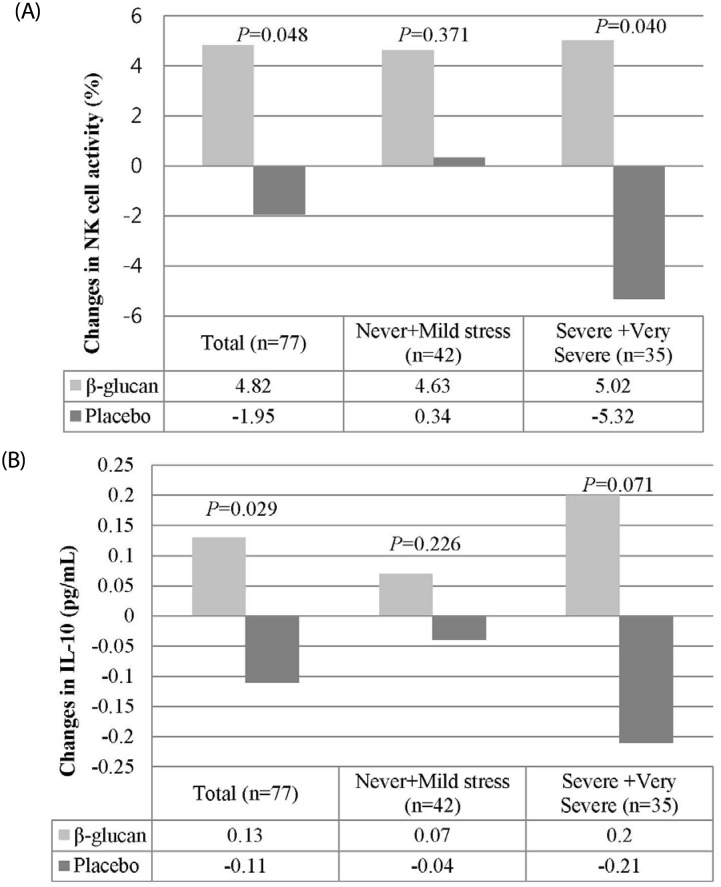
NK cell activity and cytokine concentrations in the β-glucan and placebo groups are shown in Table 2. NK cell activity was significantly higher from baseline to week 8 in the β-glucan group compared with the placebo group after adjusting for smoking and stress status (P = 0.009). The levels of IL-10 were also significantly higher from baseline to week 8 in the β-glucan group compared with the placebo group (P = 0.029). The levels of IFN-γ, IL-2, IL-4, IL-6, IL-12, TNF-α and IgG were not significantly different between the β-glucan and placebo groups. The immunological effects of β-glucan were assessed in the groups with mild stress (never + mild stress) and severe stress (severe and very severe stress), as shown in Fig. 3. In the group with severe stress, β-glucan significantly increased NK cell activity from baseline to week 8; this effect was not observed in the group with mild stress.
Table 2. Natural killer cell activity, cytokine, and IgG concentrations between β-glucan and placebo groups1).
1) Values are means ± SDs.
2) P-values for differences of changes from baseline between the β-glucan and placebo groups at week 8 were determined by the independent t-test.
3) P-values for difference of changes from baseline between the β-glucan and placebo groups at week 8 were determined by the ANCOVA after adjusting for smoking and stress status.
NK cell activity, natural killer cell activity; IFN-γ, interferon-γ; IL-2, interleukin-2; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; IL-12, interleukin-12; TNF-α, tumor necrosis factor-α; IgG, immunoglobulin G.
Fig. 3. Changes in NK cell activity (%) and in the levels of IL-10 (pg/mL) according to stress status.
(A) Changes in NK cell activity (%) from baseline to week 8 between the β-glucan and placebo groups. (B) Changes in the levels of IL-10 (pg/mL) from baseline to week 8 between the β-glucan and placebo groups. P-values were determined using an independent t-test.
Safety assessment
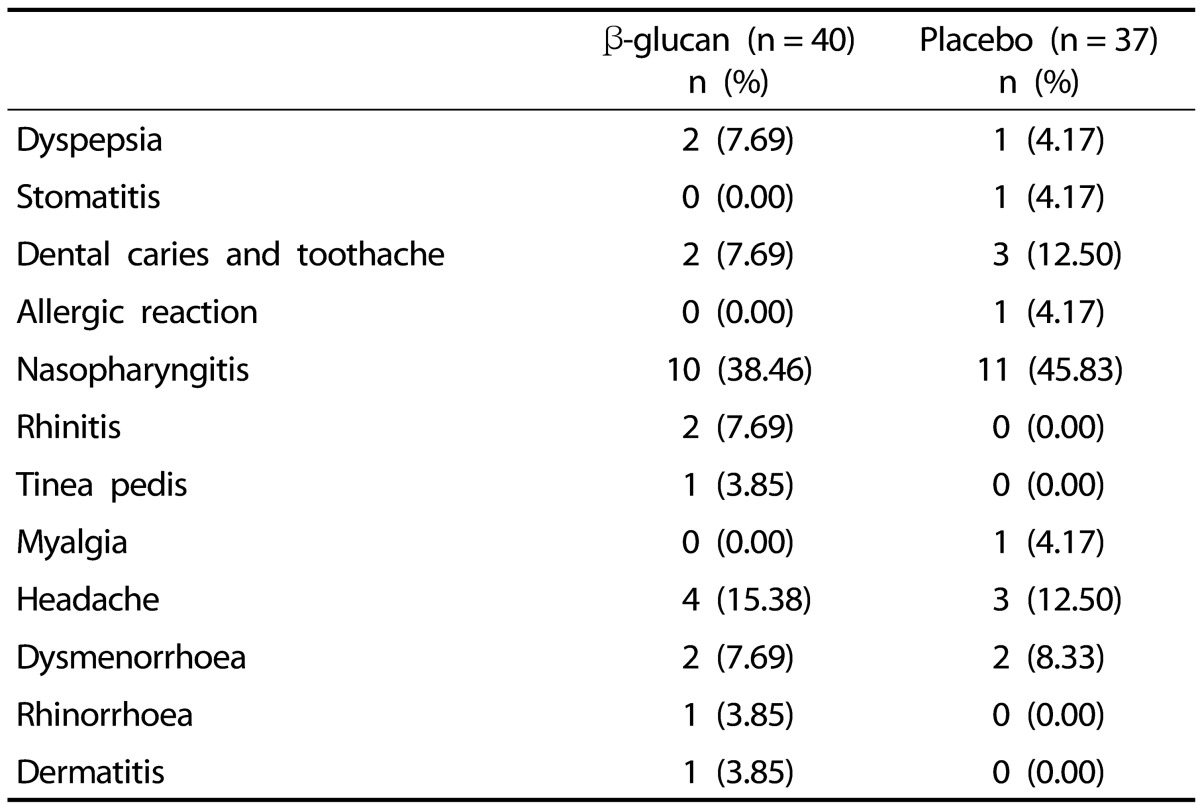
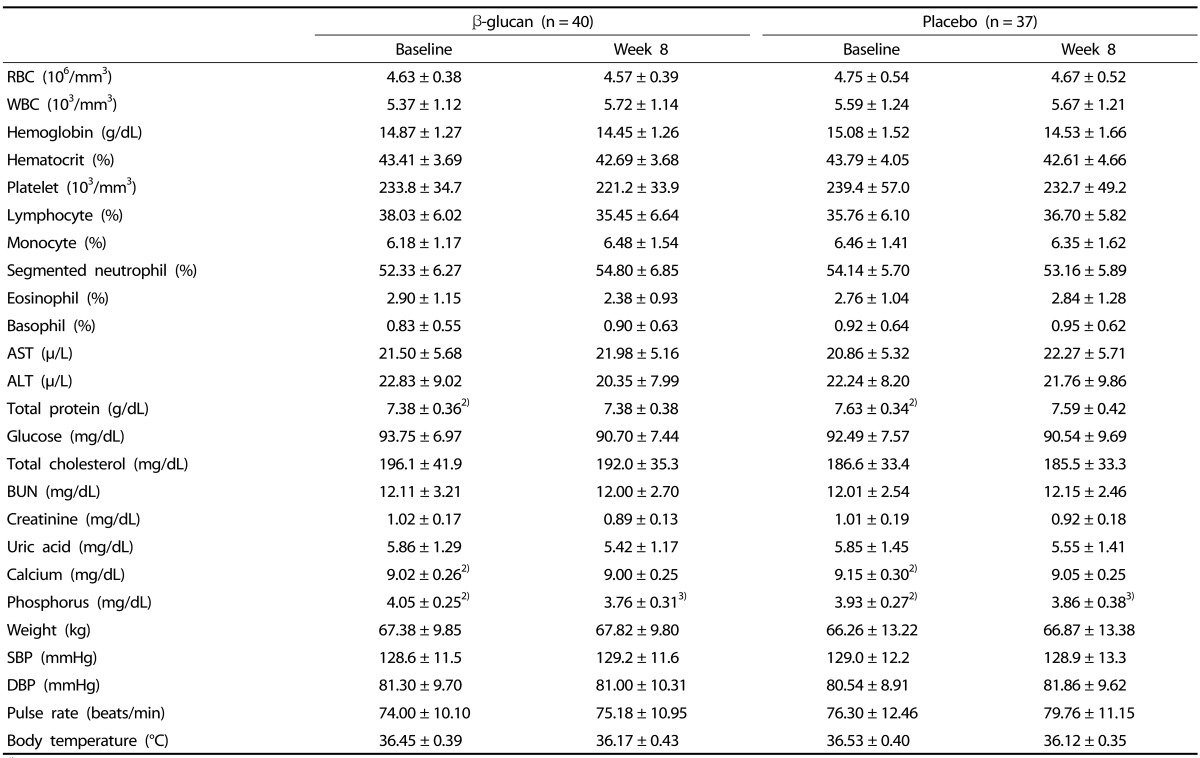
Mild adverse events were reported by 17 participants (41.46%, 26 cases) in the β-glucan group and 17 participants (40.48%, 24 cases) in the placebo groups; none of the participants withdrew due to adverse events (Table 3). All adverse events in the two groups were found to be unrelated to intervention. The hematological parameters and biochemical variables measured during the study period are presented in Table 4. Serum levels of calcium and phosphorus were significantly different between the β-glucan and placebo groups at baseline. In addition, the changes in serum phosphorus from baseline to week 8 were significantly different between the β-glucan and placebo groups. However, the levels of phosphorus were all within a normal range. There were no significant differences in any of the other biological parameters between groups. The results from all hematologic, biochemical and liver function tests were within in the normal range. Urinary parameters were also not significantly different between the groups and were within normal range (data not shown).
Table 3. Adverse events frequently occurring in participants taking either β-glucan or placebo groups.
Table 4. Safety assessments in participants of β-glucan and placebo groups1).
1) Values are means ± SDs.
2) P-values < 0.05 for differences between the β-glucan and placebo groups at baseline were determined by the independent t-test.
3) P-values < 0.05 for differences of changes from baseline between the β-glucan and placebo groups at week 8 were determined by the independent t-test.
RBC, red blood cell; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; SBP, systolic blood pressure; DBP, diastolic blood pressure.
DISCUSSION
This randomized, double-blind, placebo-controlled study demonstrated that supplementation with purified bacterial linear β-1,3-glucan significantly increased NK cell activity, compared with a placebo, without any significant adverse effects. The effect of β-1,3-glucan was greater in participants reporting severe stress than in those experiencing mild stress.
Previous studies have consistently reported that supplementation with β-1,3/1,6-glucans from yeast or mushrooms activated NK cells in mice with cancer [21] and in healthy adults [10], and prevented reductions in NK cell activity and counts during the recovery period after intensive training [22]. Although β-1,3/1,6-glucans have immunological and anticarcinogenic effects, the use of yeast, mushrooms or natural products as a source could be associated with problems such as low yield and the requirement for more expensive and complicated processes for purification [12]. Utilization of bacterial β-1,3-glucan could solve these problems, and recent studies have confirmed that bacterial linear β-1,3-glucan also modulated the immune response in in vitro [14,16] and in vivo studies [17]. Wu et al. [14] reported that bacterial linear β-1,3-glucan (curdlan) produced by Agrobacterium species inhibited the progression of human breast cancer by preventing the generation of pro-tumor iTh2 cells. Shim et al. [16] reported that bacterial β-1,3-glucan from a mutant strain of Agrobacterium species increased IFN-γ production in PBMCs of mice. Lee et al. [17] also showed that the linear β-1,3-glucan produced by a mutant strain of Agrobacterium species prevented the progression of dextran sulfate sodium (DSS)-induced inflammatory bowel disease by recovering the reduction in Tregs, the functional defect of NK cells and the excessive IgA production in mice. In agreement with the previous results, our purified bacterial β-1,3-glucan could modulate the immune response by enhancing NK cell activities in humans.
In particular, the present study showed that the effect of β-glucan on NK cell activity was greater in participants with severe stress as compared with those with mild stress. Chronic stress had been reported to be associated with suppressed cellular immunity [23], and with both pro-inflammatory and anti-inflammatory cytokines [24]. Moreover, psychological stress has been suggested to reduce NK cell activity through the increased production of circulating glucocorticoid [25]. The results of the present study suggest that administration of β-1,3-glucan could prevent the reduction of immune responses caused by stress.
In the present study, bacterial β-1,3-glucan significantly increased the serum level of IL-10, which is an anti-inflammatory cytokine that acts as a negative feedback regulator of diverse immune responses in preventing inflammatory and autoimmune pathologies [26]. The effect of a β-glucan on IL-10 concentrations has been reported for the role of Dectin-1, a receptor of β-1,3-glucan expressed on NK cells [7,27,28]. The activation of NF-κB by β-1,3-glucans leads to the release of cytokines, including IL-12, IL-6, TNF-α and IL-10 [1,29]. Supplementation with yeast-β-1,3/1,6-glucans increased plasma levels of the anti-inflammatory cytokine IL-10 mRNA in adipose tissue in overweight to obese subjects with moderately elevated C-reactive protein (CRP) levels [7]. In addition, curdlan (a linear bacterial β-1-3-glucan) has been suggested to induce IL-10 producing CD4(+) T cells and inhibit the development of eosinophilic airway inflammation [27]. As a result of previous studies, it is likely that β-1,3-glucan could enhance the immune response against pathogens by activating NK cells, and control the pro-inflammatory cytokines by increasing IL-10 secretion. Therefore, β-1,3-glucan is considered to play a regulatory role between the pro-inflammatory and anti-inflammatory cytokines.
In contrast to previous studies, the present study showed that bacterial linear β-1,3-glucan had no significant effect on the other pro-inflammatory cytokines, such as IFN-γ, IL-2, IL-4, IL-6, IL-12, TNF-α and IgG. In particular, no effect was seen on IFN-γ, a well-known pro-inflammatory cytokine produced predominantly by NK cells as part of the innate immune response, and associated with autoimmune diseases [30]. In addition to NK cell activity, IFN-γ production in human PBMC was induced by IL-12 [31], and was also secreted by activated T cells [32]. Cooper et al. [33] reported that IFN-γ was primarily produced by CD56bright NK cell subsets, which had the capacity to produce abundant cytokines. However, the majority of this cytotoxic response in NK cells was induced by CD56dim NK cell subsets, which comprise 90% of human NK cells and produce fewer cytokines compared to CD56bright NK cell subsets [33]. Thus, changes in IFN-γ may be not accompanied by increasing NK cell activity in healthy participants. IL-10 is a potent regulator of anti-inflammatory immune responses and, hence, is considered to be a down-regulator of pro-inflammatory cytokine production by Th1 cells and macrophages [34,35]. Therefore, the regulation of IL-10 could affect the concentrations of pro-inflammatory cytokines, such as IL-12 and TNF-α. Inconsistent results could be also due to the source, branches and purity of β-glucan.
This study has a few limitations. Psychological stress and smoking could be confounding factors on immune responses [25,36]. In our study there were significant differences between the two groups with respect to these two factors. To minimize bias, the statistical analyses were conducted after adjustment for stress status and smoking. In addition, we did not measure whether participant stress status changed during the study period. Another limitation was that NK cell counts and other functional properties of NK cells, such as IL-17A, MIP-1α or MIP-1β, and activation of NK cell receptors (CD94 or KIRs), were not investigated in this study.
This present study is the first randomized, double-blind, placebo-controlled study to suggest that the administration of Agrobacterium species-derived bacterial β-1,3-glucan significantly increases NK cell activity in healthy adults without causing significant adverse effects. Future clinical trials will be needed to confirm the immune responses of patients with cancer or chronic stress to bacterial linear β-1,3-glucan.
Footnotes
This research was supported by the Ministry of Agriculture, Food and Rural Affairs, and partly Korea Food Research Institute and Naturence Co., Ltd. (G0142014039), and by the National Research Foundation of Korea (2015R1D1A1A09060823).
COMPETING INTERESTS: The authors declare no potential conflicts of interests
Supplementary Material
Nutrient intake of the β-glucan and placebo groups1)
References
- 1.Chan GC, Chan WK, Sze DM. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy EA, Davis JM, Carmichael MD. Immune modulating effects of β-glucan. Curr Opin Clin Nutr Metab Care. 2010;13:656–661. doi: 10.1097/MCO.0b013e32833f1afb. [DOI] [PubMed] [Google Scholar]
- 3.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–1547. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani MS, Bellini MF, Angeli JP, Oliveira RJ, Silva AF, Ribeiro LR. β-glucans in promoting health: prevention against mutation and cancer. Mutat Res. 2008;658:154–161. doi: 10.1016/j.mrrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Yoon TJ, Koppula S, Lee KH. The effects of β-glucans on cancer metastasis. Anticancer Agents Med Chem. 2013;13:699–708. doi: 10.2174/1871520611313050004. [DOI] [PubMed] [Google Scholar]
- 6.Heinsbroek SE, Williams DL, Welting O, Meijer SL, Gordon S, de Jonge WJ. Orally delivered β-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutr Res. 2015;35:1106–1112. doi: 10.1016/j.nutres.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohl A, Gögebakan O, Möhlig M, Osterhoff M, Isken F, Pfeiffer AF, Weickert MO. Increased interleukin-10 but unchanged insulin sensitivity after 4 weeks of (1, 3)(1, 6)-β-glycan consumption in overweight humans. Nutr Res. 2009;29:248–254. doi: 10.1016/j.nutres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Nameda S, Harada T, Miura NN, Adachi Y, Yadomae T, Nakajima M, Ohno N. Enhanced cytokine synthesis of leukocytes by a β-glucan preparation, SCG, extracted from a medicinal mushroom, Sparassis crispa. Immunopharmacol Immunotoxicol. 2003;25:321–335. doi: 10.1081/iph-120024500. [DOI] [PubMed] [Google Scholar]
- 9.Bergendiova K, Tibenska E, Majtan J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur J Appl Physiol. 2011;111:2033–2040. doi: 10.1007/s00421-011-1837-z. [DOI] [PubMed] [Google Scholar]
- 10.Eom SY, Zhang YW, Kim NS, Kang JW, Hahn YS, Shin KS, Song HG, Park SY, Kim JS, Kim H, Kim YD. Effects of Keumsa Sangwhang (Phellinus linteus) mushroom extracts on the natural killer cell activity in human. Korean J Food Sci Technol. 2006;38:717–719. [Google Scholar]
- 11.Nieman DC, Henson DA, McMahon M, Wrieden JL, Davis JM, Murphy EA, Gross SJ, McAnulty LS, Dumke CL. β-glucan, immune function, and upper respiratory tract infections in athletes. Med Sci Sports Exerc. 2008;40:1463–1471. doi: 10.1249/MSS.0b013e31817057c2. [DOI] [PubMed] [Google Scholar]
- 12.Smiderle FR, Baggio CH, Borato DG, Santana-Filho AP, Sassaki GL, Iacomini M, Van Griensven LJ. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (13)-β-D-glucan. PLoS One. 2014;9:e110266. doi: 10.1371/journal.pone.0110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MK, Ryu KE, Choi WA, Rhee YH, Lee IY. Enhanced production of (1 → 3)-β-D-glucan by a mutant strain of Agrobacterium species. Biochem Eng J. 2003;16:163–168. [Google Scholar]
- 14.Wu TC, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, Anguiano E, Pedroza-Gonzalez A, Snipes GJ, O'Shaughnessy J, Nishimura S, Liu YJ, Pascual V, Banchereau J, Oh S, Palucka K. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. 2014;2:487–500. doi: 10.1158/2326-6066.CIR-13-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rui K, Tian J, Tang X, Ma J, Xu P, Tian X, Wang Y, Xu H, Lu L, Wang S. Curdlan blocks the immune suppression by myeloid-derived suppressor cells and reduces tumor burden. Immunol Res. 2016;64:931–939. doi: 10.1007/s12026-016-8789-7. [DOI] [PubMed] [Google Scholar]
- 16.Shim JH, Choi WA, Sang BC, Yoon DY. Immune stimulating efficacy of insoluble β-1,3-glucan from Agrobacterium sp. R259 KCTC 10197BP. Yakhak Hoeji. 2002;46:459–465. [Google Scholar]
- 17.Lee KH, Park M, Ji KY, Lee HY, Jang JH, Yoon IJ, Oh SS, Kim SM, Jeong YH, Yun CH, Kim MK, Lee IY, Choi HR, Ko KS, Kang HS. Bacterial β-(1,3)-glucan prevents DSS-induced IBD by restoring the reduced population of regulatory T cells. Immunobiology. 2014;219:802–812. doi: 10.1016/j.imbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Fries R, König J, Schäfers HJ, Böhm M. Triggering effect of physical and mental stress on spontaneous ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Clin Cardiol. 2002;25:474–478. doi: 10.1002/clc.4960251007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auinger A, Riede L, Bothe G, Busch R, Gruenwald J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body's defence against pathogens: a double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur J Nutr. 2013;52:1913–1918. doi: 10.1007/s00394-013-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colker CM, Swain M, Lynch L, Gingerich DA. Effects of a milk-based bioactive micronutrient beverage on pain symptoms and activity of adults with osteoarthritis: a double-blind, placebo-controlled clinical evaluation. Nutrition. 2002;18:388–392. doi: 10.1016/s0899-9007(01)00800-0. [DOI] [PubMed] [Google Scholar]
- 21.Vetvicka V, Vetvickova J. Glucan supplementation has strong anti-melanoma effects: role of NK cells. Anticancer Res. 2015;35:5287–5292. [PubMed] [Google Scholar]
- 22.Bobovčák M, Kuniaková R, Gabriž J, Majtán J. Effect of Pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl Physiol Nutr Metab. 2010;35:755–762. doi: 10.1139/H10-070. [DOI] [PubMed] [Google Scholar]
- 23.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priyadarshini S, Aich P. Effects of psychological stress on innate immunity and metabolism in humans: a systematic analysis. PLoS One. 2012;7:e43232. doi: 10.1371/journal.pone.0043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush KA, Krukowski K, Eddy JL, Janusek LW, Mathews HL. Glucocorticoid receptor mediated suppression of natural killer cell activity: identification of associated deacetylase and corepressor molecules. Cell Immunol. 2012;275:80–89. doi: 10.1016/j.cellimm.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima S, Hirose K, Iwata A, Takahashi K, Ohkubo A, Tamachi T, Ikeda K, Kagami S, Nakajima H. β-glucan curdlan induces IL-10-producing CD4+ T cells and inhibits allergic airway inflammation. J Immunol. 2012;189:5713–5721. doi: 10.4049/jimmunol.1201521. [DOI] [PubMed] [Google Scholar]
- 28.Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8:e60086. doi: 10.1371/journal.pone.0060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeković DB, Kwiatkowski S, Vrvić MM, Jakovljević D, Moran CA. Natural and modified (1-->3)-β-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol. 2005;25:205–230. doi: 10.1080/07388550500376166. [DOI] [PubMed] [Google Scholar]
- 30.Schoenborn JR, Wilson CB. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 31.Budak F, Goral G, Oral HB. Saccharomyces cerevisiae beta-glucan induces interferon-gamma production in human T cells via IL-12. Turk J Immunol. 2008;13:21–26. [Google Scholar]
- 32.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) Clin Ter. 2006;157:377–386. [PubMed] [Google Scholar]
- 33.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 34.Ala Y, Pasha MK, Rao RN, Komaravalli PL, Jahan P. Association of IFN-γ : IL-10 cytokine ratio with Nonsegmental Vitiligo pathogenesis. Autoimmune Dis. 2015;2015:423490. doi: 10.1155/2015/423490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 36.O'Shea D, Cawood TJ, O'Farrelly C, Lynch L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke. PLoS One. 2010;5:e8660. doi: 10.1371/journal.pone.0008660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nutrient intake of the β-glucan and placebo groups1)