Abstract
To understand sleep mechanisms and develop treatments for sleep disorders, investigations using animal models are essential. The sleep architecture of rodents differs from that of diurnal mammals including humans and non-human primates. Sleep studies have been conducted in non-human primates; however, these sleep assessments were performed on animals placed in a restraint chair connected via the umbilical area to the recording apparatus. To avoid restraints, cables, and other stressful apparatuses and manipulations, telemetry systems have been developed. In the present study, sleep recordings in unrestrained cynomolgus monkeys (Macaca fascicularis) and common marmoset monkeys (Callithrix jacchus) were conducted to characterize normal sleep. For the analysis of sleep–wake rhythms in cynomolgus monkeys, telemetry electroencephalography (EEG), electromyography (EMG), and electrooculography (EOG) signals were used. For the analysis of sleep–wake rhythms in marmosets, telemetry EEG and EOG signals were used. Both monkey species showed monophasic sleep patterns during the dark phase. Although non-rapid eye movement (NREM) deep sleep showed higher levels at the beginning of the dark phase in cynomolgus monkeys, NREM deep sleep rarely occurred during the dark phase in marmosets. Our results indicate that the use of telemetry in non-human primate models is useful for sleep studies, and that the different NREM deep sleep activities between cynomolgus monkeys and common marmoset monkeys are useful to examine sleep functions.
Keywords: common marmoset monkey, Cynomolgus monkey, Sleep–wake rhythm, Telemetry system
Introduction
Sleep architecture follows an alternating cycle of rapid eye movement (REM) and non-rapid eye movement (NREM) sleep throughout a typical night and is defined in the laboratory by recording the electrical field activity of large groups of neurons and muscle cells in both humans and animals [6, 26]. Electrodes on the scalp are used for electroencephalography (EEG), electrodes on skeletal muscles are used for electromyography (EMG), and electrodes placed over or near the muscles responsible for horizontal eye movement are used for electrooculography (EOG) [25]. The different types and stages of sleep can be identified using polysomnography, which simultaneously measures several body functions using EEG, EMG, and EOG. EEG traces of brain wave activity have revealed that NREM sleep is characterized by very slow but relatively high-amplitude or high-voltage oscillations (with the frequency gradually slowing and the amplitude increasing as sleep deepens), while REM sleep shows a much faster and lower amplitude trace, similar to normal waking activity. EMG traces of skeletal muscle activity show that while the body is effectively paralyzed during REM sleep, the body does make limited movements during NREM sleep. EOG traces indicate little or no eye movement during NREM sleep and rapid eye movements during REM sleep.
Appropriate animal models may help better understand the regulation of sleep and the mechanisms underlying sleep disorders. Currently, the most widely used animal models in sleep research are rodents, particularly rats and mice [12, 32]. However, unlike humans, these rodents are nocturnal. Thus, diurnal non-human primates represent a valuable and more translational animal model of sleep. Indeed, although sleep studies have been conducted in a number of non-human primates, these sleep assessments were performed on animals placed in a restraint chair connected via the umbilical area to the recording apparatus [2, 3, 23]. Such recording methodologies lead to difficulties during EEG recording for extended periods of time in sleep architecture [4, 17]. Recent sleep studies have confirmed the relevance of EEG recordings using telemetry implants in non-human primates [9, 18]. Telemetry involves the surgical implantation of electrodes relayed to a biosensor device (transmitter) positioned intraperitoneally, intramuscularly, or subcutaneously, enabling electronic capture of biological signals in freely moving animals for an extended time period. Accordingly, such a remote system does not involve restraint, and the animal can be kept in its home cage, leading to reduced stress and alterations of sleep characteristics.
Approximately 40 million years ago, the Simiiformes infraorder split into the parvorders Platyrrhini (New World monkeys) and Catarrhini (apes and Old World monkeys). New World monkeys are found in Central and South America and comprise five families: Callitrichidae, Cebidae, Aotidae, Pitheciidae, and Atelidae. Old World monkeys are native to Africa and Asia and include many of the most familiar non-human primate species, such as baboons and macaques. Although Old and New World monkeys evolved independently, commonalities exist in their social structure and behavior, thought to be due to parallel evolution. For example, because of their cognitive development, cynomolgus monkeys (Macaca fascicularis, Old World monkeys) and common marmoset monkeys (Callithrix jacchus; New World monkeys) are capable of using tools. Therefore, it is of interest to compare their sleep patterns.
In this study, we evaluated the use of a telemetry system for recording sleep architecture and circadian rhythm in non-human primates and characterized the behaviors of different species, including rats, cynomolgus monkeys, and common marmoset monkeys, in a comparative study under a standard 12-h light/dark cycle.
Materials and Methods
Ethical declaration
This research was conducted in accordance with the Declaration of Helsinki and was approved by the Animal Experiments Committee of the RIKEN Brain Science Institute and by HAMRI Co., Ltd. All animals were cared for and treated humanely in accordance with the Institutional Guidelines for Experiments using Animals.
Animals
Sprague-Dawley rats from Charles River Japan (Kanagawa, Japan) were housed in individual cages (RC2016, W250 × H360 × D360 mm, Shintoyo Seisakusho Ltd., Saitama, Japan) maintained at 24 ± 3°C and 50 ± 20% humidity under a 12-h light/dark cycle (light period: 7:00–19:00 h). Rats were allowed ad libitum access to tap water and food pellets (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan). Body weight was measured once a week using a scale (CS-2000, CUSTOM Co., Ltd., Tokyo, Japan).
Cynomolgus monkeys from Apes Inc. (Ibaraki, Japan) were housed in individual stainless steel cages (CyM2016, W550 × H815 × D735 mm, Shintoyo Seisakusho Ltd.) and maintained at 24 ± 3°C and 50 ± 20% humidity under a 12-h light/dark cycle (light period: 7:00–19:00 h). Cynomolgus monkey were allowed ad libitum access to tap water and food pellets (PS-A, Oriental Yeast Co., Ltd.). Body weight was measured once a week using a scale (DP-6200, Yamato Scale Co., Ltd., Chiba, Japan).
Common marmoset monkeys were provided by the RIKEN Brain Science Institute (Saitama, Japan). They were housed in individual stainless steel cages (SNBL2016, W420 × H700 × D700 mm, Shintoyo Seisakusho Ltd.), and maintained at 24 ± 3°C and 50 ± 20% humidity under a 12-h light/dark cycle (light period: 8:00–20:00 h). Heat retention equipment was present in the cage. Marmoset monkeys were allowed ad libitum access to tap water and food pellets (CMS-1M, Clea Japan Inc., Tokyo, Japan) and were given a piece of Calorie Mate (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) or castella (Castella, Imuraya Co., Ltd., Mie, Japan) each day. Body weight was measured once a week using a scale (SH-2000, A&D Co., Ltd., Tokyo, Japan).
Surgery
EEG and EMG electrodes were implanted in rats (7 males, 8 weeks old, 276–316 g body weight) as described previously [29]. Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (somnopentyl, 55 mg/kg body weight, Kyoritsu Seiyaku Corporation, Tokyo, Japan) and stabilized in a stereotaxic apparatus (SR-6R, NARISHIGE Group, Tokyo, Japan). The head was disinfected before a midline incision was made to expose the skull. Three small holes were drilled into the skull, and three gold-plated screw-electrodes (Unique Medical Co., Tokyo, Japan) were placed through the skull on the cortices (anterior/posterior to bregma, +2.5 mm; lateral to midline, +2.0 mm, anterior/posterior to bregma, −2.0 mm; lateral to midline, +0.5 mm, anterior/posterior to bregma, −4.0 mm; lateral to midline, +3.0 mm) for EEG recordings. Each electrode was fixed to the skull using dental acrylic resin (GC Co., Tokyo, Japan) for protection and insulation. In addition, two stainless steel hook-electrodes were inserted into the cervical portion of the trapezius muscle for EMG recordings. During and at the end of surgery, 40,000 U penicillin G potassium (Meiji Co., Ltd., Tokyo, Japan) were administered.
Cynomolgus monkeys (5 males, 3–4 years old, 2.8–3.8 kg body weight) were subcutaneously implanted with a transmitter (TL10M3-D70-EEE, Data Sciences International, MN, USA), which consisted of a sterile, disk-shaped, three-channel biopotential device for measuring EEG, EOG, and EMG signals. Biopotential signals were transmitted to receivers (RMC-1, Data Sciences International) mounted on the cage, forwarded to a data exchange matrix serving as a multiplexer, and connected through a ribbon cable to a computer for data recording and storage. Cynomolgus monkeys were anesthetized using an intramuscular injection of ketamine (Ketalar, Daiichi Sankyo Propharma Co., Ltd., Tokyo, Japan) and maintained using 2–5% isoflurane (Pfizer Japan Inc., Tokyo, Japan) in 100% oxygen with tracheal insertion. Following disinfection and an abdominal skin incision, a telemetry transmitter was sutured on the inside wall of the abdominal cavity using suture thread (Alfresa Pharma Co., Osaka, Japan). Next, the head was disinfected, a midline incision was made in the head skin, and the electrode biopotential leads from the transmitter were guided subcutaneously from the left side of the abdomen to the head. An antibiotic (penicillin G potassium, Meiji Co., Ltd.) was placed on the abdomen, and the incision was closed. Two EEG electrodes were screwed into the skull (anterior/posterior to bregma, −5.0 mm; lateral to midline, +5.0 mm, anterior/posterior to bregma, −15.0 mm; lateral to midline, +5.0 mm), and two EOG electrodes were affixed at the level of the orbital arch bone. Each implanted electrode and the surrounding areas were covered by resin (GC Co.) for protection and insulation, and the head skin incision was closed. Next, a cervical skin incision was made following disinfection, and EMG leads from a transmitter were guided subcutaneously from the left side of the abdomen to the cervix. Two EMG electrodes were implanted on the left side of the cervical portion of the trapezius muscle for EMG recordings. Cynomolgus monkeys were administered analgesic (lepetan and buprenorphine hydrochloride, Otsuka Pharmaceutical Co., Ltd.) and anti-inflammatory agents (prednisolone, Kyoritsu Seiyaku Corporation) on postoperative days 1–3 and an antibiotic (penicillin G potassium, Meiji Co., Ltd.) on postoperative days 1–7.
Common marmoset monkeys (5 males, 22–95 months old, 285–366 g body weight) were subcutaneously implanted with a transmitter (TL11M2-F40-EET, Data Sciences International) consisting of a sterile, two-channel biopotential device for measuring body temperature, locomotor activity, and EEG and EOG signals. The marmosets could only accommodate measurements using two channels due to their size. Marmosets were anesthetized via inhalation of 5% isoflurane (Pfizer, Japan Inc.) in 100% oxygen and maintained with 2–5% isoflurane. Following disinfection and an abdominal skin incision, a telemetry transmitter was sutured on the inside wall of the abdominal cavity using suture thread (Alfresa Pharma Co.). Next, following disinfection and a midline incision in the head skin, the leads from the transmitter were guided subcutaneously from the left side of the abdomen to the head. An antibiotic (penicillin G potassium, Meiji Co., Ltd.) was placed on the abdomen, and the incision was closed. Two EEG electrodes were implanted into the calvaria (anterior/posterior to bregma, −3.0 mm; lateral to midline, +3.5 mm, anterior/posterior to bregma, −7.0 mm; lateral to midline, +3.5 mm), and two EOG electrodes were affixed at the level of the orbital arch bone. Postoperative management was performed as for the cynomolgus monkeys.
Recordings
Following surgery, the rats were placed in individual recording cages (RC2016, Shintoyo Seisakusho Ltd.) in an electromagnetically shielded chamber, and recording was initiated 3 weeks postoperatively. EEG and EMG signals were amplified (AB-610J; Nihon Kohden Co., Tokyo, Japan), digitized at a sampling rate of 128 Hz, and recorded using the SleepSign software, version 2 (Kissei Comtec Co., Ltd., Nagano, Japan). EEG and EMG signals and locomotor activity (Digital acquisition system; NS-AS01, Neuroscience Inc., Tokyo, Japan) were examined consecutively for 24 h.
Following surgery, cynomolgus monkeys were moved to individual monitoring cages (CyM2016, Shintoyo Seisakusho Ltd.), and at 3 weeks postoperatively, EEG, EOG, and EMG signaling and locomotor activity were recorded consecutively for 24 h and analyzed using the telemetry system (Dataquest A.R.T. analysis software Dataquest ART, Data Sciences International) via a receiver (RMC-1, Data Sciences International). These recordings were also analyzed using the SleepSign software, version 2 (Kissei Comtec Co., Ltd.).
Following surgery, common marmoset monkeys were moved to individual cages for monitoring (SNBL2016, Shintoyo Seisakusho Ltd.). At 3 weeks postoperatively, EEG and EOG signaling, locomotor activity, and body temperature were recorded for 24 h using the telemetry system (Dataquest ART, Data Sciences International) with a reception board (RMC-1, Data Sciences International) to evaluate EEG and EOG traces and circadian rhythm. These recordings were also analyzed using the SleepSign software, version 3 (Kissei Comtec Co., Ltd.).
Sleep–wake states
The duration of each vigilance state was averaged over the full 24 h and additionally separated into the 12-h light and dark periods, as well as by hour. The mean duration of the vigilance state episodes (consecutive epochs not interrupted by another state) and the number of episodes were averaged over 24 h and over the 12-h light and dark periods. Epochs with prominent muscular or ocular artifacts were simultaneously identified and excluded from the analyses.
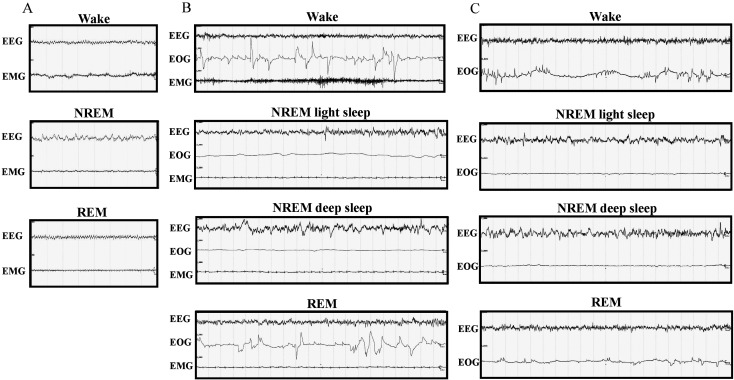
Identification of the vigilance states in rats was performed over 8-s epochs, and three different states were identified (Fig. 1A): wakefulness, NREM sleep, and REM sleep. Wakefulness was characterized by low-amplitude, high-frequency EEG activity accompanied by high-amplitude EMG activity. NREM sleep was scored when EEG showed high-amplitude and mixed-frequency activity accompanied by low EMG activity. REM sleep was characterized by low-voltage, mixed-frequency activity within the 4–8 Hz range (theta) accompanied by low or no EMG activity.
Fig. 1.
Recordings of sleep stages. The images represent recording samples over 8-s epochs in rats (A), 20-s epochs in cynomolgus monkeys (B), and 20-s epochs in common marmoset monkeys (C). Wake: wakefulness, NREM: non-rapid eye movement sleep, REM: rapid eye movement sleep, EEG: electroencephalogram, EMG: electromyogram, EOG: electrooculogram.
Identification of the vigilance states in cynomolgus monkeys was performed over 20-s epochs, and four different states were identified (Fig. 1B): wakefulness, NREM light sleep, NREM deep sleep, and REM sleep. NREM light and deep sleep were combined into the category “NREM sleep”. Wakefulness was characterized by low-amplitude, high-frequency EEG activity accompanied by high-amplitude EOG and EMG activities. NREM light sleep was characterized by low-amplitude, mixed-frequency EEG accompanied by low EOG and EMG activities. NREM deep sleep was characterized by slow waves (high-amplitude, low-frequency waves: <2 Hz, >75 µV) appearing as >20% of an epoch on the EEG signal accompanied by very low EOG and EMG activity. REM sleep was characterized by low-voltage, mixed-frequency activity on EEG accompanied by high-amplitude EOG activity and low or no EMG activity.
Identification of the vigilance states in common marmoset monkeys was performed over 20-s epochs, and four different states were identified (Fig. 1C): wakefulness, NREM light sleep, NREM deep sleep, and REM sleep. NREM light and deep sleep were combined into the category “NREM sleep”. Wakefulness was characterized by low-amplitude, high-frequency EEG activity accompanied by high-amplitude EOG activity. NREM light sleep was characterized by low-amplitude, mixed-frequency EEG activity accompanied by low EOG activity. NREM deep sleep was characterized by slow waves (high-amplitude, low-frequency waves: <2 Hz, >75 µV) appearing as >20% of an epoch on the EEG signal accompanied by very low EOG activity. REM sleep was characterized by low-amplitude, mixed-frequency activity on EEG accompanied by high-amplitude EOG activity.
Non-REM sleep was evaluated in humans according to the methods developed by Rechtschaffen and Kales [22] and was categorized into four different sleep stages. Upon adaptation of the human criteria, stages 1 and 2 were combined into “light sleep” and stages 3 and 4 into “deep sleep.”
Data analysis
Data are presented as means ± standard errors of the means. All statistical analyses were performed using GraphPad Prism Software version 6 (CA, USA). Data were analyzed using Dunnett’s test, where appropriate. A P-value <0.05 was considered to indicate statistical significance.
Results
To evaluate the general sleep patterns of rats, cynomolgus monkeys, and common marmoset monkeys, data were collected and scored visually. The total time and percentages of time spent in total sleep, REM sleep, NREM sleep, light sleep, and deep sleep were averaged to yield group means.
Sleep–wake rhythm in rats
We observed that rats spent 53.5 ± 1.7% of the recording time in total sleep over a 24-h period (Fig. 2). During the 12-h dark period, the sleep–wake state pattern confirmed the predominance of wakefulness (wakefulness, 63.4 ± 2.1%; sleep, 36.6 ± 2.1%), with NREM and REM sleep taking precedence. The time spent in NREM sleep was ~3.4-fold greater than that in REM sleep (NREM, 28.3 ± 1.5%; REM, 8.3 ± 0.7%). During the 12-h light phase, rats spent the majority of time sleeping (wakefulness, 29.5 ± 1.8%; sleep, 70.5 ± 1.8%). However, the time spent in NREM sleep increased, becoming ~6.5-fold greater than that in REM sleep (NREM, 61.2 ± 1.8%; REM, 9.3 ± 0.9%).
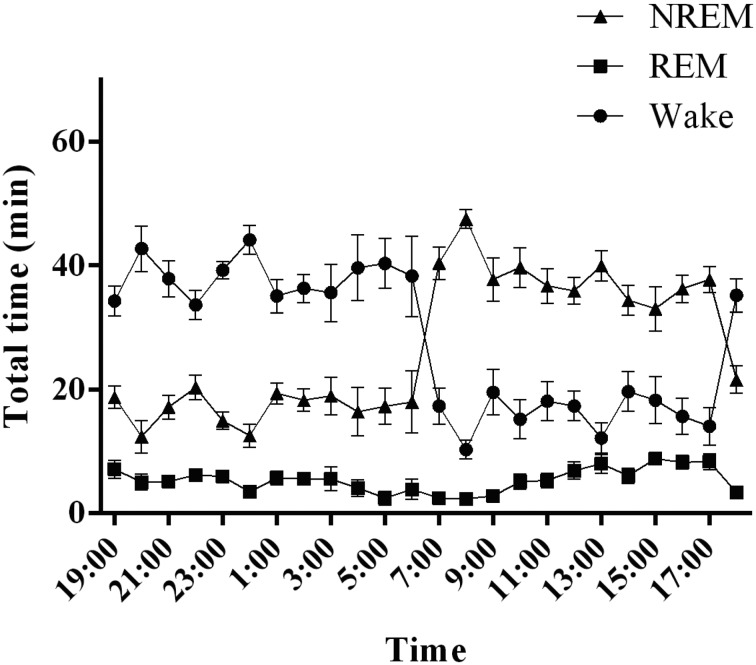
Fig. 2.
Sleep–wake activity in rats. The graph shows the hourly distributions of NREM, REM, and wakefulness during the day. NREM: non-rapid eye movement sleep, REM: rapid eye movement sleep, Wake: wakefulness.
Sleep-wake rhythm in cynomolgus monkeys
Cynomolgus monkeys spent more than a third of the 24-h day asleep, as sleep occupied 49.3 ± 2.3% of the 24-h recording (Fig. 3). The sleep–wake state pattern confirmed the predominance of wakefulness during the 12-h light period, with naps taking place during the middle of the light period. Animals quickly fell asleep at the beginning of the dark period. NREM sleep was predominant during the first half of the 12-h dark period and peaked 1–3 h after the lights were turned off, followed by a progressive decrease over the 12-h dark period. In contrast, the incidence of REM sleep increased over the 12-h dark period. The diurnal preference of cynomolgus monkeys was confirmed, as they spent 90.9 ± 3.6% of the 12-h light period awake. Naps during the 12-h light period comprised mainly NREM sleep (9.1 ± 3.6% of total sleep during the 12-h light period). REM sleep occurred rarely over the 12-h light period. During the 12-h dark period, NREM sleep occupied 78.6 ± 2.6% and REM sleep 10.9 ± 2.1% of the total sleep time. The remainder of the 12-h dark period was spent in wakefulness (10.5 ± 2.0%).
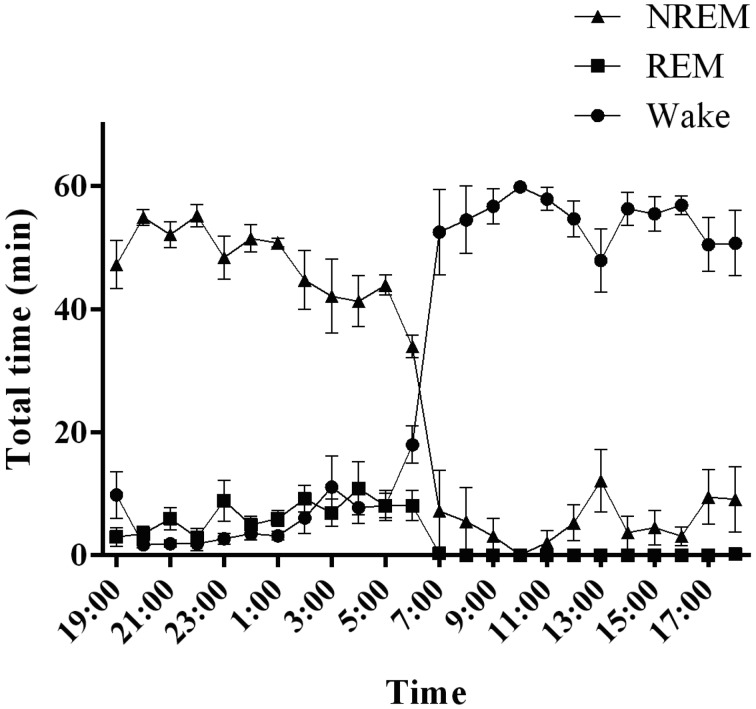
Fig. 3.
Sleep–wake activity in cynomolgus monkeys. The graph shows the hourly distributions of NREM, REM, and wakefulness during the day. NREM: non-rapid eye movement sleep, REM: rapid eye movement sleep, Wake: wakefulness.
Sleep-wake rhythm in common marmoset monkeys
Common marmoset monkeys spent half of the 24-h day asleep, with sleep occupying 51.6 ± 1.2% of the 24-h recording period (Fig. 4A). The sleep–wake state pattern confirmed the predominance of wakefulness during the 12-h light period, with few naps taken during the light period. The diurnal preference of marmoset monkeys was confirmed, as 93.0 ± 2.7% of the 12-h light period was spent awake. Naps during the 12-h light period comprised mainly NREM sleep (7.0 ± 2.7% of total sleep during the 12-h light period). REM sleep occurred rarely over the course of the 12-h light period. When the lights were turned off, the animals fell asleep quickly. NREM sleep was predominant over the 12-h dark period (82.8 ± 1.7% of total sleep during the 12-h dark period), and REM sleep occupied 13.4 ± 1.2% of the total dark period. The remainder of the 12-h dark period was spent awake (3.6 ± 0.6%).
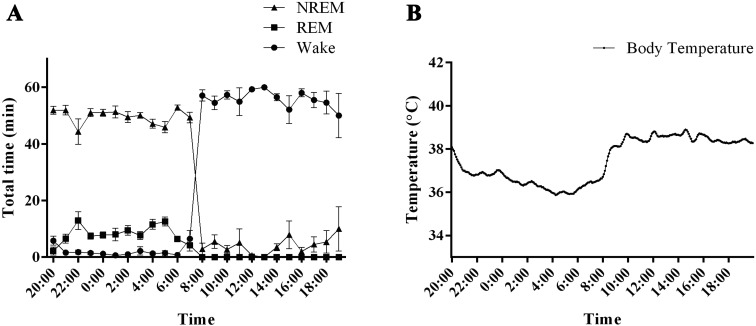
Fig. 4.
Sleep–wake activity and body temperature in common marmoset monkeys. (A) The graph shows the hourly distributions of NREM, REM, and wakefulness during the day. NREM: non-rapid eye movement sleep, REM: rapid eye movement sleep, Wake: wakefulness. (B) The graph shows the body temperature fluctuations.
Body temperature in common marmoset monkeys
A distinct rhythm was observed in the body temperature of common marmoset monkeys, with a difference of approximately 3°C between the light and dark periods (Fig. 4B). Within the first 2 h of turning the lights on, body temperature increased gradually to a stable daytime temperature of approximately 39°C. Body temperature began to decrease 5 h prior to dark onset. This trend continued for the first 8 h of the dark period until a stable nighttime temperature of approximately 36°C was reached. During the last 2 h of the dark phase, body temperature began to rise.
NREM sleep state in cynomolgus monkeys
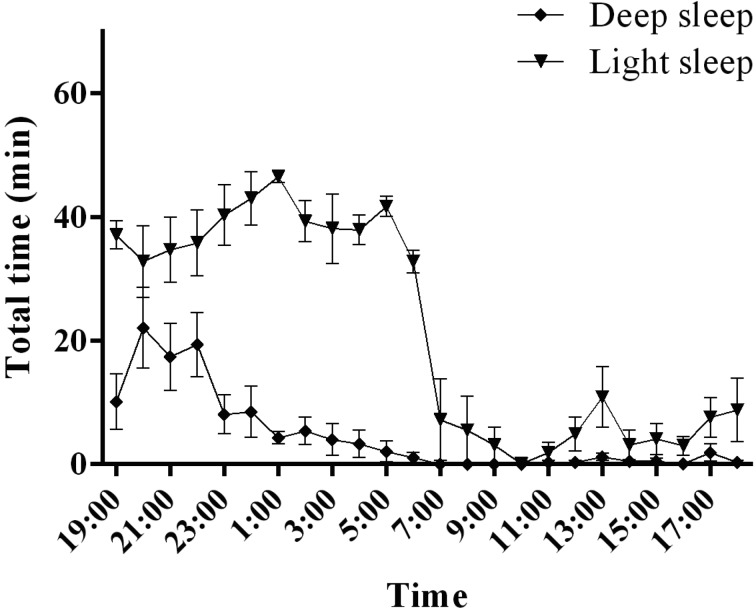
Cynomolgus monkeys exhibited higher levels of light sleep than deep sleep and wakefulness during the 12-h dark period (Fig. 5). Deep sleep reached its maximum 1 h after the lights were turned off, followed by a progressive decrease over the 12-h dark period. NREM sleep, occupying 78.6 ± 2.6% of the total 12-h dark period (Fig. 3), was composed of deep sleep and light sleep (deep sleep, 14.7 ± 3.7%; light sleep, 63.9 ± 2.9% of total sleep time). NREM sleep occupied 9.1 ± 3.6% of the total 12-h light period (Fig. 3). Most of this NREM sleep was composed of light sleep, with deep sleep occurring rarely (deep sleep, 0.7 ± 0.3%; light sleep, 8.4 ± 3.6% of total sleep time).
Fig. 5.
Light sleep and deep sleep activities in cynomolgus monkeys. The graphs show the hourly distributions of light sleep and deep sleep during the day.
NREM sleep state in common marmoset monkeys
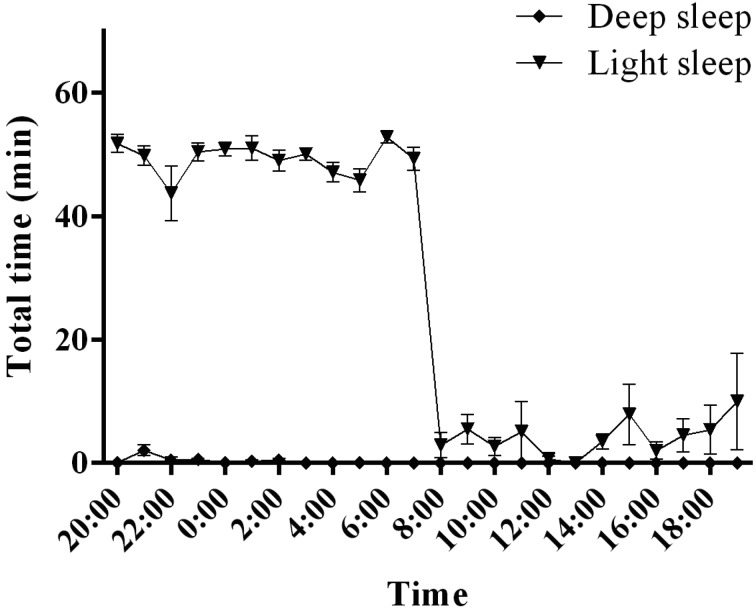
In common marmoset monkeys, NREM sleep occupied 82.8 ± 1.7% of the total 12-h dark period (Fig. 4). Most of this NREM sleep was composed of light sleep, with deep sleep occurring rarely (deep sleep, 0.6 ± 0.3%; light sleep, 82.2 ± 1.5% of total sleep time) (Fig. 6). NREM sleep occupied 7.0 ± 2.7% of the total 12-h light period (Fig. 4). Most of this NREM sleep was composed of light sleep, with no deep sleep occurring (deep sleep, 0.0 ± 0.0%; light sleep, 7.0 ± 2.7% of total sleep time).
Fig. 6.
Light sleep and deep sleep activities in common marmoset monkeys. The graphs show the hourly distributions of light sleep and deep sleep during the day.
Discussion
Sleep distribution in vertebrates is generally considered to be either polyphasic or monophasic with respect to the number, duration, timing, and distribution of sleep cycles that occur during a sleep period over a 24-h period [7, 28]. Sleep distributed as multiple periods across a 24-h day constitutes a polyphasic sleep pattern. It has been reported that laboratory rodents sleep during both the light and dark periods of a 24-h light/dark cycle, which was also observed in the present study. Appropriate animal models are helpful to better understand the regulation of sleep and the mechanisms underlying sleep disorders. Sleep disorders typically occur with a variety of mental and neurological disorders, such as depression, anxiety disorders, and dementia [20, 24, 31]. Currently, the most widely used animal models in sleep research are rodents, particularly rats and mice. However, unlike humans, rodents are nocturnal and exhibit polyphasic sleep–wake cycles [15, 19, 27]. Thus, non-human primates would represent valuable and more translational animal models of sleep. For sleep stage scoring, EEG, EMG, and EOG signal measurements and recordings are costly and complicated. In addition, scoring of the sleep stages is tedious and time consuming. The complexity of the signal acquisition and processing tasks can be simplified by using fewer signal channels, typically one or two EEG or EOG signal channels [9, 21]. Here, we used EEG and EOG signals captured by a two-channel telemetry system to evaluate the sleep quality of common marmoset monkeys.
In this study, we validated the use of telemetry to evaluate the 24-h sleep–wake distribution in non-human primates, cynomolgus and common marmoset monkeys. Additionally, we observed that when evaluated in their home cages, undisturbed and unrestrained freely moving monkeys exhibited a diurnal pattern of activity, as the majority of their wakefulness occurred during the light period, with few daytime naps. As described above, sleep studies in non-human primate models commonly use restraint chairs during sleep assessments [1, 4, 17]. A consequence of the use of restraints is the difficulty in recording sleep–wake states for extended time periods, as continual restraint poses problems in terms of scientific quality and animal welfare. To overcome the limitations associated with restraints, studies have recently begun to use telemetry methodologies to record sleep–wake states in freely moving monkeys [9, 18, 33]. Telemetry implants permit the recording of EEG, EMG, and EOG signals under physiological conditions by limiting potential stress and allowing animals to remain in their home cages. Totally implantable telemetry systems have no external connectors or cables; thus, in addition to no restriction of movement, there are no exit wounds, reducing the risk of infection. Indeed, our results support that sleep assessed by telemetry in non-human primates represents a powerful model to investigate the physiological and molecular regulatory mechanisms of sleep. The very similar wakefulness and sleep stage patterns observed between animals, which was highlighted by low between-animal variations, and the finding that sleep occupied more than 45% of the total 24-h recording session indicate that stress levels remained low under these recording conditions.
Humans have a monophasic sleep pattern that involves one block of sleep during a single 24-h sleep–wake cycle. Our results showed a more fragmented and polyphasic sleep pattern in cynomolgus monkeys compared with humans, which was demonstrated by the distribution of wakefulness during the dark period (human: 5% of the dark phase [8]; cynomolgus monkeys: 10.5 ± 2.0% of the dark phase). On the other hand, marmosets exhibited a monophasic sleep pattern (wakefulness: 3.6 ± 0.6% of the dark phase). These results indicate that sleep patterns in the marmoset are closer to those in humans than those in cynomolgus monkeys. Longer durations of wakefulness during the main rest period are commonly considered a survival adaptation, given that constant vigilance may be needed in natural environments to reduce the risks of predation and to maintain social hierarchy. The common marmoset is entirely arboreal and lives in stable extended families consisting of one female, one male, their offspring, and their adult relatives [13]. On the other hand, cynomolgus monkeys, which prefer forested areas near water, are found at higher densities near riverbanks, lakeshores, or along seacoasts. Cynomolgus monkeys live in groups comprising 3–20 females, one or several males, and their offspring. A clear dominance hierarchy is evident, and group living appears to be maintained solely for the purposes of sexual activity and safety against predation [30]. These results suggest that cynomolgus monkeys may be more nervous and may have a more intricate social structure and hierarchy than those of marmoset monkeys.
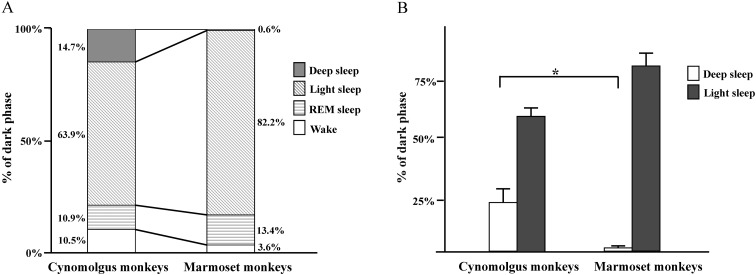
There were no great differences in sleep patterns between humans [8] and non-human primates. During the 12-h dark period, humans spend the majority of time sleeping (wakefulness, 5%; sleep, 95%). The time spent in NREM sleep was ~3-fold greater than that spent in REM sleep (NREM, 60–83%; REM, 20–25%). Light sleep is predominant in the NREM light–deep state pattern (deep, 13–23%; light, 47–60%). The distributions of sleep–wake states during the 12-h dark phase in cynomolgus monkeys and common marmoset monkeys are shown in Fig. 7. The NREM sleep (deep sleep and light sleep) duration in cynomolgus and common marmoset monkeys, representing more than approximately half of the time spent during sleep (Fig. 7A), was similar to that of other non-human primates, including squirrel monkeys [5] and rhesus monkeys (Macaca mulatta) [10, 18]. Sleep in non-human primates also may be regulated by circadian and homeostatic influences in a manner analogous to that in humans [11]. In the present study, a clear diurnal rhythm was observed in body temperature, with the highest temperatures observed during the light phase and the lowest during the dark phase in common marmoset monkeys. The differences in temperature that we observed between the light and dark phase were consistent with previous studies [16]. Marmosets showed body temperature changes that paralleled the pattern of wakefulness. Our results showed that NREM sleep activity in sleep–wake rhythms, especially that of deep sleep activity, between cynomolgus monkeys and common marmoset monkeys was different (Fig. 7B). During the rest period, deep sleep was detected in cynomolgus monkeys but rarely in common marmoset monkeys. The sleep–wake patterns of a particular species evolve to match environmental demands through adaptation of physiological functions controlled by the brain. NREM deep sleep is comprised of stages 3 and 4 [14]. Stage 3 NREM sleep is defined as at least 20% but not more than 50% slow EEG waves. Stage 4 NREM sleep has attributes similar to those of stage 3, except for a greater proportion of slow EEG waves, which make up 50% or more of the recording during stage 4. According to these criteria, we detected both stages 3 and 4 NREM sleep in the cynomolgus monkeys. On the other hand, in the common marmoset monkeys, we detected stage 3 but not stage 4. Stage 4 involves the deepest sleep, characterized by a lack of eye movement and decreased muscle activity; yet, muscles retain their ability to function. It is difficult to be awakened during deep sleep. Blood pressure falls, breathing slows, and body temperature drops even lower, with the body becoming immobile. Marmosets might not experience the same amount of deep sleep as a means to protect themselves. Future studies are warranted to clarify any variations between monkeys.
Fig. 7.
The distribution of sleep–wake states during the 12-h dark phase in cynomolgus monkeys and common marmoset monkeys. (A) The distribution of sleep–wake states during the 12-h dark phase. (B) The durations of deep sleep and light sleep during the 12-h dark phase. *P<0.05, compared with the appropriate control.
In conclusion, we reported differences in sleep–wake rhythms between cynomolgus and common marmoset monkeys using telemetry systems. This method more closely mimics natural sleep–wake states and would be an effective tool for examining sleep mechanisms and developing treatments for sleep disorders in non-human primates. The different NREM deep sleep patterns between cynomolgus monkeys and common marmoset monkeys are interesting and may be useful for examining sleep mechanisms and brain physiology.
Acknowledgments
We thank Dr. Kazuo Owada for useful technical supports and discussion in this study. We also thank Yo Aoyama and Takuro Yoshimoto for useful comments on an earlier draft of this manuscript.
References
- 1.Almirall H., Pigarev I., de la Calzada M.D., Pigareva M., Herrero M.T., Sagales T.1999. Nocturnal sleep structure and temperature slope in MPTP treated monkeys. J Neural Transm. Vienna 106: 1125–1134. doi: 10.1007/s007020050228 [DOI] [PubMed] [Google Scholar]
- 2.Balzamo E., Santucci V., Seri B., Vuillon-Cacciuttolo G., Bert J.1977. Nonhuman primates: laboratory animals of choice for neurophysiologic studies of sleep. Lab. Anim. Sci. 27: 879–886. [PubMed] [Google Scholar]
- 3.Bert J., Kripke D.F., Rhodes J.1970. Electroencephalogram of the mature chimpanzee: twenty-four hour recordings. Electroencephalogr. Clin. Neurophysiol. 28: 368–373. doi: 10.1016/0013-4694(70)90229-4 [DOI] [PubMed] [Google Scholar]
- 4.Bouyer J.J., Dedet L., Debray O., Rougeul A.1978. Restraint in primate chair may cause unusual behaviour in baboons; electrocorticographic correlates and corrective effects of diazepam. Electroencephalogr. Clin. Neurophysiol. 44: 562–567. doi: 10.1016/0013-4694(78)90123-2 [DOI] [PubMed] [Google Scholar]
- 5.Breton P., Gourmelon P., Court L.1986. New findings on sleep stage organization in squirrel monkeys. Electroencephalogr. Clin. Neurophysiol. 64: 563–567. doi: 10.1016/0013-4694(86)90195-1 [DOI] [PubMed] [Google Scholar]
- 6.Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W.2012. Control of sleep and wakefulness. Physiol. Rev. 92: 1087–1187. doi: 10.1152/physrev.00032.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell S.S., Tobler I.1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8: 269–300. doi: 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- 8.Carskadon M.A., Dement W.C.1994. Normal human sleep: an overview. pp. 16–25. In: Principles and practice of sleep medicine (Kryger, M.H., Roth, T., Dement, W.C., eds.). W.B. Saunders Company, Philadelphia. [Google Scholar]
- 9.Crofts H.S., Wilson S., Muggleton N.G., Nutt D.J., Scott E.A., Pearce P.C.2001. Investigation of the sleep electrocorticogram of the common marmoset (Callithrix jacchus) using radiotelemetry. Clin. Neurophysiol. 112: 2265–2273. doi: 10.1016/S1388-2457(01)00699-X [DOI] [PubMed] [Google Scholar]
- 10.Daley J.T., Turner R.S., Freeman A., Bliwise D.L., Rye D.B.2006. Prolonged assessment of sleep and daytime sleepiness in unrestrained Macaca mulatta. Sleep 29: 221–231. [PubMed] [Google Scholar]
- 11.Dijk D.J., Czeisler C.A.1995. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15: 3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi M.2012. Circadian clock-deficient mice as a tool for exploring disease etiology. Biol. Pharm. Bull. 35: 1385–1391. doi: 10.1248/bpb.b12-00364 [DOI] [PubMed] [Google Scholar]
- 13.Ferrari S.F., Digby L.J.1996. Wild Callithrix group: stable extended families? Am. J. Primatol. 38: 19–27. doi: [DOI] [PubMed] [Google Scholar]
- 14.Genzel L., Kroes M.C., Dresler M., Battaglia F.P.2014. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 37: 10–19. doi: 10.1016/j.tins.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Hiyoshi H., Terao A., Okamatsu-Ogura Y., Kimura K.2014. Characteristics of sleep and wakefulness in wild-derived inbred mice. Exp. Anim. 63: 205–213. doi: 10.1538/expanim.63.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann K., Coolen A., Schlumbohm C., Meerlo P., Fuchs E.2012. Remote long-term registrations of sleep-wake rhythms, core body temperature and activity in marmoset monkeys. Behav. Brain Res. 235: 113–123. doi: 10.1016/j.bbr.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 17.Holcombe V., Sterman M.B., Goodman S.J., Fairchild M.B.1979. The immobilization response in rhesus monkey: a behavioral and electroencephalographic study. Exp. Neurol. 63: 420–435. doi: 10.1016/0014-4886(79)90136-5 [DOI] [PubMed] [Google Scholar]
- 18.Hsieh K.C., Robinson E.L., Fuller C.A.2008. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep 31: 1239–1250. [PMC free article] [PubMed] [Google Scholar]
- 19.Mileva-Seitz V.R., Louis R.P., Stephenson R.2005. A visual aid for computer-based analysis of sleep-wake state in rats. J. Neurosci. Methods 148: 43–48. doi: 10.1016/j.jneumeth.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Papadimitriou G.N., Linkowski P.2005. Sleep disturbance in anxiety disorders. Int. Rev. Psychiatry 17: 229–236. doi: 10.1080/09540260500104524 [DOI] [PubMed] [Google Scholar]
- 21.Pearce P.C., Crofts H.S., Muggleton N.G., Scott E.A.1998. Concurrent monitoring of EEG and performance in the common marmoset: a methodological approach. Physiol. Behav. 63: 591–599. doi: 10.1016/S0031-9384(97)00494-0 [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A., Kales A.1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. pp. 1–57. In: Brain Information Service, Brain Research Institute, University of California, Los Angeles (Rechtschaffen, A., and Kales, A. eds.), University of California, California. [Google Scholar]
- 23.Reite M.L., Rhodes J.M., Kavan E., Adey W.R.1965. Normal sleep patterns in Macaque monkey. Arch. Neurol. 12: 133–144. doi: 10.1001/archneur.1965.00460260023003 [DOI] [PubMed] [Google Scholar]
- 24.Riemann D., Berger M., Voderholzer U.2001. Sleep and depression--results from psychobiological studies: an overview. Biol. Psychol. 57: 67–103. doi: 10.1016/S0301-0511(01)00090-4 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J.R., Roth T.2008. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr. Neuropharmacol. 6: 367–378. doi: 10.2174/157015908787386050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel J.M.2005. Clues to the functions of mammalian sleep. Nature 437: 1264–1271. doi: 10.1038/nature04285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson R., Caron A.M., Cassel D.B., Kostela J.C.2009. Automated analysis of sleep-wake state in rats. J. Neurosci. Methods 184: 263–274. doi: 10.1016/j.jneumeth.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 28.Tobler I.1995. Is sleep fundamentally different between mammalian species? Behav. Brain Res. 69: 35–41. doi: 10.1016/0166-4328(95)00025-O [DOI] [PubMed] [Google Scholar]
- 29.Unno K., Ozaki T., Mohammad S., Tsuno S., Ikeda-Sagara M., Honda K., Ikeda M.2012. First and second generation H1 histamine receptor antagonists produce different sleep-inducing profiles in rats. Eur. J. Pharmacol. 683: 179–185. doi: 10.1016/j.ejphar.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 30.van Schaik C.P., van Noordwijk M.A., de Boer R.J., den Tonkelaar I.1983. The effect of group size on time budgets and social behavior in wild long-tailed macaques. Behav. Ecol. Sociobiol. 13: 173–181. doi: 10.1007/BF00299920 [DOI] [Google Scholar]
- 31.Wulff K., Gatti S., Wettstein J.G., Foster R.G.2010. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11: 589–599. doi: 10.1038/nrn2868 [DOI] [PubMed] [Google Scholar]
- 32.Yasenkov R., Deboer T.2012. Circadian modulation of sleep in rodents. Prog. Brain Res. 199: 203–218. doi: 10.1016/B978-0-444-59427-3.00012-5 [DOI] [PubMed] [Google Scholar]
- 33.Yukuhiro N., Kimura H., Nishikawa H., Ohkawa S., Yoshikubo S., Miyamoto M.2004. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. 1027: 59–66. doi: 10.1016/j.brainres.2004.08.035 [DOI] [PubMed] [Google Scholar]