Abstract
To identify subgroups of individuals with similar trajectories in blood pressure (BP) from childhood to young adulthood, and to determine the relationship of BP trajectories with carotid intima-media thickness (IMT) and left ventricular mass index (LVMI). BP was measured up to 16 times over a 23-year period in 683 participants from childhood to young adulthood. IMT and LVMI were measured in 551 participants and 546 participants respectively. Using latent class models, three trajectory groups in BP from childhood to young adulthood were identified, including high-increasing (HI), moderate-increasing (MI) and low-increasing (LI) group. We found that trajectory of systolic blood pressure (SBP) was a significant predictor of both IMT and LVMI with increased rate of growth in SBP associated with higher levels of IMT and LVMI (P for trend <0.001). Similar to the BP trajectory groups from childhood to young adulthood, three trajectory groups in BP during childhood (≤18 years) were identified and participants in the HI group had thicker IMT (P <0.001) and increased LVMI (P =0.043) in comparison with those in the LI group. Results were similar for Mid-BP trajectories but not for diastolic BP trajectories. Our results suggested that different BP trajectories exist from childhood to young adulthood, and the trajectories were independently associated with IMT and LVMI. We for the first time reported the association between SBP trajectories derived from childhood with subclinical cardiovascular risk in young adulthood, indicating that monitoring trajectories of BP from childhood may help identify a high cardiovascular risk population in early life.
Keywords: blood pressure trajectories, intima-media thickness, left ventricular mass index, youth cohort, longitudinal study
INTRODUCTION
High blood pressure (BP) has been well established as a major risk factor for cardiovascular disease (CVD) and stroke,1, 2 which are the leading causes of death and disability worldwide.3 In addition to the BP levels, which are usually measured only one time at the baseline, recent longitudinal studies indicate that patterns of BP over time (i.e., BP trajectories) are associated with an increased risk of CVD. For example, Allen et al. have identified subgroups with differential BP trajectories among young adults and suggested that higher BP trajectories (i.e., BP increased more rapidly) were significantly associated with the presence of subclinical atherosclerosis in middle age.4 Numerous studies have demonstrated that levels of BP in childhood could predict coronary atherosclerosis risk in adulthood.5, 6 However, little is known about the varied BP trajectory patterns from childhood to young adulthood and their effects on CVD risk. Moreover, whether routine screening for primary hypertension should be performed in children and adolescents is still debated due to false-positive results.7, 8 Therefore, further research to examine the association between longitudinal BP and CVD risk, could shed some light on the necessity of BP routine screening from childhood. Taking advantage of a 23-year longitudinal cohort with up to 16 visits from childhood to young adulthood, we aimed to identify subgroups of individuals with differential trajectories in BP from childhood through young adulthood, and to determine the associations of BP trajectories with the risk of subclinical CVD, indexed by intima-media thickness (IMT) and left ventricular mass index (LVMI).9–14
METHODS
Study Population
The participants were from the Georgia Stress and Heart (GSH) study, an ongoing longitudinal study designed to evaluate the development of CV risk factors in youth and young adults.15 Recruitment and evaluation of participants have been described in detail elsewhere.15–17 Briefly, participants who met the following criteria were recruited: (1) aged 5 to 16 years in 1989, (2) African or European ancestry, (3) normotensive for age and gender based on BP screening, and (4) apparently healthy based on parental reports of the child’s medical history. All participants were recruited using family health history questionnaires obtained from a county-wide (Richmond County, Georgia) public school screening of children in kindergarten through grade 8 whose families were interested in health research. A high participation rate was obtained, with 96.3% of those contacted agreeing to participate. Our data encompass a 23-year period (1989 to 2012) in which 16 visits were conducted. Participants with 3 or more BP measurements were included in the analysis, and 75% of them completed more than 8 measurements (Table 1). The Institutional Review Board of the Medical College of Georgia have approved the study and an informed consent was provided by all subjects, or by parents if subjects were <18 years old.
Table 1.
Characteristics of participants by SBP trajectory group at baseline visit
| Characteristics | Low-increasing | Moderate-increasing | High-Increasing | P value |
|---|---|---|---|---|
| No. of participants | 334 | 266 | 83 | |
| Participants with > 8 visits, % | 252(75.4) | 200(75.2) | 65(78.3) | 0.836 |
| Age (years) | 11.6±3.6 | 12.3±3.5 | 12.4±3.4 | 0.041 |
| Sex (female, %) | 230(68.9) | 94(35.3) | 18(21.7) | <0.001 |
| Height (cm) | 146.5±18.9 | 153.4±19.1 | 155.9±18.2 | <0.001 |
| Weight (kg) | 45.2±20.3 | 54.5±24 | 58.8±22.6 | <0.001 |
| BMI (kg/m2) | 20.0±5.5 | 22.1±6.5 | 23.3±6.1 | <0.001 |
| SBP (mmHg) | 101.6±7.9 | 109.9±8.9 | 118.7±11.0 | <0.001 |
| DBP (mmHg) | 57.0±5.8 | 59.2±6.6 | 62.8±5.3 | <0.001 |
| Race | ||||
| European American (%) | 202(60.5) | 124(46.6) | 29(34.9) | <0.001 |
| African American (%) | 132(39.5) | 142(53.4) | 54(65.1) | |
| Father’s education level, % | ||||
| ≤11 | 39(11.9) | 51(19.6) | 20(24.7) | 0.004 |
| 12–15 | 224(68.1) | 147(56.5) | 50(61.7) | |
| ≥16 | 66(20.1) | 62(23.9) | 11(13.6) | |
SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI= body mass index
Procedure and Measurements
On each laboratory visit, demographic information was collected. Participants’ height and weight were measured with a Healthometer medical scale that was calibrated daily. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Body surface area (BSA) was calculated as SQRT ([height (cm) * weight (kg)]/3600).18 BP was measured with an automated oscillatory BP system (Dinamap Vital Signs Monitor, Model 1846 SX; Criticon Incorporated, Tampa, Fla), using an appropriately sized BP cuff that was placed on the participants’ right arm. BP measurements were taken at the end of the 11th, 13th, and 15th minutes during a 15-minute relaxation period in which participants were instructed to relax as completely as possible while lying (supine) with their head resting on a pillow. The average of the last two readings (at 13 and 15 minutes) was used to represent resting systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively. In addition to SBP and DBP, Mid-BP was also calculated ([SBP+DBP]/2) because it has been shown to have the greatest predictive utility for CVD in adults.19
IMT and LVM were measured in 551 participants (1215 measurements, 1015 measurements were eligible for analysis) and 546 participants (1229 measurements, 1107 measurements were eligible for analysis) respectively at visit 12, 14 and 15. Hewlett-Packard Sonos 5500 (Andover, MA) equipped with a 7.5 MHz linear array probe was used to measure the common carotid artery IMT. Left and right common carotid, carotid bulb, internal carotid and external carotid were first visualized in transverse then in longitudinal planes. Measurements were made at a point 2 cm proximal to the bifurcation on both near and far wall that showed the intima-media boundaries most clearly. Images were saved on high quality VHS tapes. IMT were derived from a computer program Vascular Tool (Medical Imaging Application, Iowa City, Iowa). This system uses an automated method for near and/or far wall border detection. Common carotid‘s IMT was measured as the distance from leading edge of first echogenic line to that of the second echogenic line. Ten frames of common carotid artery were analyzed by one experienced sonographer. The mean carotid IMT for left far wall was the primary outcome of interest in this analysis. Sector-guided M-mode echocardiograms were performed with a Hewlett Packard Sonos 1500 echocardiograph to measure the LVM. Left ventricular posterior wall in diastole, interventricular septum in diastole, and left ventricular internal diameter in diastole were measured according to the American Society of Echocardiography convention.20 LVM was calculated using the necropsy-validated formula of Devereux et al and normalized to BSA to obtain LVMI,21, 22 Intra- and interrater coefficients of variation for all cardiac structures assessed were 10%.23
Childhood socioeconomic status (SES) was indexed by father’s education level, because this measure remained highly stable across the years of this longitudinal cohort and was available for all participants used in the present study. We used the father’s education, measured at the midpoint of visits 1 to 12, as a representative for the whole study period. Father’s education was measured in years on a 7-point scale that ranged from less than high school to postgraduate education and was subsequently divided into 3 categories: low (<12 years), medium (≥12 and <16 years), and higher (≥16 years).
Statistical Analysis
Latent class modeling was used to identify subgroups that share a similar underlying trajectory in BP.24, 25 A Stata plugin program (Traj) was used for estimating group-based trajectory model using the maximum likelihood method.24, 26 Briefly, this method is designed to identify clusters of individuals following a similar developmental trajectory on an outcome of interest, based on a semiparametric group-based approach.27 Age was used as a timescale for the trajectories. Linear and quadratic terms of age were considered and evaluated based on their significance level. Selection of the best-fitting trajectory model was assessed using the Bayesian Information Criterion. We found that the model with 3 classes with up to quadratic order terms fit best. Then the associations of the trajectory groups with IMT and LVMI were examined by using a mixed linear regression model with unstructured covariance. Sequential models were constructed, including (1) unadjusted (trajectory group only), (2) adjusted for age, race, and gender; (3) additionally adjusted for BMI and fathers’ education level, and (4) additionally adjusted for BP levels. IMT and LVMI at visit 12, 14 and 15 were defined as the dependent variables. Age, BMI and BP at three visits were included as time-dependent variables. Race, gender, and the trajectory group were treated as categorical and time-invariant variables. Visits were treated as random effects in these models. The mixed linear models have the advantage to deal with missing values because they use all of the available data from an individual during follow-up. Furthermore, to examine the relationship of BP developmental patterns in childhood with atherosclerosis risk in young adulthood, we modeled the BP trajectories in childhood in 626 participants who had at least 3 BP measurements prior to 18 years old, and repeated all the analyses. The interactions between sex and SBP trajectories for IMT and LVMI were tested, and a sex-specific analysis was performed considering the occurrence of sex-differences in BP as early as adolescence. In a sensitivity analysis, we excluded 2976 measurements with obesity and 44 measurements taking anti-hypertensive medications. All data analyses were performed using Stata software version 12.1 (STATA Corp., TX, US). A two-sided P<0.05 was considered statistically significant.
RESULTS
Trajectory patterns of BP
The trajectories in BP were examined among 683 participants with 3 or more BP measurements from childhood to young adulthood. Of these, 7604 BP measurements were available to analysis. Three trajectory groups in BP from childhood to young adulthood were identified, including a high-increasing group (HI), a moderate-increasing (MI) and a low-increasing group (LI).
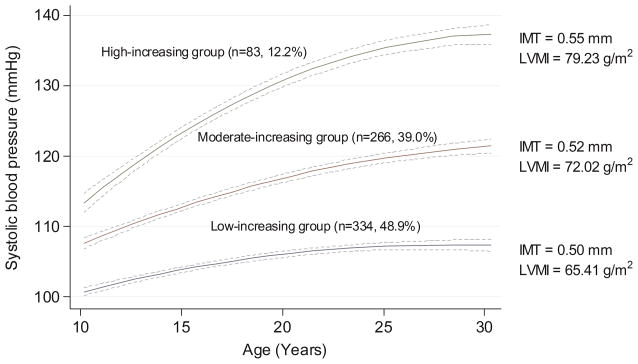
As shown in Figure 1, 83 (12.2%) participants started with a high level and had relatively fast increase in SBP level from childhood to young adulthood (HI group); 266 (39.0%) participants started with a moderate level and experienced a moderate increase in SBP (MI group); and 334 (48.9%) participants started with a low level and maintained a low increase in SBP (LI group). Overall, LI group maintained a small increase in mean level of SBP (9.1 mmHg) across 23 years. The MI group experienced an average of 13.9 mmHg increase, and the HI group had rapid increase over time (22.0 mmHg) in SBP (Table S1). As shown in Table 1, participants in the HI group tend to be male, black, higher BMI, and more likely to have a father with a lower educational level (P < 0.05). The trends remained the same when participants were followed up into adulthood. In addition, the percent of hypertension (SBP ≥ 140 or DBP ≥ 90 mmHg, or taking antihypertensive medications) was 42.2% in HI group, significantly higher than 1.8% in LI and 6.0% in MI group (Tables S2). The trajectories identified in Mid-BP and DBP were shown in Figure S1A and Figure S2A.
Figure 1. Trajectory groups identified for systolic blood pressure (SBP) from childhood to adulthood.
Their patterns by age, the number and percentage were shown for each group. The mean levels of IMT and LVMI in adulthood were also shown for each group. Dash lines are 95% confidence interval lines.
The association between trajectories of BP with IMT and LVMI
Table S3 shows the differences between the participants who were used to identify the BP trajectories and those who are not included in the association analyses later. The results show that sex and race were significantly different for IMT, whereas race and father’s education level were significantly different for LVMI. The mean IMT and LVMI in the HI and MI group were higher than in the LI group (0.55, 0.52 and 0.50 mm for IMT; 79.23, 72.02 and 65.41g/m2 for LVMI) (Figure 1). Table 2 presents results of mixed regression models for trajectories of SBP with IMT and LVMI respectively. Increased rate of growth in SBP was significantly associated with increased IMT and LVMI in young adulthood (P for trend <0.001). Compared to the LI group, individuals in the MI and HI groups showed higher IMT (β=0.019, P=0.007 for the MI group; β=0.051, P =0.012 for the HI group) and LVMI (β=2.785, P=0.019 for the MI group; β=7.451, P <0.001 for the HI group), respectively. These associations were independent of covariates, including age, race, sex, BMI, father’s education, and BP levels. The associations between trajectories of Mid-BP with IMT (adjusted β=0.006, P=0.384 for the MI group and β=0.028, P =0.007 for the HI group compared with the LI group) and LVMI (adjusted β=0.409, P=0.726 for the MI group and β=3.672, P =0.035 for the HI group compared with the LI group) were also statistically significant (Table S4). However, the associations between DBP trajectories with IMT or LVMI were not significant (Table S5).
Table 2.
The associations between systolic BP trajectories with carotid intima-media thickness and left ventricular mass index
| Trajectories | Intima-media thickness
|
Left ventricular mass index
|
||||
|---|---|---|---|---|---|---|
| N | β (95% CI) | P value | N | β (95% CI) | P value | |
| Model 1 | N=551 | N=546 | ||||
| Low-increasing | 268 | Reference | 269 | Reference | ||
| Moderate-increasing | 215 | 0.018 (0.006–0.030) | 0.003 | 212 | 6.824 (4.545–8.941) | <0.001 |
| High-increasing | 68 | 0.047 (0.029–0.064) | <0.001 | 65 | 14.313 (10.873–17.508) | <0.001 |
| P for trend =0.001 | P for trend <0.001 | |||||
| Model 2 | N=551 | N=546 | ||||
| Low-increasing | 268 | Reference | 269 | Reference | ||
| Moderate-increasing | 215 | 0.017 (0.005–0.030) | 0.006 | 212 | 3.582 (1.428–5.840) | 0.001 |
| High-increasing | 68 | 0.046 (0.028–0.065) | <0.001 | 65 | 9.119 (5.841–12.398) | <0.001 |
| P for trend <0.001 | P for trend <0.001 | |||||
| Model 3* | N=545 | N=539 | ||||
| Low-increasing | 265 | Reference | 265 | Reference | ||
| Moderate-increasing | 212 | 0.013 (0.001–0.026) | 0.039 | 209 | 3.225 (1.022–5.428) | 0.004 |
| High –increasing | 68 | 0.039 (0.020–0.058) | <0.001 | 65 | 8.477 (5.110–11.843) | <0.001 |
| P for trend =0.010 | P for trend <0.001 | |||||
| Model 4* | N=545 | N=539 | ||||
| Low-increasing | 265 | Reference | 265 | Reference | ||
| Moderate-increasing | 212 | 0.019 (0.005–0.032) | 0.007 | 209 | 2.785 (0.448–5.121) | 0.019 |
| High –increasing | 68 | 0.051 (0.027–0.074) | <0.001 | 65 | 7.451 (3.644–11.257) | <0.001 |
| P for trend <0.001 | P for trend <0.001 | |||||
BP = blood pressure; CI = confidence interval
Model 1= Unadjusted model; Model 2= age, race, sex; Model 3 = Model 2+ body mass index and father’s education level; Model 3= Model 4+ systolic BP and diastolic BP
Six participants were excluded due to missing values of father’s education in Model 3 and Model 4 for intima-media thickness, seven participants were excluded due to missing values of father’s education in Model 3 and Model 4 for left ventricular mass index
The association between BP trajectories during childhood with IMT and LVMI
Using BP data obtained prior to 18 years old, we re-modeled BP trajectories in 626 participants (a total of 4146 BP measurements). Ninety-seven (15.5%) participants started with a high level and had a relatively fast increase in SBP levels throughout (HI group); 292 (46.7%) participants started with moderate level and experienced a moderate increase in SBP (MI group); and 237 (37.6%) participants started with low level and maintained a low increase in SBP (LI group) (Figure 2). The trajectories identified in Mid-BP and DBP using data prior to 18 years old are shown in Figure S1B and Figure S2B. The associations of childhood SBP trajectories with IMT and LVMI were virtually unchanged. For trajectories of SBP, an average of increase of 0.040 mm for IMT (P<0.001) and 3.552 g/m2 for LVMI (P=0.043) were observed in the HI group compared to the LI group (Table 3). For trajectories of Mid-BP, an increase of 0.029 mm for IMT (P =0.004) was observed in the HI group compared to the LI group (Table S4). There was no significant association between DBP trajectories in childhood and the mean for IMT and LVMI (Table S5).
Figure 2. Trajectory groups identified for systolic blood pressure (SBP) during childhood up to age 18 years.
Their patterns by age, the number and percentage were shown for each group. The mean levels of IMT and LVMI in adulthood were also shown for each group. Dash lines are 95% confidence interval lines.
Table 3.
The associations between systolic BP trajectories during childhood with intima-media thickness and left ventricular mass index
| Trajectories | Intima-media thickness
|
Left ventricular mass index
|
||||
|---|---|---|---|---|---|---|
| N | β (95% CI) | P value | N | β (95% CI) | P value | |
| Model 1 | N=501 | N=496 | ||||
| Low-increasing | 190 | Reference | 190 | Reference | ||
| Moderate-increasing | 229 | 0.009 (−0.003–0.022) | 0.152 | 227 | 4.815 (2.324–7.232) | <0.001 |
| High-increasing | 82 | 0.043 (0.025–0.060) | <0.001 | 79 | 11.296 (7.901–14.562) | <0.001 |
| P for trend <0.001 | P for trend <0.001 | |||||
| Model 2 | N=501 | N=496 | ||||
| Low-increasing | 190 | Reference | 190 | Reference | ||
| Moderate-increasing | 229 | 0.009 (−0.004–0.022) | 0.180 | 227 | 1.815 (−0.449–4.078) | 0.116 |
| High-increasing | 82 | 0.044 (0.026–0.061) | <0.001 | 79 | 5.952 (2.771–9.132) | <0.001 |
| P for trend <0.001 | P for trend <0.001 | |||||
| Model 3* | N=496 | N=490 | ||||
| Low-increasing | 189 | Reference | 188 | Reference | ||
| Moderate-increasing | 225 | 0.007 (−0.006–0.019) | 0.311 | 223 | 1.397 (−0.880–3.674) | 0.229 |
| High –increasing | 82 | 0.037 (0.019–0.054) | <0.001 | 79 | 5.018 (1.789–8.247) | 0.002 |
| P for trend <0.001 | P for trend =0.004 | |||||
| Model 4* | N=496 | N=490 | ||||
| Low-increasing | 189 | Reference | 188 | Reference | ||
| Moderate-increasing | 225 | 0.008 (−0.005–0.021) | 0.237 | 223 | 0.783 (−1.539–3.105) | 0.509 |
| High –increasing | 82 | 0.040 (0.020–0.060) | <0.001 | 79 | 3.552 (0.108–6.998) | 0.043 |
| P for trend <0.001 | P for trend =0.066 | |||||
SBP = blood pressure; CI = confidence interval
Model 1= Unadjusted model; Model 2= age, race, sex; Model 3 = Model 2+ body mass index and father’s education level; Model 3= Model 4+ systolic BP and diastolic BP
Five participants were excluded due to missing values of father’s education in Model 3 and Model 4 for intima-media thickness, six participants were excluded due to missing values of father’s education in Model 3 and Model 4 for left ventricular mass index
Sensitivity analyses
The trajectories identified in SBP from the BP data obtained prior to 18 years old were similar with those from the total cohort (Table S6), i.e. participants who displayed moderate and fast increasing BP in childhood, also showed moderate and fast increasing BP in young adulthood.
There were no interactions between sex and SBP trajectories for IMT or LVMI (P>0.05). Sex-specific analyses (3679 measurements of males, 3925 measurements of females) were performed, and the similar trends were found in our study (Table S7 and Figure S3). In addition, after excluding 3304 measurements with obesity and 44 measurements taking anti-hypertensive drugs, the results remained the same (Table S8 and Figure S4). Finally, we identified same BP trajectories using the same population with available IMT (6703 measurements) or LVMI (6608 measurements) data, and the associations with IMT or LVMI remained the same (Table S9 and Figure S5).
Adding BMI, race, gender and father’s education (BMI was included as time-dependent variables, and race, gender and father’s education were treated as time-invariant variables) to group-based trajectory model resulted in a 2% improvement in model fit (Bayesian Information Criterion= −27209.77 versus −26757.68). We further explored the risk factors affecting the SBP trajectories, and found that sex, race and change of BMI (P < 0.001) were significantly associated with the SBP trajectories (Table S10, S11).
DISCUSSIONS
Three trajectory groups in BP from childhood to young adulthood were identified. We found that trajectories of BP (SBP and Mid-BP) were significantly associated with IMT and LVMI levels in young adulthood, i.e., the faster increasing rate of growth in BP, the higher levels of IMT and LVMI, which are two accepted subclinical markers for cardiovascular risk.14, 28 Furthermore, we for the first reported significant associations between SBP trajectories derived from childhood and subclinical cardiovascular indices in young adulthood.
Several studies have elucidated that there were varied patterns of BP change in population.29, 30 Allen et al. identified 5 distinct trajectories in mid-BP and SBP over a 25-year span from young adulthood to middle age.4 In the Framingham Heart Study, 4 trajectories were identified in 890 men aged 30 to 84 years.31 However, little is known about the association between BP changes during childhood to young adulthood and cardiovascular risk. Only one longitudinal birth cohort in New Zealand identified four different SBP trajectories from childhood to young adulthood in a population of 975 participants aged 7–38 years old. The findings showed that males, participants whose mothers had pregnancy hypertension, who were first born, who had lower birth weight, who had increasing BMI and increasing daily cigarettes tended to be in the hypertensive group. The study also found that the SBP trajectories were correlated with early-midlife cardiovascular risk indicators, such as obesity, lipid level and glycohemoglobin concentration.32 Similarly, we identified three trajectory groups in BP from childhood to young adulthood in our cohort that consists of both EAs and AAs. Consistent with previous reports, we also found participants in the HI group tended to be males, African Americans4, with higher BMI 33, and more likely to have a lower parental SES34, suggesting that the subgroups do capture the persons with differing cardiovascular risk exposures.
CARDIA study demonstrated that higher BP trajectories throughout early adulthood were significantly associated with the presence of subclinical atherosclerosis (coronary artery calcification) in middle age.4 Susanne and colleagues found that ten-year BP trajectories of middle-aged men were stronger predictors of CVD mortality, all-cause mortality, and life years lost than a single, average, and usual BP in Minnesota Business and Professional Men study.35 All these results, including ours, imply that BP trajectories based on multiple BP recordings may be a stronger predictor than a single measurement. In addition, screening of CVD risk factors in younger population is necessary, because atherosclerosis has its roots in childhood.36 U.S. Preventive Services Task Force concludes that the evidence to support screening for primary hypertension in children and adolescents is insufficient, one of the major reasons is that false-positive results may occur when BP are measured in a clinical setting.7 Our data suggested that monitoring trajectories of BP from childhood may assist in a more accurate identification of individuals with the higher cardiovascular risk as early in life as possible, and a rational approach to prevent CVD should begin early in life.
A large number of longitudinal studies indicated that the association between early-life factors and the SBP trajectory group is stronger compared with the DBP group.37, 38 To some extent agreeing with these results, we didn’t observe the associations of DBP trajectories with mean IMT and LVMI. However, we found an association of Mid-BP trajectories with IMT and LVMI, which is consistent with previous studies showing that Mid-BP may provide a greater predictive utility for cardiovascular events compared to DBP.19, 39 Numerous studies have found that the SBP but not DBP was independently associated with IMT, and SBP was more closely related with LVM than DBP.40–45 These findings suggest that at younger age SBP may increase left ventricular afterload with ensuing cardiac structural and functional changes. DBP elevation reflects more an increase of vascular resistance which is a chronic adaptive process leading to vascular remodeling46, 47. Elevated blood pressure either directly or indirectly induces vasoconstriction with adaptive wall thickening. Since our study population is still young meaning with low likelihood of vascular remodeling, a longer follow-up period might unveil the DBP effect on CVD health indicators.
Strengths and Limitations
A major strength of the present study is that it involves up to 16 BP measurements over a 23-year period from childhood to young adulthood. A second feature is that we used two well accepted markers of subclinical CVD and mutually confirmed the associations between SBP/Mid-BP trajectories and cardiovascular risk. Third, the latent class model, an innovative and powerful method, was used to identify subgroups in a cohort with long-term patterns of BP that share a similar latent growth rate. This enables us to evaluate the associations between subclinical cardiovascular markers and BP changes from childhood to young adulthood. However, several limitations should be noted. First, our cohort only includes European and African Americans. The trajectory groups identified may not be generalizable to other populations. In addition, not all participants have BP information available at all visits. However, over 75% of the participants had BP measured more than 8 times. Therefore, we believe the results are unlikely to be biased due to the missing BP measurements. Finally, a national representative and large sample cohort study is needed to build a reference of the rate of growth for SBP to screen the high cardiovascular risk population.
Perspectives
Our study confirmed that subgroups with different BP trajectories exist in population, and found trajectories of BP (SBP and Mid-BP) throughout childhood and young adulthood were significant predictors of subclinical CVD. We for the first time report significant associations between SBP trajectories derived from childhood and subclinical cardiovascular indices in young adulthood. Monitoring trajectories of BP from childhood may provide an important approach to identify population with higher risk for developing hypertension and CVD. Early prevention and intervention in these population may effectively reduce their cardiovascular risks in adulthood.
Supplementary Material
Novelty and Significance.
What Is New?
This study involves up to 16 BP measurements over a 23-year period from childhood to young adulthood.
Trajectories of BP (SBP and Mid-BP) were significantly associated with IMT and LVMI in young adulthood, i.e., the faster increasing rate of growth in BP, the higher levels of IMT and LVMI.
We for the first time report that SBP and Mid-BP trajectories during childhood were statistically significantly associated with subclinical CVD risk in young adulthood.
What Is Relevant?
Monitoring trajectories of BP from childhood may provide an important approach to identify a population with higher risk for developing hypertension and CVD.
Summary
SBP trajectories during childhood were significantly associated with subclinical cardiovascular indices in young adulthood, indicating that monitoring trajectories of BP from childhood may help to identify a high cardiovascular risk population in early life.
Acknowledgments
We would like to thank all the participants in the study and the staff at the Georgia Prevention Institute. We would also like to thank Dr. James Halbert for the careful review of our manuscript.
Funding Sources
The present study was supported in part by NIH/NHLBI HL69999 and HL125577.
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. Us population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR, Jr, Liu K, Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The bogalusa heart study. Am J Cardiol. 2002;90:3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 6.Newman WP, 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The bogalusa heart study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA Force USPST. Screening for primary hypertension in children and adolescents: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2013;159:613–619. doi: 10.7326/0003-4819-159-9-201311050-00725. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: A new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–169. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The rotterdam study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 12.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the stanislas cohort: Effect of age, sex, anthropometry and blood pressure. J Hypertens. 1998;16:1593–1602. doi: 10.1097/00004872-199816110-00005. [DOI] [PubMed] [Google Scholar]
- 13.Lee DG, Han JH, Kwon KY, Kim JH, Han KH, Lee EJ. Association of 10-year atherosclerotic cardiovascular disease risk score with carotid intima-media thickness and plaque. Korean J Fam Med. 2015;36:310–315. doi: 10.4082/kjfm.2015.36.6.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agabiti-Rosei E, Muiesan ML, Salvetti M. Evaluation of subclinical target organ damage for risk assessment and treatment in the hypertensive patients: Left ventricular hypertrophy. J Am Soc Nephrol. 2006;17:S104–108. doi: 10.1681/ASN.2005121336. [DOI] [PubMed] [Google Scholar]
- 15.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: A 10-year longitudinal study. J Pediatr. 2002;141:770–779. doi: 10.1067/mpd.2002.128113. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: Results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 17.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: The georgia stress and heart study. Circulation. 2015;131:1674–1681. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 19.Mosley WJ, 2nd, Greenland P, Garside DB, Lloyd-Jones DM. Predictive utility of pulse pressure and other blood pressure measures for cardiovascular outcomes. Hypertension. 2007;49:1256–1264. doi: 10.1161/HYPERTENSIONAHA.106.083592. [DOI] [PubMed] [Google Scholar]
- 20.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: Predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reis G, Alderman MH, Laragh JH. Standardization of m-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol. 1984;4:1222–1230. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Cook BB, Treiber FA, Mensah G, Jindal M, Davis HC, Kapuku GK. Family history of hypertension and left ventricular mass in youth: Possible mediating parameters. Am J Hypertens. 2001;14:351–356. doi: 10.1016/s0895-7061(00)01275-9. [DOI] [PubMed] [Google Scholar]
- 24.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 25.Nagin DS, Odgers CL. Group-based trajectory modeling (nearly) two decades later. J Quant Criminol. 2010;26:445–453. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BL, Nagin DS. A note on a stata plugin for estimating group-based trajectory models. Socio Meth Res. 2013;42:608–613. [Google Scholar]
- 27.JONES BL, NAGIN DS, ROEDER K. A sas procedure based on mixture models for estimating developmental trajectories. Socio Meth Res. 2001;29:374–393. [Google Scholar]
- 28.Baldassarre D, Hamsten A, Veglia F, de Faire U, Humphries SE, Smit AJ, Giral P, Kurl S, Rauramaa R, Mannarino E, Grossi E, Paoletti R, Tremoli E, Group IS. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: Results of the improve (carotid intima media thickness [imt] and imt-progression as predictors of vascular events in a high risk european population) study. J Am Coll Cardiol. 2012;60:1489–1499. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Wills AK, Lawlor DA, Muniz-Terrera G, Matthews F, Cooper R, Ghosh AK, Kuh D, Hardy R, Team FAS. Population heterogeneity in trajectories of midlife blood pressure. Epidemiology. 2012;23:203–211. doi: 10.1097/EDE.0b013e3182456567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safar ME, Lange C, Tichet J, Blacher J, Eschwege E, Balkau B D. E.S.I.R. Study Group F. The data from an epidemiologic study on the insulin resistance syndrome study: The change and the rate of change of the age-blood pressure relationship. J Hypertens. 2008;26:1903–1911. doi: 10.1097/HJH.0b013e32830b8937. [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The framingham heart study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 32.Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, Cutfield W, Williams MJ, Harrington H, Moffitt TE, Caspi A, Milne B, Poulton R. Childhood to early-midlife systolic blood pressure trajectories: Early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108–1115. doi: 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Law C, Power C. Body mass index throughout the life-course and blood pressure in mid-adult life: A birth cohort study. J Hypertens. 2007;25:1215–1223. doi: 10.1097/HJH.0b013e3280f3c01a. [DOI] [PubMed] [Google Scholar]
- 34.Blane D, Hart CL, Smith GD, Gillis CR, Hole DJ, Hawthorne VM. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ. 1996;313:1434–1438. doi: 10.1136/bmj.313.7070.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tielemans SM, Geleijnse JM, Menotti A, Boshuizen HC, Soedamah-Muthu SS, Jacobs DR, Jr, Blackburn H, Kromhout D. Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: The minnesota business and professional men study and the zutphen study. J Am Heart Assoc. 2015;4:e001378. doi: 10.1161/JAHA.114.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juonala M, Magnussen CG, Venn A, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: The cardiovascular risk in young finns study, the childhood determinants of adult health study, the bogalusa heart study, and the muscatine study for the international childhood cardiovascular cohort (i3c) consortium. Circulation. 2010;122:2514–2520. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 37.Hardy R, Kuh D, Langenberg C, Wadsworth ME. Birthweight, childhood social class, and change in adult blood pressure in the 1946 british birth cohort. Lancet. 2003;362:1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- 38.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: A longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 39.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 40.Manios E, Michas F, Stamatelopoulos K, Koroboki E, Stellos K, Tsouma I, Vemmos K, Zakopoulos N. Association of isolated systolic, isolated diastolic, and systolic-diastolic masked hypertension with carotid artery intima-media thickness. J Clin Hypertens (Greenwich) 2015;17:22–26. doi: 10.1111/jch.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palu C, Hansson L, Magnani B, Rahn KH, Reid J, Rodicio J, Safar M, Eckes L, Ravinetto R. Risk factors associated with alterations in carotid intima-media thickness in hypertension: Baseline data from the european lacidipine study on atherosclerosis. J Hypertens. 1998;16:949–961. doi: 10.1097/00004872-199816070-00008. [DOI] [PubMed] [Google Scholar]
- 42.Valero FA, Martinez-Vea A, Bardaji A, Gutierrez C, Garcia C, Richart C, Oliver JA. Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10:1020–1026. doi: 10.1681/ASN.V1051020. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Lahiguera FJ, Rodilla E, Costa JA, Gonzalez C, Martin J, Pascual JM. Relationship of central and peripheral blood pressure to left ventricular mass in hypertensive patients. Rev Esp Cardiol (Engl Ed) 2012;65:1094–1100. doi: 10.1016/j.recesp.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Malcolm DD, Burns TL, Mahoney LT, Lauer RM. Factors affecting left ventricular mass in childhood: The muscatine study. Pediatrics. 1993;92:703–709. [PubMed] [Google Scholar]
- 45.Ferreira JP, Girerd N, Bozec E, Machu JL, Boivin JM, London GM, Zannad F, Rossignol P. Intima-media thickness is linearly and continuously associated with systolic blood pressure in a population-based cohort (stanislas cohort study) J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17:1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.